Abstract
The emerald ash borer (EAB), Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), is one of the most destructive invasive forest pests in North America, where it has killed hundreds of millions of ash (Fraxinus spp.) trees since its first detection in 2002. Native to Asia, female EAB adults lay their eggs between bark crevices or under loose bark of their host trees. Because of this cryptic egg-laying behavior, field detection of EAB eggs has been extremely difficult, resulting in knowledge gaps of EAB egg-laying behavior. In the present study, I tested the potential use of strips of burlap fabric (0.03 m × 1 m) or polypropylene curling ribbon (0.005 m × 2 m) to induce female EAB adults to lay eggs on the trunks or branches of ash covered with these materials in the field. The burlap trap tested in this study detected more than twice as many EAB eggs (4.9 ± 1.1 eggs per burlap trap) as did the polypropylene ribbon trap (1.9 ± 0.5 eggs per ribbon trap). When the surface area of each trap is standardized to one square meter, however, both burlap fabric and polypropylene ribbon traps are equally effective in inducing female EAB adults to lay eggs onto the covered surface of ash trunks or branches. Potential applications of the oviposition traps for effective detection and control of EAB and other jewel beetles are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The emerald ash borer (EAB), Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), is one of the most destructive invasive forest pests in North America, where it is responsible for the death of hundreds of millions of ash (Fraxinus spp.) trees since its first detection in 2002 (Herms and McCullough 2014 and Emerald Ash Borer Information 2023). This borer also invaded Moscow in 2003 and has since spread throughout European Russia and eastern Ukraine, causing widespread mortality of native European ash trees (Orlova-Bienkowskaja and Bieńkowski 2022). Native to Asia, gravid female EAB adults lay their eggs in bark crevices or under loose layers of bark on host trees (Anulewicz et al. 2006; Jennings et al. 2014). Eggs hatch in approximately 2–3 weeks and newly hatched larvae bore into the phloem/cambium layers under the bark, where they develop over 1–2 growing seasons. Feeding inside the phloem/cambium layers by EAB larvae interrupts nutrient and water transportation, causing tree mortality even at moderate larval densities.
The cryptic nature of adult egg laying and larval feeding poses serious challenges not only to the early detection and prevention of EAB establishment but also to effective management via insecticide treatment and biological control. Largely because of the lack of effective means to observe EAB eggs in the field, no studies have been able to reliably examine the seasonal pattern or phenology of egg laying by gravid females of EAB under field conditions (Barker et al. 2023).
In laboratory rearing, researchers have been able to induce gravid EAB adults to lay eggs on coffee filter papers through a nylon window screen (~ 1 mm2 mesh) placed on the opening of a rearing jar or cup (150–300 L) containing fresh ash foliage (Duan et al. 2013). Under similar laboratory rearing conditions, gravid EAB adults will also lay their eggs on the surface of ash bolts wrapped with spiraling strips (~ 0.5 cm width) of polypropylene curling ribbon (Duan et al. 2011). Based on these laboratory observations, I hypothesize that gravid female EAB adults might be stimulated to lay eggs on the surface of ash trunks or branches that are covered by spiraling strips of burlap fabrics or polypropylene curling ribbons. The rough surface of burlap fabric and the spaces created between wraps of polypropylene curling ribbons or burlap fabric may serve as super-stimuli associated with potential oviposition sites for gravid female EAB adults. In the present study, I tested the potential of using strips of burlap fabrics or polypropylene ribbons to lure gravid EAB adults to lay eggs on the covered surface of ash tree trunks or branches under field conditions.
Materials and methods
Study sites
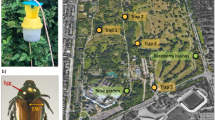
The study was conducted in an urban landscape woodlot near the USDA ARS Beneficial Insects Introduction Research Unit (BIIRU, Newark, Delaware), where white ash (Fraxinus americana L.) trees were planted approximately 15 years ago, mixed with maple (Acer spp.) and white birch (Betula papyrifera Marsh). The emerald ash borer was first detected in Delaware the summer of 2016 in an ash-dominated forest ~ 10 km away from BIIRU, and symptoms of emerald ash borer infestation (D-shaped adult exit holes and woodpecker feedings signs) were first observed in 2018 on ash trees at the BIIRU landscape woodlot. Before this study, the crown classes of all ash trees used for trap installation in this experiment were assessed, using the crown decline and dieback scale of 1–5 developed by Knight et al. (2013), with a score of 1.0 representing an ash with a healthy crown, and the score of 5.0 representing a dead or dying crown. The crown class of ash trees used for trap installation was between 1.5 and 3.5, indicating light to moderate EAB infestations.
Oviposition traps and their installation
Two types of traps were tested. One consisted of a strip (0.03 m wide × 1 m long) of burlap fabric (ULINE Co., Pleasant Prairie, WI) and the other of a piece (0.005 m wide × 2 m long) of polypropylene curling ribbons (Amazon Co. Seatle, WA). Strips of burlap fabric or curling ribbon (hereafter termed as oviposition traps) were wound tightly around tree trunks or branches (at 0.5–2.5 m above the ground) in a spiraling pattern with ~ 5 cm between neighboring bands (Fig. 1). Both ends of each oviposition trap (strip) were stapled to the tree trunks or branches. Each tree trunk or branch had one burlap trap and one curling ribbon trap in alternating (up or down) positions ~ 30 cm apart. A total of 32 pairs of traps were installed on 20 different ash trees with diameter at breast height (DBH) values ranging from 8 to 20 cm. While all the ash trees had one pair of traps on the main trunk, additional large branches (diameter = 9–15 cm) from six larger trees also had a pair of traps installed—i.e., more than one pair of traps were installed on those large trees. All traps were installed on June 1, 2023, approximately one week after the first EAB emergence (D-exit holes) was observed on a monitoring (EAB-infested) ash tree at BIIRU.
Observations
Observation of EAB eggs laid on both the covered and uncovered surfaces of ash trunks or branches between or under the spiraling wraps of each trap were conducted on a weekly basis from June 8 to August 16, 2023. At each observation time, surface area not covered between the spiraling wraps of each trap was first examined for any EAB eggs. Then, each trap (strip) was unwound after removing the staple at one end, and the covered surface area of ash trunk or branch was examined for EAB eggs. EAB eggs are oval to round, ~ 1 mm in diameter and can be recognized without the assistance of optic magnification (Fig. 2). Although eggs laid within less than 24 h are white, they rapidly turn golden or reddish orange in a few days. However, unfertilized EAB eggs remain white until they desiccate. In addition, EAB eggs attacked by the introduced parasitoid Oobius agrili Zhang and Huang turn dark black in five to seven days. Throughout the study, all EAB eggs were collected and placed in the ventilated plastic vials (5 ml), and the fate or hatching of each egg was determined after approximately four weeks of incubation in a growth chamber under normal rearing conditions (25 °C, photoperiod L:D 16:8 h, and 65% RH).
Data analyses
The number of EAB eggs collected throughout the entire experimental season (June 9 to August 16, 2023) for each trap was first summarized and analyzed using mixed linear model for ANOVA, which included the trap type as a fixed effect and trap location (tree trunk or branch) as a random effect. The Fit Model platform in JMP 17.01 Pro was used for statistical analysis (SAS 2023).
Results and discussion
Throughout the study period (June 1 to August 16), a total of 157 and 61 EAB eggs were observed and collected on the surface area of ash trunks or branches covered by burlap and ribbon oviposition traps, respectively (Table 1). In contrast, no EAB eggs were found on the uncovered surface area of ash trunks or branches in the spaces between the burlap or ribbon wraps. The mean number of EAB eggs (4.9 ± 1.1) per burlap trap was significantly higher than that per ribbon trap (1.9 ± 0.5) (F = 8.67, df = 1, 48, P = 0.005). When the surface area for each trap was adjusted to an equal area basis (per 1 m2), however, there was no significant difference in the mean number of EAB eggs observed per unit area (163.5 ± 37.2 vs. 190.6 ± 46.3) between the burlap and ribbon traps (F = 0.31, df = 1, 48, P = 0.58). Among all the eggs observed from both burlap and ribbon traps (N = 218), 98% successfully hatched into EAB larvae when incubated under normal rearing conditions, whereas 2% (n = 5) did not hatch due to infertility (white, n = 3) and parasitism by O. agrili (dark, n = 2). These results indicate that both burlap fabric and polypropylene ribbon traps are equally effective in attracting EAB oviposition onto the trap-covered surface of ash trunks or branches.
Potentially, the efficacy of either burlap or polypropylene ribbon traps to stimulate EAB oviposition could be enhanced by increasing the trap surface area through lengthening and/or widening the fabric strip. However, the effective spaces created by increasing the trap surface area for EAB oviposition differ between the two types of traps tested. EAB adults can lay eggs onto oviposition substrates (e.g., coffee filter paper or ash branches) through nylon screen or burlap fabric with an approximately 1-mm2 mesh (Duan et al. 2013). In contrast, EAB adults can only lay eggs into the space or crevices created between the edges of the polypropylene ribbon strips and the oviposition substrate (e.g., ash branch) because EAB adults cannot penetrate the ribbon surface with their ovipositors. Therefore, the effective space for EAB adults to lay eggs created by the ribbon wraps on ash trunk or branch can only be increased by increasing the ribbon’s length, not its width. For these reasons, we recommend the use of burlap fabric traps rather than polypropylene ribbon traps for field detection of EAB eggs.
Gravid female EAB adults are known to lay eggs under the loose bark or bark crevices of host tree trunks and branches. However, our field and laboratory observations indicate that gravid EAB adults appear to select certain types of loose bark or bark crevices to lay eggs. They do not lay eggs under pieces of bark that are too loosely attached to the tree trunk (which do not offer protection of their eggs) or too tightly attached, such that they cannot insert their ovipositors (JJD, unpublished data). I thus hypothesize that EAB adults may select appropriate oviposition sites based on both the surface roughness of the substrates and the pressure or tightness of the space between the substrate crevices. Studies to characterize the “super” stimuli associated with EAB oviposition sites are currently being conducted at BIIRU and may further improve the design of effective oviposition traps based on burlap fabric and/or polypropylene ribbon strips.
Worldwide, there are over 14,800 valid jewel beetle (Buprestidae) species including over 3000 species in the genus Agrilus (Nelson et al. 2008). While most jewel beetles are not considered to be pests of agriculture or forests, EAB and several of its congeners, such as the gold spotted oak borer (A. auroguttatus Shaefer), European oak borer (A. sulcicollis Lacordaire), and bronze birch borer (A. anxius Gory), are serious forest pests in both their native home and/or newly invaded areas (Jendek and Grebennikov 2009; Coleman et al. 2011; Evans et al. 2020). Like EAB, those jewel beetles also lay eggs under the loose bark or bark crevices of their host plants. Therefore, it is conceivable that the burlap and/or ribbon traps for EAB oviposition may also be an effective tool in detecting eggs of other jewel beetles, particularly Agrilus spp.
Currently, visual traps such as purple or yellow prism traps, double-decker prism traps, and Lindgren funnel traps have been used in combination with various lures to catch EAB adults foraging for food plants or seeking mates (e.g., Poland et al. 2019; review in Silk et al. 2019). One type of visual trap—the green Lindgren funnel—has also been modified to serve as an auto-dissemination device (FraxiProtec™) for controlling emerald ash borer with the common entomopathogenic fungus [Beauveria bassiana (Balsamo) Vuillemin] in Canada (Srei et al. 2020). Although these visual traps have been frequently used in delineating the distribution of EAB as well as its seasonal abundance in both established and newly invaded regions, their effectiveness in detecting EAB at low densities have not been well studied. In contrast, the oviposition traps are much smaller and easier to deploy in the field than the large and cumbersome visual traps that are currently being used. The efficacy of oviposition traps for detecting EAB in low density sites should be determined by simultaneous comparison with traps currently deployed for detection of EAB adults at sites across a range of EAB population densities ranging from very low (i.e., not yet detected) to moderate. Furthermore, the oviposition traps might also be easily modified to serve as an auto-dissemination device for control of EAB with agents such as B. bassiana.
References
Anulewicz AC, McCullough DG, Miller DL (2006) Oviposition and development of emerald ash borer (Agrilus planipennis) (Coleoptera: Buprestidae) on hosts and potential hosts in no-choice bioassays. Great Lakes Entomol 59:99–112
Barker BS, Coop L, Duan JJ, Petrice TR (2023) An integrative phenology and climatic suitability model for emerald ash borer. Front Insect Sci 3:1239173. https://doi.org/10.3389/finsc.2023.1239173
Coleman TW, Grulke NE, Daly M (2011) Coast live oak, Quercus agrifolia, susceptibility and response to goldspotted oak borer, Agrilus auroguttatus, injury in southern California”. For Ecol Manage 261:1852–1865
Duan JJ, Bauer LS, Ulyshen MD, Gould JR, Van Driesche R (2011) Development of methods for the field evaluation of Oobius agrili (Hymenoptera: Encyrtidae) in North America, a newly introduced egg parasitoid of the emerald ash borer (Coleoptera: Buprestidae). Biol Control 56:170–174
Duan JJ, Watt T, Taylor P, Larson K, Lelito JP (2013) Effects of ambient temperature on egg and larval development of the invasive emerald ash borer (Coleoptera: Buprestidae): implications for laboratory rearing. J Econ Entomol 106:2101–2108
Emerald Ash Borer Information. Emerald Ash Borer Information. Emerald ash borer information network. Available online 2013 : http://www.emeraldashborer.info/ (accessed on 15 January 2023).
Evans HF, Williams D, Hoch G, Loomans A, Marzan M (2020) Developing a European Toolbox to manage potential invasion by emerald ash borer (Agrilus planipennis) and bronze birch borer (Agrilus anxius), important pests of ash and birch. Forestry 93:187–196. https://doi.org/10.1093/forestry/cpz074
Herms DA, McCullough DG (2014) Emerald ash borer invasion of North America: history, biology, ecology, impact, and management. Ann Rev Entomol 59:13–30
Jendek E, Grebennikov VV (2009) Agrilus sulcicollis (Coleoptera: Buprestidae), a new alien species in North America. Canadian Entomol 141:236–245
Jennings DE, Tylor PH, Duan JJ (2014) The mating and oviposition behavior of the invasive emerald ash borer (Agrilus planipennis), with reference to the influence of host tree condition. J Pest Sci 87:71–78
Knight KS, Brown JP, Long RP (2013) Factors affecting the survival of ash (Fraxinus spp.) trees infested by emerald ash borer (Agrilus planipennis). Biol Invas 15:371–383
Nelson GH, Walters GC Jr, Haines RD, Bellamy CL (2008) A Catalog and Bibliography of the Buprestoidea of America North of Mexico. Coleopterists Society, North Potomac, Maryland, USA
Orlova-Bienkowskaja MJ, Bieńkowski AO (2022) Southern range expansion of the emerald ash borer, Agrilus planipennis, in Russia threatens ash and olive trees in the Middle East and Southern Europe. Forests 13(4):541. https://doi.org/10.3390/f13040541
Poland TM, Petrice TR, Ciaramitaro TM (2019) Trap designs, colors, and lures for emerald ash borer detection. Front Forests Global Change. https://doi.org/10.3389/ffgc.2019.00080
SAS Institute Inc. JMP® Pro 17.0.1 Statistical Software. 2023. SAS Institute Inc, SAS Campus Drive, Cary, NC 27513, USA.
Silk P, Mayo P, Ryall K, Roscoe L (2019) Semiochemical and communication ecology of the Emerald Ash Borer, Agrilus planipennis (Coleoptera: Buprestidae). Insects 10(10):323. https://doi.org/10.3390/insects10100323
Srei NC, Guertin G, Lavallée R, Lajoie M-E, Brousseau C, Bergevin R, Miller F, McMillin K, Trudel T (2020) Microbial control of the emerald ash borer (Coleoptera: Buprestidae) using Beauveria bassiana (Hypocreales: Cordycipitaceae) by the means of an autodissemination device. J Econ Entomol 113:2657–2665
Acknowledgements
I am grateful for the assistance of University of Delaware students (Smith Eaton, Peyton Easton and Grace Delseni) and USDA ARS technician Jonnathan Schmude in installation of the traps and weekly observation of EAB eggs from those traps. Roy van Driesche (University of Massachusetts) provided helpful comments on the earlier version of the manuscript. Mitchell Green (University of Massachusetts) and Heather Callahan (University of Delaware) provided comments on the last revision of the manuscript. This project was supported by the USDA ARS appropriated funds (CRIS-8010-00022-31D).
Author information
Authors and Affiliations
Contributions
Jian J Duan conceived the ideas, designed methodology, collected and analyzed the data, a prepared Tables and Figures, and drafted the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Tim Haye.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duan, J.J. A novel oviposition trap for studying the egg-laying behavior of emerald ash borer. J Pest Sci 97, 1087–1091 (2024). https://doi.org/10.1007/s10340-024-01770-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-024-01770-5