Abstract
In the present study, the seed extract of Vitis vinifera L. (grape) prepared with the methanol-ethanol mixture (volume ratio of 1:1) was evaluated for antibacterial activity on some Gram-positive and Gram-negative strains. In addition, the activity of the extract on the production potential of Quorum Sensing (QS)-dependent virulence factors on Pseudomonas aeruginosa PAO1 and Chromobacterium violaceum was investigated. The phytochemical analysis resulted in the identification of 23 phenolic phytochemicals; epicatechin, catechin and p-hydroxybenzoic acid were determined as the major components in the extract, respectively. The mode of action for the anti-QS activity was explored by molecular docking. The binding affinity of the three major phytoconstituents towards LasR and CviR was determined. Computational analysis demonstrated that the three components (epicatechin, catechin, p-hydroxybenzoic acid) could bind to LasR and CviR. The binding affinity towards LasR was higher than CviR. Hence, the activity of the extract on P. aeruginosa and C. violaceum could result from the competitive inhibition of LasR and CviR by the major components, respectively. Considering the literature and the computational analysis, it is thought that the antibacterial and anti-QS activity of the seed extract may be related to the synergistic effect of the phenolic phytochemicals it contains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term “antibiotic resistance” encompasses defence demonstrated by bacteria to antibiotics (Sharma et al. 2005). The inexorable rise in levels of this resistance is associated with high health care costs and mortality rates. As the biggest challenge in the treatment of infectious diseases, antibiotic resistance is recognized as a major concern for public health at the global level and requires international approaches (Getahun et al. 2020). To combat this worldwide threat, understanding the resistance mechanisms of bacteria remains the key to scientific progress. Among these, the QS system associated with bacterial virulence is the most remarkable mechanism in recent decades (Medarametla et al. 2021).
QS is a regulatory communication mechanism that allows bacterial populations to coordinate their behaviors by regulating specific genes that can play important roles in bacteria such as biofilm formation and production of virulence factors (such as elastase and pyocyanin) and leads to the development of drug resistance in bacteria. Therefore, inhibition of this mechanism is being investigated as an alternative treatment strategy in drug discovery programs to overcome antibiotic resistance (Tonkin et al. 2021).
P. aeruginosa PAO1 is a medically important Gram-negative opportunistic rod that shows resistance to antibiotics and is classified as a major cause of nosocomial infections (Lyczak et al. 2000). This resistance results from the production of virulence factors and biofilm formation all under the control of the QS system (Pompilio et al. 2015). Therefore, inhibition of QS pathways is considered a promising strategy for the control of microbial pathogenesis. In fact, many pathogenic bacteria use this system, including Stenotrophomonas maltophilia, Escherichia coli, Vibrio cholerae, Acinetobacter baumannii and C. violaceum. Although rare, it has also been reported that C. violaceum cause lung infections and metastatic abscesses of the spleen, brain and lymph nodes.
With increasing microbial resistance against antibiotics and environmental conditions, applying natural products (especially extracts) could provide a promising means of treatment. It is now well known that grape seed is a rich source of polyphenol compounds. Therefore, its antimicrobial activity has been investigated. In a study, the antibacterial activities of grape seed extracts against 15 bacteria were investigated. All tested bacteria were inhibited by grape seed extracts using the agar-well diffusion method. There are regions in Italy where alcoholic beverages obtained from V. vinifera L. are used to treat diseases related to the digestive system. In addition, wine, vinegar, and spirit obtained from the plant extract are used as an ointment, poultice, and mouthwash in the Republic of Cyprus (Baydar et al. 2006; Insanu et al. 2021).
To date, bioactive compounds of plant-based natural products have provided new drug leads used for the treatment of various diseases, including bacterial infections, and the discovery of QS mechanism has triggered the searches for anti-QS phytocompounds (Zahin et al. 2010).
In the present research described here, we aimed to evaluate the antibacterial activity of the Vitis vinifera L. (grape) seed extract on some Gram-positive and Gram-negative strains. In addition, the effect of the grape seed extract on the production potential of QS-dependent virulence factors on P. aeruginosa PAO1 and C. violaceum was investigated. To the best of our knowledge, there is no published comprehensive study in the literature on these activities of grape seed. We hope the results of our study will shed light on the development of new anti-infective therapeutic agents.
Materials and Methods
Plant Material and Extract Preparation
The grapes whose seeds were used in the study were commercially supplied and identified as Vitis vinifera L. by the botanist. The seeds were separated from the succulent parts of the fruits manually, dried in shade at 25 °C for 5 days, and ground into powder by using a grinding machine (Waring 8011 EB). After that, 8.8 g of seed powder was subjected to ultrasonic extraction with 88 mL methanol-ethanol mixture (volume ratio of 1:1) for 45 min. The extract was filtered and the filtrate was evaporated to dryness at 36 °C using a rotary evaporator (Heidolph Hei-Vap Rotary Evaporator [Germany]). At the end of the process, the crude plant extract remaining in the flask was weighed and the amount recorded, then dissolved with dimethylsulfoxide (DMSO) and transferred to a vial.
Phytochemical Screening
The phytochemical analysis of the seed extract was carried out using the high-performance liquid chromatography (HPLC) technique. HPLC conditions were presented in Table 1.
Screening Seed Extract for Antibacterial Activity
In our study, some Gram-positive and Gram-negative strains obtained from the bacterial stock available (P. aeruginosa PAO1; Bacillus cereus ATCC 11778; Enterococcus faecalis ATCC 29212; Staphylococcus aureus ATCC 25923; Methicillin-Resistant Staphylococcus aureus (MRSA); ATCC 43300; Enterobacter aerogenes ATCC 13048; and Escherichia coli ATCC 25922) were used. Antibacterial effects of grape seed extract were tested on these strains using the agar-well method (Holder and Boyce 1994). Bacteria grown in Luria-Bertani (LB) medium at 37 °C overnight were prepared the next day according to 0.5 McFarland turbid value. A total of 100 μL of the bacterial suspension prepared in 0.5 McFarland turbidity was added into 5 mL soft agar (0.5% agar) and poured onto the Müller-Hinton agar medium prepared in Petri dishes. The wells were opened with the help of a 6-mm diameter glass pipette on the media that were left to dry for a while and 100 μL of the seed extract was added into the wells. Antibacterial activity was determined by measuring the zone diameters formed at the end of 24‑h incubation at 37 °C. All experiments were repeated three times unless otherwise mentioned.
Screening Seed Extract for QS Inhibitory Activity
Biofilm Formation Assay
The effect of seed extract on biofilm formation was investigated on P. aeruginosa PAO1 strain using the crystal violet method (Önem et al. 2018; O’Toole 2010). In all, 10 µL of an overnight culture of PAO1 (Optic Density [OD, Radiant Power] at 600 nm = 0.05) was added to a 96-well microplate containing 160 µL of freshly prepared Luria–Bertani Broth (LBB) medium and 20 µL of the seed extract. The microplate was incubated at 37 °C for 48 h. After incubation, the culture on the plates was drained and washed three times with sterile water. By adding 125 µL aqueous solution of crystal violet (0.1%) to the wells, the biofilm layer was dyed for 30 min, then the paint was poured and the excess was washed with distilled water. A total of 200 µL of 95% ethanol was added and the reaction mixture was read spectrophotometrically at 570 nm. The reference PAO1 strain was used as a positive control in this experiment. The negative control was sterile LBB.
The following formula was used to determine the inhibition of biofilm formation:
- OD:
-
Optic Density
Elastolytic Assay
The elastolytic activity of the extract was determined with the Elastin Congo Red (ECR) test according to the method of Ohman et al. (Ohman et al. 1980). This test helps to measure the elastase activity in the supernatant of bacteria culture using ECR as substrate. Elastase B degrades elastin and this causes the Congo red dye to be released into the supernatant. The activity is assessed by spectrophotometric quantification.
Adhering to the mentioned procedure, 100 µL of the extract was mixed with 10 mL LBB containing OD 0.05 at 600 nm PAO1 culture and left to incubate at 37 °C by shaking for 16–18 h. A total of 100 µL of the supernatant part of this culture was transferred to a tube and 900 μL ECR buffer was added on. This mixture was incubated at 37 °C for 3 h with shaking at 200 rpm. After incubation, the sample was centrifuged at 4500 rpm for 5 min. The supernatant of the sample was transferred to a cuvette and its optical absorption at 495 nm wavelength was read spectrophotometrically (BioTek-Epoch 2 Microplate Spectrophotometer). PAO1 culture and LBB were used as positive and negative controls, respectively.
Pyocyanin Inhibition Assay
The effect of the extract on pyocyanin pigment production was determined as described by Essar et al. (Essar et al. 1990). A total of 100 µL of plant extract were added to 10 mL of LBB medium and incubated at 37°C in a shaker incubator overnight. After the incubation period, 5 mL of chloroform was added to the medium and vortexed for 30 s. The sub-phase formed in the medium and separated from chloroform was transferred to tubes as 2 mL. In all, 1 mL HCl-water mixture (0.2 mol/L HCl) was added on it and once again vortexed for 30 s. The absorbance of the pink phase formed on the upper part of the tubes was recorded at 520 nm. Untreated PAO1 served as the positive control.
Inhibition of Violacein Production
In order to quantify violacein inhibitory activity of V. vinifera L. seed extract on C. violaceum 12472, spectrophotometric flask incubation assay was used (Choo et al. 2006). A total of 100 µL of plant extract were added to 5 ml LBB medium and incubated at 30 °C in a shaking incubator overnight. At the end of the incubation, 1 mL bacteria culture was centrifuged at 13,000 rev min−1 for 10 min. Then the supernatant was removed and 1 mL of DMSO was added on pellet and vortex for 30 s. After vortex again at 13,000 rev min−1 for 10 min, supernatant with violacein was read at 585 nm.
Statistical Analysis
The experiments were carried out in triplicate according to the randomized plot design and the data obtained were subjected to variance analysis using the JMP 8 packet statistics program. Statistical differences were marked by the LSD multiple comparison test.
Molecular Docking
Molecular docking was performed with AutoDock Vina (Trott and Olson 2010). The crystal structure of LasR with PDB code of 6MWL, (Paczkowski et al. 2019) and the structure of CviR with PDB code of 3QP1 (Chen et al. 2011) was obtained from a protein data bank (PDB). The structures of the ligands were obtained from PubChem (Kim et al. 2021). Prior to docking, grid boxes that encompass the ligands bound in the structure of the proteins were determined. The protein structures obtained from a PDB were prepared by removing water, adding polar hydrogens, and assigning Gasteiger charges. Similarly, the ligands were prepared by adding polar hydrogens and assigning Gasteiger charges. Then, AutoDock Vina was run (Onem et al. 2021). Docking outcomes were visualized and analyzed with Biovia Discovery Studio.
Results
Results of Antibacterial Activity Test
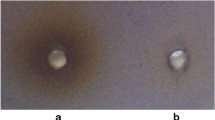
According to the data obtained, while the extract showed different antibacterial effects on Gram-positive strains, no effect was observed on Gram-negative strains (Table 2).
Confirmation of Anti-QS Activity
Fig. 1 presents the results of the antibiofilm formation assay. The extract inhibited biofilm formation of PAO1 by 38%.
The results of the elastolytic assay are given in Fig. 2. The percentage of elastase inhibition of the extract was calculated as 79%.
Results of the pyocyanin inhibition assay are shown in Fig. 3. The pyocyanin inhibition rate of the extract was found as 89%.
Violacein Inhibition
As a result of the experiment in which the inhibition effect of violet pigment production by the C. violaceum 12472 with QS control was investigated with grape seed extract and 60% inhibition was determined at 50 mg concentration (Fig. 4).
Results of HPLC Analysis
The HPLC chromatogram of the grape seed extract (Fig. 5) shows the presence of 23 phenolic components, all of which are given in Table 3. Epicatechin had the highest concentration (2346 µg/mL), followed by catechin with a concentration of 1204 µg/mL, then p-hydroxybenzoic acid with a concentration of 414 µg/mL.
Molecular Docking
The mode of action for the activity of the extract on the QS system was investigated through molecular docking. For this purpose, the most abundant three phytochemical constituents (epicatechin, catechin, and p-hydroxybenzoic acid) were docked on LasR and CviR. The docking outcomes showed that these phytoconstituents had better interaction with LasR. The interactions of LasR with epicatechin and p-hydroxybenzoic were given in Fig. 6 and Table 4. The interaction of catechin with LasR was investigated in a previous study (Table 4) (Gürağaç Dereli et al. 2022).
The interactions of the three most abundant molecules were also compared with the interactions of the natural ligand of LasR, N‑3-(oxododecanoyl)-l-homoserine lactone (OdDHL). The results were analyzed accordingly (Table 4).
The binding mode of epicatechin, catechin, and p-hydroxybenzoic acid with CviR was also studied. There were interactions of the three abundant molecules with the protein. However, the binding ability of the ligands with CviR was found to be less than that of LasR (Fig. 7). The binding of the components was compared with its natural ligand, N‑hexanoyl-L-homoserine lactone (C6-HSL). The binding energies of epicatechin, catechin, p-hydroxybenzoic acid, and C6-HSL were found to be −9.1 kcal/mol, −6.9 kcal/mol, −6.7 kcal/mol, −6.9 kcal/mol, respectively.
Discussion
Hospital infections caused by multi-drug resistant bacteria resulting from the overuse and misuse of antibiotics are a nightmare for physicians all over the world. The emergence of bacteria resistance to existing conventional antibiotics and the inadequacy of antibiotics in the treatment of infections caused by these types of bacteria indicate the urgent need for new strategies to develop effective therapeutic options (Saleem et al. 2010). The use of plants for medicinal purposes has been going on for thousands of years (Solecki 1975). They have formed the basis of many traditional medicine systems to date and continue to offer new hope to humanity in terms of treatment today (Jachak and Saklani 2007). Recently, the importance of phytochemicals to reduce bacterial virulence in P. aeruginosa has been realized and researches in this field have gained momentum. Grape plant V. vinifera L. is an economically and medicinally important member of the family Vitaceae. It is one of the most planted fruit plants all over the world and represents a precious resource of nutraceuticals and pharmaceuticals (Vivier and Pretorius 2002). Especially the leaves and seeds of the plant, the fruit of which is consumed as a nutritional supplement, have been the subject of scientific research. Recent studies have demonstrated that the seed part of the plant possesses a broad spectrum of pharmacological activities such as antioxidant, antidiabetic, cardioprotective, neuroprotective, hepatoprotective, anticancer, anti-inflammatory, antiviral, and antimicrobial activities (Insanu et al. 2021). The reports of the studies to determine the chemical content of seeds indicate the presence of large amounts of phenolic compounds, predominantly epicatechin, catechin, procyanidin, and gallic acid (Monagas et al. 2003). It has been shown that partially hydrophobic phenolic compounds in the seeds interact with the bacterial cell wall and lipopolysaccharide interfaces by reducing membrane stability and are directly related to antibacterial activity (Baydar et al. 2006). In the current study, grape seed extract was evaluated for both antibacterial and anti-QS activity. There is no other study in the literature so comprehensively investigating the effectiveness of grape seed against bacteria.
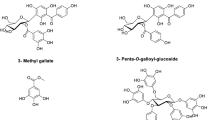
The results of the experiments carried out proved that the extract has antibacterial activity on the Gram-positive bacteria used in this study and the extract has inhibitory activity on the regulation of virulence and biofilm formation. Phytochemical analysis performed on the extract resulted in the identification of 23 phenolic phytocomponents, with epicatechin (Fig. 8a), catechin (Fig. 8b), and p-hydroxybenzoic acid (Fig. 8c) being determined as the major compounds in the extract.
The phenolic phytoconstituents have attracted scientific interest in terms of their diverse biological roles, including antibiotic and anti-QS activities. In a study by Vandeputte and colleagues, catechin was found to have inhibitory activity on elastase and pyocyanin production and biofilm formation by down-regulating QS gene expression in PAO1. On the other hand, it was determined that this compound had an inhibitory effect on pyocyanin production (Vandeputte et al. 2010). In the study conducted by Lahiri and associates, it has been determined that catechin obtained from Azadirachta indica leaf extract is highly active in preventing dental biofilm, and this compound can be used in the treatment of chronic biofilm-associated infections (Lahiri et al. 2021). Furiga and coworkers proved that catechin and its epimer epicatechin inhibited the formation of multi-species biofilms composed of oral bacteria (Furiga et al. 2014, 2008).
In the phytochemical content analysis, epicatechin, catechin, and p-hydroxybenzoic acid were found to be the three abundant components of the grape seed. The anti-QS activity investigation demonstrated that the extracts were effective on both P. aeruginosa and C. violaceum. The experimental activity test results were explained through molecular docking. This was done by the docking of the three abundant components with LasR and CviR.
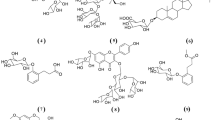
The molecular docking of epicatechin, catechin, and p-hydroxybenzoic acid with LasR revealed the high binding affinity of the phytochemicals; epicatechin in particular interacted with seven conventional hydrogen bonds. The interactions of the components were better than the interaction of the natural ligand, OdDHL. Furthermore, catechin and epicatechin had slightly lower binding energy than OdDHL. The three components had common interaction residues with OdDHL (Arg55, Tyr58, Thr69) (Fig. 6). The high binding affinity with similar binding points implicated that these components would inhibit the LasR by competitively interfering with the binding of the autoinducer, OdDHL. Therefore, the anti-QS activity on P. aeruginosa could be the result of the LasR inhibition.
The molecular docking of epicatechin, catechin, and p-hydroxybenzoic acid demonstrated that these components had binding affinity with CviR. However, the ability to bind to CviR was found to be lower than their binding affinity towards LasR. The three components exhibited better interaction with LasR in binding energy and interaction strength. These components had almost all the interactions of the natural ligand, C6-HSL (Leu57, Leu72, Val75, Tyr80, Tyr88, Asp97, Ser155) with CviR. The binding energies of the molecules were close to each other.
The components had less interaction than C6-HSL separately (Fig. 7). Together with this, the cumulative interactions of the three constituents were higher than C6-HSL. Hence, the anti-QS effect on C. violaceum might result from the inhibition of CviR by the constituents competitively. In the experimental study, grape seed extract was found to be more active on P. aeruginosa than C. violaceum. Similarly, in the computational study, the three phytoconstituents had a better binding affinity towards LasR than CviR. Hence, the computational analysis confirmed the outcomes of the activity study. As a result, considering the literature data and the computational analysis results, it is thought that the antibacterial and anti-QS activity of the grape seed extract may be due to the synergistic effect of the phenolic phytochemicals in its content. Hopefully, the outcomes of this study will provide new insight into the treatment of infectious diseases.
References
Baydar NG, Sagdic O, Ozkan G, Cetin S (2006) Determination of antibacterial effects and total phenolic contents of grape (Vitis vinifera L.) seed extracts. Int J Food Sci Tech 41:799–804. https://doi.org/10.1111/j.1365-2621.2005.01095.x
Chen G, Swem LR, Swem DL, Stauff DL, O’Loughlin CT, Jeffrey PD, Bassler BL, Hughson FM (2011) A strategy for antagonizing quorum sensing. Mol Cell 42:199–209. https://doi.org/10.1016/j.molcel.2011.04.003
Choo JH, Rukayadi Y, Hwang JK (2006) Inhibition of bacterial quorum sensing by vanilla extract. Lett Appl Microbiol 42:637–641. https://doi.org/10.1111/j.1472-765X.2006.01928.x
Essar DW, Eberly L, Hadero A, Crawford IP (1990) Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: Interchangeability of the two anthranilate synthase and evolutionary implications. J Bacteriol 172:884–900. https://doi.org/10.1128/jb.172.2.884-900.1990
Furiga A, Lonvaud-Funel A, Dorignac G, Badet C (2008) In vitro anti-bacterial and anti-adherence effects of natural polyphenolic compounds on oral bacteria. J Appl Microbiol 105:1470–1476. https://doi.org/10.1111/j.1365-2672.2008.03882.x
Furiga A, Roques C, Badet C (2014) Preventive effects of an original combination of grape seed polyphenols with amine fluoride on dental biofilm formation and oxidative damage by oral bacteria. J Appl Microbiol 116:761–771. https://doi.org/10.1111/jam.12395
Getahun H, Smith I, Trivedi K, Paulin S, Balkhy HH (2020) Tackling antimicrobial resistance in the COVID-19 pandemic. Bull World Health Organ 98:19–20. https://doi.org/10.2471/BLT.20.268573
Gürağaç Dereli FT, Önem E, Özaydin AG, Arın E, Muhammed MT (2022) Persea americana Mill.: as a potent quorum sensing inhibitor of Pseudomonas aeruginosa PAO1 virulence. Int J Second Metab 9(1):14–26. https://doi.org/10.21448/ijsm
Holder IA, Boyce ST (1994) Agar well diffusion assay testing of bacterial susceptibility to various antimicrobials in concentrations non-toxic for human cells in culture. Burns 20:426–429. https://doi.org/10.1016/0305-4179(94)90035-3
Insanu M, Karimah H, Pramastya H, Fidrianny I (2021) Phytochemical compounds and pharmacological activities of vitis vinifera L.: An updated review. Biointerface Res Appl Chem 11:13829–13849. https://doi.org/10.33263/BRIAC115.1382913849
Jachak SM, Saklani A (2007) Challenges and opportunities in drug discovery from plants. Curr Sci 92:1251–1257
Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L, Zhang J, Bolton EE (2021) PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res 49:D1388–D1395. https://doi.org/10.1093/nar/gkaa971
Lahiri D, Nag M, Dutta B, Mukherjee I, Ghosh S, Dey A, Banerjee R, Ray RR (2021) Catechin as the most efficient bioactive compound from azadirachta indica with antibiofilm and anti-quorum sensing activities against dental biofilm: an in vitro and in Silico study. Appl Biochem Biotechnol 193:1617–1630. https://doi.org/10.1007/s12010-021-03511-1
Lyczak JB, Cannon CL, Pier GB (2000) Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect 2:1051–1060. https://doi.org/10.1016/S1286-4579(00)01259-4
Medarametla P, Kronenberger T, Laitinen T, Poso A (2021) Structural characterization of LsrK as a quorum sensing target and a comparison between X‑ray and homology models. J Chem Inf Model 61:1346–1353. https://doi.org/10.1021/acs.jcim.0c01233
Monagas M, Gómez-Cordovés C, Bartolomé B, Laureano O, Ricardo Da Silva JM (2003) Monomeric, oligomeric, and polymeric flavan-3-ol composition of wines and grapes from vitis vinifera L. Cv. graciano, tempranillo, and Cabernet Sauvignon. J Agric Food Chem 51:6475–6481. https://doi.org/10.1021/jf030325
Ohman DE, Cryz SJ, Iglewski BH (1980) Isolation and characterization of a Pseudomonas aeruginosa PAO mutant that produces altered elastase. J Bacteriol 142:836–842. https://doi.org/10.1128/jb.142.3.836-842.1980
Önem E, Dündar Y, Ulusoy S, Noyanalpan N, Bosgelmez-Tinaz G (2018) Anti-quorum sensing activity of 1, 3‑dihydro-2H-benzimidazol-2-one derivatives. Fresenius Environ Bull 27:9906–9912
Onem E, Soyocak A, Muhammed MT, Ak A (2021) In vitro and in Silico assessment of the potential of Niaouli essential oil as a quorum sensing inhibitor of biofilm formation and its effects on fibroblast cell viability. Braz Arch Biol Technol 64:1–11
O’Toole GA (2010) Microtiter dish Biofilm formation assay. J Vis Exp. https://doi.org/10.3791/2437
Paczkowski JE, Mccready AR, Cong J, Li Z, Jeffrey PD, Smith CD, Henke BR, Hughson FM, Bassler BL (2019) An autoinducer analog reveals an alternative mode of ligand binding for the LasR quorum-sensing receptor. ACS Chem Biol 14:378–389. https://doi.org/10.1021/acschembio.8b00971.An
Pompilio A, Crocetta V, De Nicola S, Verginelli F, Fiscarelli E, Di Bonaventura G (2015) Cooperative pathogenicity in cystic fibrosis: Stenotrophomonas maltophilia modulates Pseudomonas aeruginosa virulence in mixed biofilm. Front Microbiol 6:1–13. https://doi.org/10.3389/fmicb.2015.00951
Saleem M, Nazir M, Ali MS, Hussain H, Lee YS, Riaz N, Jabbar A (2010) Antimicrobial natural products: an update on future antibiotic drug candidates. Nat Prod Rep 27:238–254. https://doi.org/10.1039/b916096e
Sharma R, Sharma CL, Kapoor B (2005) Antibacterial resistance: current problems and possible solutions. Indian J Med Sci 59:120–129. https://doi.org/10.4103/0019-5359.15091
Solecki RS (1975) Shanidar IV, a Neanderthal flower burial in northern Iraq. Science 190:880–881. https://doi.org/10.1126/science.190.4217.880
Tonkin M, Khan S, Wani MY, Ahmad A (2021) Quorum sensing—a stratagem for conquering multi-drug resistant pathogens. CPD 27:2835–2847. https://doi.org/10.2174/1381612826666201210105638
Trott O, Olson A (2010) Autodock vina: improving the speed and accuracy of docking. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334.AutoDock
Vandeputte OM, Kiendrebeogo M, Rajaonson S, Diallo B, Mol A, El Jaziri M, Baucher M (2010) Identification of catechin as one of the flavonoids from combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in pseudomonas aeruginosa PAQ1. Appl Environ Microbiol 76:243–253. https://doi.org/10.1128/AEM.01059-09
Vivier MA, Pretorius IS (2002) Genetically tailored grapevines for the wine industry. Trends Biotechnol 20:472–478. https://doi.org/10.1016/S0167-7799(02)02058-9
Zahin M, Hasan S, Aqil F, Khan MSA, Husain FM, Ahmad I (2010) Screening of certain medicinal plants from India for their anti-quorum sensing activity. Indian J Exp Biol 48:1219–1224
Author information
Authors and Affiliations
Contributions
All authors participated in the development and design of the study. B.M. Ibrahim analyzed the antimicrobial activity data and prepared the manuscript. F.T. Dereli took part in data collection and read the article. E. Önem was involved in data collection, HPLC analysis of data, QS activity data, and statistical analysis. E. Arin was involved in data collection and analysis of data. M.T. Muhammed participated in the molecular docking description and read the article. All authors have read and approved the final version of the article.
Corresponding author
Ethics declarations
Conflict of interest
B.M. Ibrahim, F.T. Dereli, Y. Erzurumlu, E. Önem, E. Arin, and M.T. Muhammed declare that they have no competing interests.
Additional information
Data Availability
If further information about this study is required please contact the authors.
Code availability
Not applicable.
Rights and permissions
Springer Nature oder sein Lizenzgeber (z.B. eine Gesellschaft oder ein*e andere*r Vertragspartner*in) hält die ausschließlichen Nutzungsrechte an diesem Artikel kraft eines Verlagsvertrags mit dem/den Autor*in(nen) oder anderen Rechteinhaber*in(nen); die Selbstarchivierung der akzeptierten Manuskriptversion dieses Artikels durch Autor*in(nen) unterliegt ausschließlich den Bedingungen dieses Verlagsvertrags und dem geltenden Recht.
About this article
Cite this article
Ibrahim, B.M., Dereli, F.T., Erzurumlu, Y. et al. Anti-Quorum Sensing Activity of Vitis vinifera L. Seed Extract on Some Bacteria: A Greener Alternative Against Antimicrobial Resistance. Erwerbs-Obstbau 65, 1931–1939 (2023). https://doi.org/10.1007/s10341-022-00783-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10341-022-00783-7