Abstract
The scion-rootstock incompatibility is one of the most common problems in fruit trees. The study evaluated predicted compatibility or incompatibility by internode association and callus fusion techniques. Two factorial experiments were laid out with an incompletely randomized design with three replications. Treatment includes the first factor, four levels of sweet cherry cultivars (‘Bing’, ‘Takdaneh’, ‘Siyah Mashhad’ and ‘Adli’), and the second factor, four levels of rootstocks (‘Gisela-5’, ‘Gisela-6’, ‘Mahaleb’ (M-168) and ‘GF-305’). Results showed significant differences in total phenolic content, peroxidase and starch content in internode association and callus fusion experiments. ‘GF-305’ rootstock (incompatible) had the lowest success rate of grafts on all cultivars, while ‘Gisela-6’ rootstock showed the highest success rate of grafts on ‘Siyah Mashhad’ and ‘Bing’ cultivars. Both experiments showed that in incompatible grafts, an increase in total phenol content and peroxidase activity and a decrease in grafts’ success rate were observed. The degree of compatibility was significantly and positively correlated with the success rate of grafts while significantly and negatively correlated with total phenol content and peroxidase activity. In both experiments, no clear trend was observed regarding starch content and the success rate of grafts. The regression analysis results showed that phenol compounds in both experiments significantly affected the graft's success rate and the degree of compatibility. Our findings lead to the conclusion that phenolic compounds (mostly) and peroxidase activity can be used to pre-screen for incompatible grafts. Moreover, the callus fusion technique can be a quick way to predict (in)compatibility of graft in sweet cherry.
Key Message
Results of this article indicated that phenolic and peroxidase activity can be used to pre-screen for incompatible grafts. The callus fusion technique can be a quick way to predict (in) compatibility of graft in sweet cherry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Graft incompatibility is one of the obstacles and problems in using the rootstock of fruit trees. The genetic difference between rootstock and scion is one of the important factors of graft incompatibility (Gainza et al. 2015). Although a series of biological studies have been performed on woody and herbaceous plants, this area has limited information. Moreover, the molecular and biochemical mechanisms of graft incompatibility are not well understood. The accurate and rapid prediction of graft incompatibility is essential (Hudina et al. 2014).
Global sweet cherry production has slightly increased recently (Lanauskas et al. 2023). Different studies with Prunus sp. have shown that rootstocks significantly influence the grafted cultivar. This influence is expressed as differences in vigor, yield and fruit characteristics such as firmness, fruit size, sugar and organic acid content (Lopez-Ortega et al. 2016). Sweet cherry rootstocks show different suitability for sweet cherry cultivars. Such a different pattern is highlighted by the nutrient uptake efficiency, leaf area development, chlorophyll content and subsequent photosynthetic activity (Asanica et al. 2015).
The use of new methods, such as tissue culture for the production of plants has received much attention. Tissue culture guarantees production at a high speed and with this method, it is possible to produce uniform plants similar to the mother base and free from disease and contamination (Sarropoulou et al. 2017). Jonard et al. (1990) reported the possibility of predicting graft incompatibility of apricot and lemon trees using in vitro techniques (e.g., internode association and callus fusion). Callus formation at the graft union is the first positive reaction in the graft, while failure to form callus tissue leads to graft failure (Porika et al. 2016).
Recently, different methods, such as anatomical studies (Balbi et al. 2019) and the determination of starch accumulation (Zhou et al. 2020), have been used to predict rootstock and scion incompatibility quickly. These techniques facilitate the early detection of graft incompatibility and provide a model for a more accurate analysis of the grafting phenomenon (Suryadi et al. 2020).
Some compounds are essential in the early stages of growth for the compatibility of the rootstock and scion. Many studies show the role of phenols in lignification. Xylem cell wall cells have a dynamic structure, including polysaccharides, phenolic compounds (such as lignin), minerals and proteins (Tucker et al. 2018; Mnich et al. 2020).
Porika et al.(2016) showed different phenols in the branches of apple, pear, plum, cherry, apricot and peach trees, and the amount of the metabolites depends on the branches' growth degree. Prabpree et al.(2018) have reported phenolic compounds as markers for evaluating graft compatibility between rootstock and scion. Cheng et al. (2017) said the number of phenolic compounds significantly differed at the top and bottom of the graft union. Due to the significant relationship between arbutin accumulation at the top and bottom of the graft union, this combination can be used to perform pre-screening graft incompatibility.
Many reports have shown peroxidase’s role in inhibiting growth (Guclu and Koyuncu 2012). Researchers reported that peroxidase levels in incompatible grafts were higher than in compatible grafts (Zarrouk et al. 2010). Peroxidase profiles in grafted pears were not similar compatible and incompatible grafts, which caused abnormal lignification at the graft union and incompatibility.
Different opinions exist about starch accumulation at the compatible and incompatible grafts. Studies have shown that starch accumulation above the graft union and its absence or lack at the bottom of the graft union destroys the phloem. However, it should be noted that in some rootstock and scion interactions, signs of graft incompatibility may be observed. Still, there is no accumulation of starch at the top of the graft union, or the opposite may occur (Melnyk et al. 2018).
This study aimed to predict the (in)-compatibility by internode association and callus fusion techniques and compare the amount of total phenol, peroxidase and starch accumulation in compatible and incompatible grafts of sweet cherry cultivars.
Materials and methods
This experiment was carried out on four sweet cherry cultivars (‘Bing’, ‘Takdaneh’, ‘Siyah Mashhad’, and ‘Adli’), one of Iran's important sweet cherry cultivars. Four rootstocks (‘Gisela-5’, ‘Gisela-6’, ‘Mahaleb’(M-168), and ‘GF-305’) in the tissue culture laboratory of Khorasan Razavi Agricultural and Natural Resources Research and Education Center, Iran.
’Gisela’ rootstock from the interspecific P. cerasus × P. avium and ‘Gisela 6’ rootstock interspecific hybridization P. cerasus × P. Canescens is compatible with a wide range of cherry cultivars. In this experiment, ‘Gisela-6’ rootstock was considered a compatible control and ‘GF-305’ an incompatible control. This study was performed in two independent experiments, internode association and callus fusion, under in vitro conditions.
Internode association experiment
The internode (1.0 cm) was sterilized with 70% ethanol for 1 min, washed three times with distilled water 5% NaClO for 10 min and cleaned more than three times with sterile water. With respected polarity rootstock and scion, side-veneer (spliced-side) grafts joined each other. A sloping cut is made at the rootstock. The scion is cut to be paired with the rootstock, and the graft union site is covered with sterile glue until fusion.
The explants were cultured in Murashige and Skoog (MS) sterilized-medium modified supplemented with 0.2 mg L−1 6-Benzylaminopurine (BAP) and 1 mg L−1 naphthaleneacetic acids (NAA) (Murashige and Skoog 1962). After culturing in the test glass tube, including 10 ml medium, the samples were transferred to the growth chamber (16/8 photoperiod and temperature 25 ± 1 °C) (Fig. 1).
Approximately four weeks after grafting, grafting's success rate and total phenol, peroxidase, and starch content at the graft union were measured.
Callus fusion experiment
The internode explant (1.0 cm) was sterilized like in the previous experiment and cultured in MS medium supplemented with BAP (0,1, 2, 3 mg L−1) and 2,4-D (0, 1, 2, 3 mg L−1) concentrations. Twenty explants were in each treatment (10 glass Petri dishes included two explants), and ten treatments and 200 explants were used in the callus formation stage. After culturing in a petri dish, the explants were transferred to a growth chamber (16/8 photoperiod and temperature 25 ± 1 °C). After about a week, callus formation started in the sample of different treatments (Fig. 2).
Three weeks later, determined the best treatment, the rootstock produced calluses and the scions were placed next to each other, with side-veneer (spliced-side) grafts joined to each other and in MS medium supplemented with 2 mg L−1 BAP and 3 mg L−1 2,4-D to determine the rootstock's compatibility and incompatibility with the scion. After ten days, the compatible calli were grown and merged, and the incompatible calli remained apart (Fig. 3), and the degree of compatibility of the rootstock and scion (DC) was determined in Eq. (1):
GIRS: the percentage increase in the rootstock's mass from rootstock and sweet cherry scion cultivar from the rootstock’s co-culture. GIRC (the rootstock control): the percentage increase in the group of the rootstock. GISR: the percentage increase in the mass of the scion from the co-culture of sweet cherry cultivar and rootstock and GIsc (the scion control): the percentage increase in the scion’s mass cultivar (Pedersen 2006).
Approximately 4 weeks after grafting, grafting’s success rate and the amount of total phenol, peroxidase and starch content at the graft union were measured.
Measurement of total phenol
The measurement of total phenolic compounds was determined according to the Folin-Ciocalteu method (Singleton et al. 1999). Finally, the absorption of samples was calculated using the standard curve for gallic acid (Guimaraes et al. 2020).
Extraction of enzymatic antioxidant activity
Antioxidant enzyme extraction was defined following Sunohara and Matsumoto’s (2004) method. The filtrate was collected to determine the peroxidase activity (EC 1.11.1). Peroxidase activity was assayed using a method proposed by Polle et al. (1997).
Measurement of starch content
To extract the starch, the method of Whistler (1964) was used with some modification; the absorbance of all samples was measured at 630 nm (max-plu 5384 UV–VIS). Antron reagent was used as a control.
Statistical analysis
After checking the data distribution normality (Kolmogorov–Smirnov and Shapiro–Wilk test) assumption, the statistical analyses to determine the individual effect were conducted using Statistical Analysis System software (SAS Institute, Cary, NC, USA, ver. 9.2). The least significant difference (LSD) test was applied to compare the means (at the 0.05 probability level). Simple correlations (Pearson) and regression analysis between traits were performed using SAS and Minitab (v. 19) software.
Results
First experiment: internode association
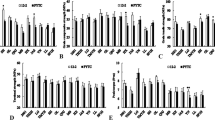
As shown in Table 1, rootstocks, cultivars and interaction effects of rootstocks and cultivars were significant on the rate of success grafting, phenol content, peroxidase activity and starch content. The highest success rate of grafts was related to the ‘Bing’ cultivars on the ‘Gisela-6’ (compatible control) rootstock. The lowest success rate of grafts was related to the ‘GF-305’ (incompatible control) on all cultivars (Fig. 4).
The highest amount of total phenol was achieved in union’s grafting ‘Takdaneh’ and ‘Siyah Mashhad’ cultivars on ‘GF-305’ rootstock with an average of 6.81 and 6.67 mg g−1 FW. There was no significant difference in the amount of total phenol in the grafting ‘Bing’ cultivar on the ‘GF-305’ rootstock (6.42 mg g−1 FW). The lowest total phenol was found in the grafting ‘Bing’ cultivar on the ‘Gisela-6’ rootstock with an average of 2.34 mg g−1 FW. The results showed that grafting ‘Mahaleb’ and ‘GF-305’ rootstocks on all cultivars increased the phenol content (Fig. 5).
The lowest peroxidase activity was observed in the ‘Bing’ cultivar on ‘Gisela-5’. Moreover, the highest peroxidase activity was related to the grafting ‘Siyah Mashhad’ cultivar on ‘GF-305’ rootstock (Fig. 6). In general, the highest level of peroxidase was obtained in grafting sweet cherry cultivars on ‘GF-305’ incompatible control.
The highest starch accumulation was observed in the ‘Takdaneh’ cultivar union`s grafting on the ‘GF-305’ (0.12%). The lowest accumulation of starch was observed in the ‘Adli’ cultivar grafting union on the ‘Gisela-6’ (0.06%) (Fig. 7). Despite the significant difference in starch accumulation at the unions grafts between cultivars on rootstocks, there was no significant relationship between compatible and incompatible graft in starch accumulation. In other words, starch accumulation in all ‘GF-305’ grafts (incompatible control) was not higher than in other grafts.
Table 2 shows significant negative and positive correlations among grafts’ success rate, phenol content, peroxidase activity and starch content. For example, the success rate of grafts was significantly and negatively correlated with phenol content, peroxidase activity, and starch content. Phenol content was highly and significantly correlated.
Regression analysis between graft success rate and other traits was performed separately to understand the relationships between characteristics better. The results showed that phenol content, peroxidase activity and starch content harmed grafts’ success rate and the highest R2 (0.34) was related to phenol content (Fig. 8). These results were in line with the correlation analysis findings between traits because the highest negative correlation coefficient was observed between phenol content and grafts’ success rate.
Second experiment: callus fusion
The mean comparison of callus formation in sweet cherry explant in MS medium under the effect of 2,4-D and BAP in different concentrations showed a statistically significant difference. The highest percentage of callus formation was treating 3 mg L−1 2,4-D and 2 mg L−1 BAP in all cultivars and rootstocks (Table 3). Callus production time was 6 to 8 days after planting, depending on the type of hormone. ‘Gisela-6’ rootstock, ‘Siyah Mashhad’ and ‘Takdaneh’ cultivars started callus formation after six days, and ‘GF-305’ started callus formation after eight days.
As shown in Table 4, rootstocks, cultivars, and interaction effects of rootstocks and cultivars were significant on the degree of compatibility, grafts’ success rate, phenol content, peroxidase activity and starch content (p ≤ 0.01).
In the interaction effects of cultivars and rootstocks, the highest degree of compatibility (1.74) was related to the grafting of ‘Takdaneh’ on ‘Gisela-5’. Moreover, ‘Bing’ on ‘GF-305’ grafting showed the lowest degree of compatibility (0.50). The results showed that ‘Gisela-5’ and -6 rootstocks increased the degree of compatibility in each cultivar, while ‘GF-305’ rootstocks decreased the degree of compatibility (Fig. 9).
Results indicated that the highest success rate was the grafting of ‘Adli’, ‘Bing’, ‘Siyah Mashhad’, and ‘Takdaneh’ cultivars on ‘Gisela-6’ rootstocks. The calluse fusion of the ‘Bing’ cultivar on ‘Gisela-6’ had the highest success rate (83.33%). On the other hand, ‘GF-305’ rootstocks' application reduced the success rate of calluse fusion, which was very noticeable in applying ‘GF-305’ rootstocks on cultivars (Fig. 10). Predicting the possibility of incompatibility between rootstock and scion in fruit trees in orchards may take a long time. Still, by using the tissue culture technique, graft incompatibility can be predicted in a shorter period. The callus fusion technique is a quick way to predict graft compatibility or incompatibility.
As shown in Fig. 11, the interaction of cultivars and rootstocks showed that the phenol content in the ‘Takdaneh’ cultivar was much higher than in other cultivars. The highest phenol content was related to the ‘Takdaneh’ cultivar's grafting on the ‘GF-305’ rootstock by 28.76 mg g−1 FW. In all cultivars, the application of ‘Gisela-6’ compatible rootstock decreased phenol content. The grafting of ‘Adli’ on ‘Gisela-6’ and ‘Mahaleb’ (M-168) rootstocks had the lowest phenol content (2.05 and 2.5 mg g−1 FW, respectively).
Peroxidase activity in ‘GF-305’ incompatible rootstocks showed a significant increase compared to other rootstocks. The highest peroxidase activity was related to ‘Takdaneh’ cultivar grafting on ‘GF-305’ rootstocks with an average of 0.754 U mg protein−1 min. On the other hand, application of ‘Gisela-6’ (compatible rootstock) resulted in a significant reduction in the activity of this enzyme, so that the lowest peroxidase activity was achieved in the ‘Adli’ cultivar on ‘Gisela-6’ rootstock with an average of 0.105 U mg protein−1 min (Fig. 12).
The highest starch content was observed in the grafting of ‘Siyah Mashhad’ on Gisela- 5 (0.1%) and ‘Takdaneh’ on Gisela- 6 (0.9%), respectively. The lowest starch accumulation was in ‘Adli’ on ‘GF-305’ (0.06%) (Fig. 13). Despite the significant difference in starch accumulation at the unions grafts between cultivars on rootstocks. Still, there was no significant relationship between compatible and incompatible grafts in starch accumulation. In other words, starch accumulation in all ‘GF-305’ grafts (incompatible control) was not higher than other grafts.
As shown in Table 5, the degree of compatibility was significantly and positively correlated with the success rate of grafts (0.64**) while significantly and negatively correlated with phenol content (− 0.34*) and peroxidase activity (− 0.33*). The degree of compatibility showed a non-significant correlation with starch content.
As shown in Fig. 14, the regression changes in the success rate of grafts, phenol content, peroxidase activity and starch content with differences in the degree of compatibility under the callus fusion experiment showed that the characteristics such as phenol content and peroxidase activity were negatively related with the degree of compatibility. In other words, by increasing the range of these compounds, the degree of graft compatibility decreased. However, the R2 obtained from this analysis had a very low coefficient compared to the first experiment. On the other side, the relationship between the degree of compatibility and the success rate of the positive relation (R2 = 0.45).
Discussion
Not many studies were performed on the graft incompatibility mechanism and its prediction methods in sweet cherry trees. Sweet cherries are mainly grown by amateur growers and are rarely planted in commercial orchards (Lanauskas et al. 2023). This study aimed to investigate the changes in some metabolites and techniques needed to understand this mechanism better. Callus tissue is formed at the junction of the rootstock and scion to the graft 4 to 5 days after grafting, while the lack of callus tissue formation at the intersection leads to graft failure (Porika et al. 2016). Using tissue culture tests helps us detect transplant compatibility in a shorter period and prevent damage to gardeners.
The grafts' degree of compatibility showed that the grafts are incompatible when the degree of compatibility is less than 1. If the degree of compatibility is more than 1 (Fig. 9), the grafts are compatible. The highest degree of compatibility was recorded in ‘Takdaneh’ and ‘Siyah Mashhad’ cultivars on the ‘Gisela-6’ rootstock. The degree of compatibility in ‘Gisela-5’ rootstocks was less than ‘Gisela-6’, which indicates that the ‘Takdaneh’ and ‘Siyah Mashhad’ cultivars are more compatible with ‘Gisela-6’(Fig. 9).
Plants rapidly increase their antioxidant capacity when stress occurs to increase resistance and tolerance to the conditions created. It can be said that the plant’s ability to disperse excess energy and neutralize free radicals is impaired, thus increasing the antioxidant capacity to increase stress resistance, so incompatible grafts increase phenolic compounds and peroxidase to minimize oxidative damage compared to compatible grafts (Zarrouk et al. 2010; Baron et al. 2019). Since the ‘GF-305’ rootstock was an incompatible control in this experiment, higher phenol content was observed in grafting sweet cherry cultivars on the ‘GF-305’ rootstock. Moreover, the total phenol content in sweet cherry cultivars on the ‘Gisela-6’ rootstock, which was a compatible control, was lower than that of the incompatible control. The amount of total phenol in the transplants performed on the ‘Mahaleb’ (M-168) rootstock was higher than the compatible control and less than the incompatible control. Generally, the total phenol in the grafts on ‘Gisela-6’ (compatible control) and ‘Gisela-5’ rootstocks was less than ‘GF-305’ (incompatible control).
The lowest phenol in the first experiment in ‘Bing/Gisela 6’ (Fig. 5) and the second experiment was ‘Adli/Gisela 6’ (Fig. 11). ‘GF-305’ is incompatible with sweet cherry cultivars and it seems that phenolic compounds can be used to pre-screen incompatible grafts. According to high storage theories, phenolic compounds such as catechins above the graft union can be used as a biochemical marker in diagnosing graft incompatibility (Canas et al. 2015; Baron et al. 2019). Moreover, Hudina et al. (2014) have reported phenolic compounds as markers to evaluate the grafts’ compatibility between the rootstocks and scion.
Peroxidase levels in incompatible grafts were higher than in compatible grafts. The peroxidase enzyme can be used to predict incompatible grafts quickly. Peroxidases are enzymes that play essential biochemical and physiological roles in plants, including plant growth, differentiation and development, auxin catabolism, ethylene biosynthesis, plasma membrane regeneration, cell wall development, ligninification and response to pathogens (Pandey et al. 2017). Preliminary analyses of peroxidase indicate that this enzyme is essential in forming cell wall constituents and ligninification. By creating cross-linking between cell wall phenolic polymers, the next step reduces cell wall flexibility. This process causes irreversible hardening of the cell wall (Suchy et al. 2010).
The result of present study showed that the peroxidase enzyme activity in grafting cultivars on ‘GF-305’ rootstocks that were incompatible was higher than grafting cultivars on ‘Gisela-6’ (compatible control), ‘Gisela-5’ and Mahaleb. The lowest peroxidase in the first experiment was in ‘Bing/Gisela 5’ (Fig. 6), and in the second experiment was ‘Adli/ Gisela 6’ (Fig. 12).
Many studies have shown the role of peroxidase in inhibiting growth and reported that peroxidase levels in incompatible grafts are higher than incompatible grafts (Zarrouk et al. 2010; Baron et al. 2019). The researchers noted that the lack of similarity in the isoperoxidase composition between the rootstock and scion could lead to abnormal ligninification and the absence of vascular attachment at the graft union, leading to incompatible grafts (Zarrouk et al. 2010).
Some research findings on the relationships between peroxidase isoenzyme patterns and graft incompatibility in different plants suggest that the matching of isoperoxidase grafts between the rootstock and the scion can be used as an indicator of graft proliferation (Dogra et al. 2018). In the present study, starch accumulation at the union’s grafting did not perform well in predicting the graft's incompatibility. Different results have been reported for starch accumulation at the union’s grafting (Deng et al. 2019).
In some of them, starch accumulation occurred in incompatible grafting. Due to genetic differences and cultivar type, starch accumulation was not a good sign of graft incompatibility as in the present study. Starch accumulation above the graft union and its absence or lack at the bottom of the graft union cause damage to the phloem vessel, resulting from the starch collection in the phloem vessel (Deng et al. 2019). Researchers have shown that the grafts union's total starch content changes dramatically (Hudina et al. 2014). The relative starch content of rootstock and scion tissues is affected by the type of graft composition. In incompatible compounds, the relative starch content of scion wood is higher than rootstock wood. Accumulation of starch and soluble sugars in scion has also been observed in transmitted incompatibility. Low accumulation of starch at the top and bottom of the graft causes better boiling of the graft and adequate transfer of nutrients to the roots and vice versa (Karimi and Hassanpour 2017). However, in the grafting of cherries on the ‘Mahaleb’ (M-168) rootstock, although signs of incompatibility were seen, no difference was found between the starch at the graft union's top and bottom.
The lowest starch in the first experiment was in ‘Siyah Mashhad/6’ (Fig. 7). The second experiment was the lowest Starch, ‘Adli/ Mahaleb’ (Fig. 13).
The callus fusion experiment showed that hormones (2,4-D and BAP) are necessary for callus production, and callus was not produced in the culture medium without hormones.
The callus fusion technique is a quick way to predict graft compatibility or incompatibility. The rootstock and scion callus are fully integrated with the compatible grafts after about ten days, but the calluses do not merge if the graft is incompatible. The present results showed that the ‘Gisela-6’ rootstock grows more than other rootstocks with scion in the culture medium. It seems that in compatible sweet cherry grafts, a growth-promoting substance is secreted to which the compatible rootstock reacts. Determination of graft incompatibility using callus culture in some plants was reported by Gainza et al. (2015). Callus tissue formation at the graft union is the first positive reaction in the graft (Porika et al. 2016). Irisarri et al. (2015) used callus fusion to predict early graft compatibility in apricot and lemon trees. Early and accurate prediction of transplant incompatibility is essential because of the need to avoid incompatible compounds and select compatible ones (Zarrouk et al. 2010). However, the metabolic and physiological mechanisms involved in maladaptive responses are not well understood and require further research.
The highest total phenol and peroxidase levels at the graft union were higher on the ‘GF-305’ incompatible rootstock than on the ‘Gisela-6’ compatible rootstock. Therefore, our finding leads to the conclusion that phenolic compounds and peroxidase activity can be used to pre-screen for incompatible grafts.
Conclusion
The finding of this study leads to the following conclusions: The rootstock and scion degree of compatibility increased with graft success rate and decreased with phenol content and peroxidase activity. Increases in phenol content and peroxidase activity were observed in incompatible grafts.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- MS:
-
Murashige and skoog medium
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- BAP:
-
6-Benzylaminopurine
- NAA:
-
1-Naphthaleneacetic acid
- DC:
-
Degree of compatibility
References
Asanica A, Badulescu L, Tudor V (2015) The synthesis potential of some sweet cherry cultivars under the influence of different rootstocks. J Agric Agric Sci Proc 6:102–109. https://doi.org/10.1016/j.aaspro.2015.08.045
Balbi RV, Pio R, da Hora FD, de Melo ET, Pereira MP, Pereira FJ (2019) The cell regeneration and connection of grafting between pear and quince trees are defined by the cortex and phloem. J. Sci Hortic 257:10866. https://doi.org/10.1016/j.scienta.2019.108662
Baron D, Amaro ACE, Pina A, Ferreira G (2019) An overview of grafting re-establishment in woody fruit species. J Sci Hortic 243:84–91. https://doi.org/10.1016/j.scienta.2018.08.012
Canas S, Assuncao M, Brazao J, Zanol G, Eiras-Dias JE (2015) Phenolic compounds involved in grafting incompatibility of Vitis spp., development and validation of an analytical method for their quantification. Phytochem Anal 26:1–7. https://doi.org/10.1002/pca.2526
Cheng J, Wei L, Mei J, Wu J (2017) Effect of rootstock on phenolic compounds and antioxidant properties in berries of grape (Vitis vinifera L.) cv. ‘Red Alexandria. J Sci Hortic 217:137–144. https://doi.org/10.1016/j.scienta.2017.01.037
Deng H, Achor D, Exteberria E, Yu Q, Du D, Stanton D, Liang G, Gmitter FG (2019) Phloem regeneration is a mechanism for Huanglong’Bing’-tolerance of “Bearss” lemon and “LB8-9” Sugar Belle mandarin. J Front Plant Sci 10:277. https://doi.org/10.3389/fpls.2019.00277
Dogra K, Kour K, Kumar R, Bakshi P, Kumar V (2018) Graft-incompatibility in horticultural crops. J Int Curr Microbiol Appl Sci 7:1805–1820. https://doi.org/10.20546/ijcmas.2018.702.218
Gainza F, Opazo I, Munoz C (2015) Graft incompatibility in plants, metabolic changes during formation and establishment of the rootstock/scion union with emphasis on Prunus species. J Chil Agric Res 75:28–34. https://doi.org/10.4067/S0718-58392015000300004
Guclu S, Koyuncu F (2012) Peroxidase isozyme profiles in some sweet cherry rootstocks and ‘0900 Ziraat’cherry variety. J Afr Biotechnol 11:678–681. https://doi.org/10.5897/AJB11.2335
Guimaraes KC, Salgado DL, Carvalho EN (2020) Evaluation of different methodologies for the determination of phenolic compounds in tropical fruits. J Food Technol. https://doi.org/10.1590/1981-6723.01519
Hudina M, Orazem P, Jakopic J, Stampar F (2014) The phenolic content andits involvement in the graft incompatibility process of various pear rootstocks (Pyrus communis L.). J Plant Physiol 171:76–84. https://doi.org/10.1016/j.jplph.2013.10.022
Irisarri P, Binczycki P, Errea P, Martens HJ, Pina A (2015) Oxidative stress associated with rootstock–scioninteractions in pear/quince combinations during early stages of graft development. J Plant Physiol 176:25–35. https://doi.org/10.1016/j.jplph.2014.10.015
Jonard R, Lukman D, Schall F, Villemur P (1990) Early testing of graft incompatibilities in apricot and lemon trees using in vitro techniques. J Sci Hortic 43:117–128. https://doi.org/10.1016/0304-4238(90)90043-E
Karimi H, Hassanpour N (2017) Effects of salinity, rootstock, and position of sampling on macro nutrient concentration of pomegranate cv. Gabri. J Plant Nutr 40:2269–2278. https://doi.org/10.1080/01904167.2016.1263324
Lanauskas J, Kviklys D, UeslisStanys N (2023) Performance of sweet cherry cultivars and advanced selections on gisela 5 rootstock in young orchards. Plants 12(3):614. https://doi.org/10.3390/plants12030614
Lopez-Ortega G, Garcia-Montiel F, Bayo-Canha A, Frutos-Ruiz C, Frutos-Tomas D (2016) Rootstock effects on the growth, yield and fruit quality of sweet cherry cv. ‘Newstar’ in the growing conditions of the Region of Murcia. J Sci Hortic 198:326–335. https://doi.org/10.1016/j.scienta.2015.11.041
Melnyk CW, Gabel A, Hardcastle TJ, Robinson S, Miyashima S, Grosse I, Meyerowitz EM (2018) Transcriptome dynamics at Arabidopsis graft junctions reveal an intertissue recognition mechanism that activates vascular regeneration. Proc Natl Acad Sci 115:E2447–E2456
Mnich E, Bjarnholt N, Eudes A, Harholt J, Holland C, Jorgensen B, Larsen FH, Liu M, Manat R, Meyer AS, Mikkelsen JD, Motawia MS, Muschiol J, Moller BL, Moller SR, Perzon A, Petersen BL, Ravn JL, Ulvskov P (2020) Phenolic cross-links: building and de-constructing the plant cell wall. Nat Prod Rep 37(7):919–961. https://doi.org/10.1039/c9np00028c
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Pandey V, Awasthi M, Singh S, Tiwari S, Dwivedi U (2017) A comprehensive review on function and application of plant peroxidases. Biochem J Anal Biochem 6(1):1–16. https://doi.org/10.4172/2161-1009.1000308
Pedersen BH (2006) Determination of graft compatibility in sweet cherry by a co-culture method. J Hortic Sci Biotechnol 81:759–764. https://doi.org/10.1080/14620316.2006.11512134
Polle A, Eiblmeier M, Sheppard L, Murray M (1997) Responses of antioxidative enzymes to elevated CO2 in leaves of beech (Fagus sylvatica L.) seedlings grown under a range of nutrient regimes. J Plant Cell Environ 20:1317–1321. https://doi.org/10.1046/j.1365-3040.1997.d01-23.x
Porika H, Nimbolkar P, Rajashekar B, Hussain S (2016) Graft compatibility-incompatibility in fruit crops: mechanism and determination techniques. J Asian Hortic 11:252–260. https://doi.org/10.15740/HAS/TAJH/11.1/252-260
Prabpree A, Sangsil P, Nualsri C, Nakkanong K (2018) Expression profile of phenylalanine ammonia-lyase (PAL) and phenolic content during early stages of graft development in bud grafted Hevea brasiliensis. J Biocatal Agric Biotechnol 14:88–95. https://doi.org/10.1016/j.bcab.2018.02.010
Sarropoulou V, Dimassi-Theriou K, Therios L (2017) Effects of the exogenous polyamines on micropropagation of cherry rootstocks. Indian J Plant Physiol 22(2):227–239
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol 299:152–178
Suchy M, Kontturi E, Vuorinen T (2010) Impact of drying on wood ultrastructure: similarities in cell wall alteration between native wood and isolated wood-based fibers. J Biomacromol 11:2161–2168. https://doi.org/10.1021/bm100547n
Sunohara Y, Matsumoto H (2004) Oxidative injury induced by the herbicide quinclorac on Echinochloa oryzicola Vasing. and the involvement of antioxidative ability in its highly selective action in grass species. J Plant Sci 167:597–606. https://doi.org/10.1016/j.plantsci.2004.05.005
Suryadi R, Pribadi E, Darwati I (2020) The effect of ascorbic acid on clove (Syzigium aromaticum) grafting. IOP Conf Ser Earth Environ Sci. https://doi.org/10.1088/1755-1315/418/1/012026
Tucker MR, Lou H, Aubert MK, Wilkinson LG, Little A, Houston K, Pinto SC, Shirley NJ (2018) Exploring the roleof cell wall-related genes and polysaccharides during plant development. J Plants 7:42–52
Whistler RL (1964) Methods in carbohydrate chemistry. Academic publisher Inc, New York, p 335. https://doi.org/10.1002/ange.19650770520
Zarrouk O, Testillano PS, Risueno MC, Moreno MA, Gogorcena Y (2010) Changes in cell/tissue organization and peroxidaseactivity as markers for early detection of graft incompatibility in peach/plum combinations. J Am Soc Hortic Sci 135:9–17
Zhou Q, Gao B, Li WF, Mao J, Yang SJ, Li W, Ma ZH, Zhao X, Chen B (2020) Effects of exogenous growth regulators and bud picking on grafting of grapevine hard branches. J Sci Hortic 264:109–186
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest related to this article.
Additional information
Communicated by Henryk Flachowsky.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jalali, A., Moghaddam, E.G. & Marjani, A. Early detection of graft incompatibility in sweet cherry by internode association and callus fusion techniques. Plant Cell Tiss Organ Cult 156, 47 (2024). https://doi.org/10.1007/s11240-023-02663-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11240-023-02663-8