Abstract
In modern pear cultivation, clonal quince and pear rootstocks are preferred because they are easy to maintain and harvest. Also, they form dwarf plants and improve fruit quality compared to pear seedling rootstocks. However, graft incompatibility can be involved between different species or genera. The aim of the study was to determine the graft compatibility of the ‘Deveci’ and ‘Williams’ pear cultivars with different pear and quince rootstocks by carbohydrate analysis. Carbohydrate accumulation in the graft union was also observed with iodized potassium iodide (KI) staining. In terms of rootstocks, there were no differences in starch and carbohydrate content, but statistically differences were found in sugar contents. Significant differences were also found between cultivars and graft union in terms of the examined traits. Sugar content was highest in OHxF 333 and lowest in seedling rootstocks. There were no statistical differences in the starch content between the graft unions of the ‘Deveci’ cultivar, while starch accumulation was higher above the graft union than below and graft union in the ‘Williams’ cultivar grafted on the quince rootstock. In the study, it was determined that there were higher carbohydrate accumulation in the scion and graft union than below the graft union in ‘Williams’ grafted quince rootstocks, which was also confirmed by staining with KI. As a result of the study, it was concluded that carbohydrate accumulation analysis can be used to determine the graft compatibility of the pear cultivars with the different quince and pear rootstocks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pear (Pyrus communis L.) is one of the most cultivated temperate fruit species after grapes and apples on the world (Jackson 2003; Ozcagiran et al. 2005). Turkey is the origin center of many fruit species including pear. Turkey has a rich variety of pear with more than 600 genotypes (Ercisli 2004; Ozcagiran et al. 2005). According to the FAO data provided in 2019, world’s pear production is about 23.9 million tons and Turkey has a 2.2% share with 530,723 tons production. Turkey is the 4th important producer after China, Argentina and USA (FAO 2021).
In modern pear cultivation, clonal quince and pear rootstocks are preferred because they are easy to maintain and harvest. Also, they form dwarf plants and improve fruit quality compared to pear seedling rootstocks. In the world, pear cultivation is mostly done by grafting on Pyrus or Cydonia rootstocks. Quince (Cydonia) rootstocks have been widely used due to some beneficial characteristics such as size reduction, yield precocity and improvements in fruit quality and size. On the other hand, Pyrus rootstocks produce larger trees, but they have a longer precocity period than Cydonia rootstocks (Barritt 1992: Bell et al. 1996; Stern 2008; Francescatto et al. 2010; Dondini and Sansavini 2012). In modern fruit production, cultivars are grafted on different rootstocks to control growth and vigor. Grafting can be made between different cultivars of the same species, as well as between species or genera. When graft is made between different species or genera, possibility of graft incompatibility may occur between grafting partners (Hartmann et al. 2011; Darikova et al. 2011; Dogra et al. 2018). As an example, when pear grafted on quince rootstocks, graft incompatibility may occur with some incompatibility symptoms in early and late stage of growing period (Ermel et al. 1997; Errea 1998; Pina and Errea 2005; Daverynejad et al. 2008; Rahman et al. 2017; Dolkar et al. 2018). The swelling on the graft site, leaflet becoming yellowish, reduction of vegetative growth and differences in growth rate between rootstock and scion are some symptoms of the graft incompatibility. Graft incompatibility may occur due to genetically, physiologically, anatomically and biochemical reasons (Hartmann et al. 2011). This phenomenon might be due to the absence of differentiation of callus tissues into new phloem tissues or necrosis of the cells in the site of scion (Moore 1983). This can cause a miss-joining between rootstock and scion, leading to lack of lignification of cells in the site of scion. Starch accumulation above, below and in the graft union might cause decay of phloem. Graft components with poor growth not only lead to structural abnormalities in the site of scion, but are also correlated with irregularities in starch distribution (Davarynejad et al. 2008; Darikova et al. 2011). Formation of assimilates and their mobility between root and shoot is highly affected by the level of rootstock and scion incompatibility. The ratio of starch substances to total dry matter in leaves and shoots is linearly reduced by the level of incompatibility. Starch distribution between different parts of graft partners has shown that there is a relationship between incompatibility and starch metabolism. This phenomenon is a good marker for evaluation between scion and rootstock compatibility (Usenik et al. 2006; Darikova et al. 2011; Canas et al. 2014). Accumulation of starch substances above or below the graft union may be the markers of graft incompatibility (Davarynejad et al. 2008; Darikova et al. 2011; Hartmann et al. 2011; Dogra et al. 2018).
For this reason, this study was carried out to determine the graft compatibility status of the ‘Deveci’ and ‘Williams’ pear cultivars with different pear and quince rootstocks by carbohydrate analysis in Samsun Provinve, Turkey, during the 2015–2016 years.
Materials and Methods
Experiment Location
This study was conducted at Agriculture Research Station of Ondokuz Mayıs University, located in Samsun (Turkey) Province, Atakum County (North: 41°21′, East: 36°11′, Altitude: 173 m). The study was conducted in nursery parcel located in the open field. Grafting was also performed in open field. The research area was flat and had a slope of about 1%. The nursery soil structure was clayed-loam and weakly acidic and it was lime-free, unsalted, rich phosphorus and potassium content and high organic matter. The plants were mulched against the weeds and drip irrigation was implemented.
The climate of Samsun proves its temperate climate character. For many years, the highest average temperature was 27.0 oC, the lowest temperature was 3.9 oC, the annual average temperature was 14.4 oC, and the average annual rainfall was 733 mm. According to the obtained data, a large part of the precipitation falls in autumn and winter (TSMS 2018).
Plant Materials
In the study, one year old clonal rootstocks of quince (Quince BA 29), pear (OHxF 333 and Fox 11) and pear seedling were used. Rootstocks were planted at distance of 120 cm and 30 cm in February 2014 and cultivated in open field. ‘Deveci’ and ‘Williams’ pear cultivars were used as scions. The ‘Deveci’ is known as being compatible with quince rootstocks (Ozcagiran et al. 2005) but the ‘Williams’ is known as being incompatible or moderately compatible with quince rootstocks (Dondini and Sansavini 2012; Hudina et al. 2014). Scions required for grafting were taken from healthy plants of the 5‑year-old stock parcel in the field where the study was conducted at the Agriculture Research Station of Ondokuz Mayis University in 2010.
Grafting and Observations
Similar sized (for thickness) rootstocks were selected for grafting. T‑budding method, which has been used the most suitable graft method in the fall period (Westwood 1995; Hartmann et al. 2011) was used in the month of 1 September, 2014 and 2015. Grafting was performed 20 cm above the soil surface (Lewis and Alexander 2008; Hartmann et al. 2011). For each scion/rootstock combination 10 grafts were done for each replicate. Totally, 3 replicates were used and 30 grafts were made for each scion/rootstock combination. White colored, soft and silicone grafting tape was used to protect graft area. Cultivation factors such as irrigation, weed management and removal of suckers below the graft union were preformed regularly. As a ground cover, black colored and UV-added, polypropylene produced, was used between the rows for weed control. The rootstocks used in the study were irrigated during summer by drip irrigation systems. Fertilization was done fertigation, and NPK (20.10.20+ microelements [ME], 30–40 kg ha−1) fertilizer was used, 20 days intervals. Chemical spraying was not performed in the orchard.
In the study, sugar, starch and carbohydrate contents of the graft partners were determined according to previous relevant studies (Candolfi-Vasconcelos and Koblet 1990). For this aim, about 2 cm piece of the graft shoots were taken from between third and fourth internode above the graft union at the end of the vegetation period (Bates et al. 2002). The shoot parts were dried for 5–7 days at 70 °C in oven. When moisture content of shoots became stable, they were broken into pieces with the mill. 200 mg sample were taken from them and put into glass tubes. 8 ml 70% ethyl alcohol was added. After that the mixture was extracted at 60 °C for 30 min (Candolfi-Vasconcelos and Koblet 1990). 8 ml 1M perchloric acid was added into the mixture and they were stored at 60 °C for 1 h.
This procedure was repeated twice. The alcohol in the samples was removed at 37 °C and then, the two mixtures were combined. The absorbance value was determined at 620 nm with spectrophotometer. Sugar and starch content were determined with anthrone method (Scott and Melvin 1953). Glucose was used as a standard to determine sugar and starch contents of the samples. The results were expressed as mg L−1.
Determination of Starch Accumulation
The accumulation and flow of starch in the grafting area was performed according to Demirsoy and Bilgener (2006). Starch accumulation and flow were observed by examining the staining of rootstock and scion wood tissues with 1% iodized potassium iodide (KI) solution in longitudinal sections taken from the grafting site. Visually, 5 points was given for combination of the most accumulation of starch in grafting site and 0 points for the least accumulation.
Data Analysis
This study was arranged in a randomized complete block design with 3 replications, each replication contained 10 plants. Data analyses were performed using IBM SPSS Statistics ver. 21 statistical package program via the license of Ondokuz Mayis University. The differences between the averages of rootstocks and cultivars and their interaction were determined by Duncan’s Multiple Range Tests at 0.05% levels. The results are given over a two-year average in the tables.
Results and Discussion
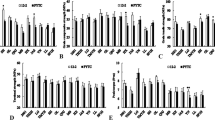
This study was aimed to determine carbohydrate analysis of graft compatibility of different rootstocks and ‘Deveci’ and ‘Williams’ pear cultivars. In the study, statistically significant differences were found between the averages of rootstocks in terms of sugar content but there were no statistically significant differences in starch and carbohydrate contents. The highest sugar content was found in the OHxF 333 (50.9 mg L−1) and the lowest in the seedling (45.7 mg L−1). It was also determined that there were no differences in terms of the averages of cultivar and graft areas (Tables 1, 2 and 3).
In ‘Deveci’/BA 29 quince rootstock combination, it was determined that there was no statistically significant difference in the starch content amongst the graft unions. On the other hand, in ‘Deveci’/Fox 11 pear rootstock combination, a higher starch content was determined in the graft union than above and below the grafting union. In ‘Deveci’/OHxF 333 combination, the starch content was highest below the graft union. In ‘Deveci’/seedling, starch content was higher below and in the graft union than above the graft union (Table 1). In ‘Williams’/BA 29 quince rootstock combination, the highest starch content was found above and the lowest below the graft union. In the ‘Williams’/Fox 11, starch accumulation was higher in the graft union than above and below the graft union. In ‘Williams’/OHxF 333 and ‘Williams’/seedling combinations, it was found that there were no statistically significant differences in starch content amongst the graft unions (Table 1).
Compared to the graft union × rootstock in terms of each cultivar and rootstock, when ‘Deveci’ pear cultivar was grafted on different rootstocks, it was determined that the starch content above and below the graft union was higher in the OHxF 333 and seedling rootstocks than in the BA 29 and Fox 11 rootstocks. In the ‘Williams’ cultivars, the highest content below the graft union was detected in the Fox 11 rootstock and the lowest in the OHxF 333 rootstock. And the highest content of starch above in the graft union was found in BA 29 (69.3 mg L−1) and lowest in the OHxF 333 rootstock (56.7 mg L−1) (Table 1).
Significantly differences were observed amongst the grafting areas in terms of sugar content in ‘Deveci’/BA 29 combination and no statistical differences were not determined in the other rootstocks. The content of sugar was the highest in the grafting site, the lowest above the graft union when ‘Deveci’ was grafted on the BA 29. In the ‘Williams’ cultivars, the highest sugar content was determined above the graft union (scion) and the lowest below the graft union (rootstock) when grafted on BA 29. On Fox 11 rootstock, the highest sugar content was observed below the graft union and the lowest above the graft union. There was no significant difference in the sugar content graft combinations with the seedling (Table 2).
Compared to the graft union × rootstock in terms of each cultivar and rootstock, the content of sugar was significantly higher below the graft union in ‘Deveci’/OHxF 333 graft combination than in the other rootstocks. In ‘Deveci’, the highest content of sugar in the graft union was measured in the OHxF 333 and the lowest in the seedling. The highest content of sugar above the graft union was found in the OHxF 333 and the lowest in the Fox 11 rootstock (Table 2). In ‘Williams’, the highest content of sugar was detected below the graft union on the Fox 11 and in the graft union on Fox 11 and BA 29 rootstocks. The sugar content above the graft union was found to be higher in the BA 29 (55.8 mg L−1) than in the other rootstocks when the ‘Williams’ cultivar grafted on the different rootstocks (Table 2).
In ‘Deveci’, carbohydrate content on BA 29 and Fox 11 was higher in the graft union than in the other grafting areas. The highest carbohydrate content was measured below the graft union on OHxF 333, and there were no differences between the grafting areas on seedling. In the ‘Williams’/BA 29 combination, the highest carbohydrate content was detected above the graft union and the lowest below the graft union. The carbohydrate content was lower below the graft union than in the other grafting areas in the ‘Williams’/Fox 11. It was determined that there were no statistically differences in terms of carbohydrate content among the grafting areas when OH ×F 333 and seedling rootstocks were used (Table 3).
Compared to the graft union × rootstock in terms of each cultivar, in ‘Deveci’, the highest carbohydrate content in the graft union and below the graft union was measured in OHxF 333 and the lowest in Fox 11 rootstock. Carbohydrate content above the graft union was higher in the OHxF 333 and seedling than in the other rootstocks. In ‘Williams’, the highest carbohydrate content was found below the graft union of Fox 11 and seedling rootstocks, and at the graft union in the Fox 11 rootstock. The carbohydrate content above the graft union was found to be higher in ‘Williams’/BA 29 (125.1 mg L−1) combination than in the others ‘Williams’ grafted rootstocks (Table 3).
The scores staining with 1% iodized potassium iodide solution of the longitudinal sections of the scion/rootstocks combinations to determine the starch accumulation and flow in the graft union are presented in Table 4. In the study, the combination of ‘Williams’/OHxF333 and ‘Deveci’/seedling and ‘Williams’/seedling had the best scores by the least starch accumulation in the grafting area (Table 4, Fig. 1b, d). They were followed by ‘Deveci’/Fox 11 and ‘Deveci’/OHxF 333 scion/rootstock combinations. Combinations with high scores means that the distribution of starch on the scion and rootstock is homogenous and no starch accumulation was found in the graft union (Fig. 1). ‘Williams’/BA 29 combination had the lowest score, followed by ‘Williams’/Fox 11 combination. In these scion/rootstock combinations, especially in the ‘Williams’/BA 29, starch accumulation was observed above the graft union (in the scion) (Table 4, Fig. 1).
The starch accumulation above the graft union was determined prominently, especially in the ‘Williams’/BA 29 scion/rootstock combination (Fig. 1a). Similarly, starch accumulation in the graft union was observed in the ‘Williams’/Fox 11 combination (Tables 1 and 4, Fig. 1c). Graft incompatibility between scion and rootstock has been more often determined for inter-specific than for intra-specific graft combinations (Darikova et al. 2011; Hartmann et al. 2011; Dogra et al. 2018; Dolkar et al. 2018). Accumulation or flow of starch in the grafting areas may indicate the level of graft incompatibility between the scion and rootstock (Demirsoy and Bilgener 2006; Davarynejad et al. 2008). Herrero (1951) cited that the accumulation of starch above the graft union is a symptom of graft incompatibility. The content of starch in the incompatible peach/plum graft combination was higher above the graft union than in the rootstock, and the content of starch in the compatible peach/plum grafting combination was less above the graft union (Breen 1975). It has been reported that more starch accumulation above the graft union was detected in some pear cultivars grafted on quince A clonal rootstock and reported that they are incompatible with quince A (Davarynejad et al. 2008). Also Moing and Gaudillere (1992) reported that soluble sugars and starch were accumulated in incompatible graft combinations. Demirsoy and Bilgener (2006) reported that starch accumulation above the graft union was higher in incompatible some peach/plum graft combinations. Hudina et al. (2014) cited that a severe incompatibility between Fox 11 rootstock and ‘Williams’ was detected by phenolic profiles investigations with HPLC. The results obtained in this study are in accordance with previous studies in peach/plum (Moing and Gaudillere 1992; Demirsoy and Bilgener 2006) and pear/quince (Davarynejad et al. 2008; Ciobotari et al. 2010). Determination of the graft compatibility status of the rootstocks and cultivars used especially in modern fruit growing is important for species that can be grafted onto different species such as pear, cherry, peach. Early identification of graft incompatibility situations that may occur in grafting of species on different species ensures that producers avoid time and economic losses (inefficiency, low quality, etc.).
Conclusion
In the present study, it has been determined that graft incompatibility may occur when ‘Williams’ is grafted on BA 29 and Fox 11 rootstock. Furthermore, the observation of graft incompatibility situation may be useful in commercial orchard of these cultivars/rootstocks. As a result of the study, it was concluded that carbohydrate accumulation could be used as an indicator in determining the graft compatibility of the pear cultivars with the different quince and pear rootstocks.
References
Barritt BH (1992) Intensive orchard management. Good fruit grower. Yakima, Washington
Bates TR, Dunst RM, Joy P (2002) Seasonal dry matter, starch, and nutrient distribution in ‘Concord’ grapevine roots. HortSci 37(2):313–316. https://doi.org/10.21273/hortsci.37.2.313
Bell RL, Quamme HA, Layne REC, Skirvin RM (1996) Pears. In: Janick J, Moore JN (eds) Tree and tropical fruits, vol I. Wiley Science, Hoboken, pp 441–514
Breen P (1975) Effect of peach/plum graft incompatibility on seasonal carbohydrate changes. J Am Soc Hortic Sci 100(3):253–259
Canas S, Assunçao M, Brazao J, Zanol G, Eiras-Dias JE (2014) Phenolic compounds involved in grafting incompatibility of Vitis spp: development and validation of an analytical method for their quantification. Phytochem Anal 26:1–7. https://doi.org/10.1002/pca.2526
Candolfi-Vasconcelos MC, Koblet W (1990) Yield, fruit quality, bud fertility and starch reserves of the wood as a function of leaf removal in Vitis vinifera—Evidence of compensation and stress recovering. Vitis 29:199–221
Ciobotari G, Brinza M, Morariu A, Gradinariu G (2010) Graft incompatibility influence on assimilating pigments and soluble sugars amount of some pear (Pyrus sativa) cultivars. Not Bot Horti Agrobo 38:187–192. https://doi.org/10.15835/nbha3814607
Darikova JA, Savvaa YV, Vaganova EA, Gracheva AM, Kuznetsova GV (2011) Grafts of woody plants and the problem of incompatibility between scion and rootstock (a review). J Siberian Fed Univ Biol 1(4):54–63. https://doi.org/10.17516/1997-1389-0185
Davarynejad GH, Shahriari F, Hamid H (2008) Identification of graft incompatibility of pear cultivars on Quince rootstock by using isozymes banding pattern and starch. Asian J Plant Sci 7(1):109–112. https://doi.org/10.3923/ajps.2008.109.112
Demirsoy H, Bilgener S (2006) Anatomically investigating of graft union in some compatible and incompatible peach/plum grafts combinations. J Fac Agric Ondokuz Mayıs Univ 21(1):89–94
Dogra K, Kour K, Kumar R, Bakshi P, Kumar V (2018) Graft-Incompatibility in Horticultural Crops. Int J Curr Microbiol App Sci 7(2):1805–1820. https://doi.org/10.20546/ijcmas.2018.702.218
Dolkar T, Mansoor A, Agleema B, Divya S, Lobzang S, Stanzin K (2018) Mitigation of temperate fruit crop problems through use of rootstock. Int J Chem Stud 6(2):880–887
Dondini L, Sansavini S (2012) European pear. In Badanes ML, Byrne DH (ed) Fruit Breeding, Series: Handbook of Plant Breeding, Vol. 8. Springer Science+Business Media, New York, pp 363–413
Ercisli S (2004) A short review of the fruit germplasm resources of Turkey. Genet Resour Crop Evol 51:419–435. https://doi.org/10.1023/b:gres.0000023458.60138.79
Ermel FF, Poessel JL, Faurobert M, Catesson AM (1997) Early scion/stock junction in compatible and incompatible pear/pear and pear/quince grafts: a histo-cytological study. Ann Bot 79:505–515. https://doi.org/10.1006/anbo/79.5.505
Errea P (1998) Implications of phenolic compounds in graft incompatibility in fruit tree species. Sci Hortic 74:195–205. https://doi.org/10.1016/s0304-4238(98)00087-9
Faostat (2021) Food and Agriculture Organization Statistical Databases, Agriculture, Crop Primary, Pear Production in the World (www.fao.org)
Francescatto P, Pazzin D, Neto AG, Fachinello JC, Giacobbo CL (2010) Evaluation of graft compatibility between quince rootstocks and pear scions. Acta Hortic 872:253–260. https://doi.org/10.17660/actahortic.2010.872.34
Hartmann HT, Kester DE, Davies Jr FT, Geneve RL (2011) Plant propagation: principles and practices. 8th edn. Regents/Prentice Hall International Editions, Englewood Cliffs, New Jersey
Herrero J (1951) Studies of compatible and incompatible graft combinations with special reference to hardy fruit trees. J Hortic Sci 26:186–237. https://doi.org/10.1080/00221589.1951.11513736
Hudina M, Orazem P, Jakopic J, Stampar F (2014) The phenolic content and its involvement in the graft incompatibility process of various pear rootstocks (Pyrus communis L.). J Plant Physiol 171:76–84. https://doi.org/10.1016/j.jplph.2013.10.022
Jackson JE (2003) Biology of apples and pears. Cambridge University Press, Cambridge
Lewis WJ, McEwan Alexander D (2008) Grafting & Budding. A Practical Guide for Fruit and Nut Plants and Ornamentals. Landlinks Press,
Moing A, Gaudillere B (1992) Carbon and nitrogen partination in peach/plum grafts. Tree Physiol 10:81–92. https://doi.org/10.1093/treephys/10.1.81
Moore R (1983) Physiological aspects of graft formation. Vegetative compatibility responses in plants. Baylor University Press, Waco, TX, pp 89–105
Ozcagiran R, Unal A, Ozeker E, Isfendiyaroglu M (2005) Pear. Temperate fruit trees, pome fruits. Ege Univ Agri Fac Pub 556(2):73–126
Pina A, Errea P (2005) A review of new advances in mechanism of graft compatibility–incompatibility. Sci Hortic 106:1–11. https://doi.org/10.1016/j.scienta.2005.04.003
Rahman J, Aftab M, Rauf MA, Rahman KU, Bilal W, Ayub FG (2017) Comparative study on compatibility and growth response of pear varieties on different rootstocks at nursery. Pure App Biol 6(1):286–292
Scott TA, Melvin EH (1953) Determination of Dextran with Anthrone. Anal Chem 25(11):1656–1661
Stern RA (2008) Pyrus betulifolia is the best rootstock for the ‘Coscia’ pear in the warm climate of Israel. Acta Hortic 800:631–638. https://doi.org/10.17660/actahortic.2008.800.84
TSMS (2018) Turkish state meteorological service official web sites. https://mgm.gov.tr/eng/forecast-cities.aspx?m=SAMSUN. Accessed 15 Dec 2018
Usenik V, Krska B, Vican M, Stampar F (2006) Early detection of graft incompatibility in apricot (Prunus armeniaca L.) using phenol analyses. Sci Hortic 109:332–338. https://doi.org/10.1016/j.scienta.2006.06.011
Westwood MN (1995) Temperate-zone pomology, physiology and culture, 3rd edn. Timber Pres, Oregon
Author information
Authors and Affiliations
Contributions
The measurements related to the research were made by N. Çoban. Statistical analysis, research method and writing design of the manuscript made by A. Öztürk.
Corresponding author
Ethics declarations
Conflict of interest
N. Çoban and A. Öztürk declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Çoban, N., Öztürk, A. Determination of Graft Compatibility of Pear Cultivars Grafted on Different Rootstocks by Carbohydrate Analyses. Erwerbs-Obstbau 64, 229–235 (2022). https://doi.org/10.1007/s10341-021-00630-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10341-021-00630-1