Abstract
Plant-emitted volatile organic compounds play an important role in plant–insect interactions. Thanks to plant-emitted volatiles, herbivores are able to find suitable hosts. Recognition and location of host plants are a key challenge for successful survival and reproduction of migrating insects, such as the plum psyllid Cacopsylla pruni. This psyllid migrates between Prunus spp. for reproduction and conifers for overwintering. C. pruni also is the only known vector of ‘Candidatus Phytoplasma prunorum’, a plant pathogen causing the European Stone Fruit Yellows, a severe plant disease. The preference of C. pruni for different Prunus species was monitored in the field. The sampling revealed a high abundance of C. pruni on Prunus spinosa, the natural host, as well as on different Prunus rootstock suckers. To investigate the influence of volatile profiles from different plants on the host preferences of C. pruni, the volatiles of two reproduction hosts and one overwintering host were sampled and analyzed by gas chromatography and mass spectrometry. The volatile compositions were compared, and important components that lead to the differentiation between plant species and growth stages were identified. Antennal responses of C. pruni females were elicited by eleven plant species and growth stage-specific volatiles, detected by electroantennography. The role of host plant volatiles on the migration behavior and the use of synthetic components in alternative control strategies are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Cacopsylla pruni is the vector of European Stone Fruit Yellows, which annually causes high economic losses in European stone fruit production.
-
Species and growth stage-specific volatiles of reproduction and overwintering hosts were identified.
-
Antennal responses of female plum psyllids to aldehydes, terpenes, acetophenone and (Z)-3-hexenyl acetate were detected.
-
C. pruni showed no olfactory preference for reproduction or overwintering hosts.
-
Results could be used for the development of innovative control strategies, such as push–pull or attract-and-kill strategies.
Introduction
Olfaction is of high importance for insects. The development of specialized and sophisticated olfactory systems enabled insects to orientate and interact with their environment. Accordingly, olfactory cues are used to detect essential resources like food sources, oviposition sites and mating partners even from a great distance (Gadenne et al. 2016). Detecting suitable host plants over distance is crucial for herbivorous insects, especially for migrating species. Migration behavior is well studied in species which show a movement over a very long distance, such as the monarch butterfly (Reppert et al. 2016) and desert locusts (Homberg 2015; Rosenberg and Burt 1999). Little is known, however, about seasonal migration occurring in psyllid species (known vectors of different plant pathogens), like the univoltine and specialized plum psyllid Cacopsylla pruni, which alternates between evergreen conifers and deciduous stone fruit trees (Prunus spp.) (Hodkinson 2009; Jarausch and Jarausch 2016; Ossiannilsson 1992; Thébaud et al. 2009). At the end of winter/early spring, C. pruni remigrants move from conifers back to Prunus ssp. for oviposition. The new generation develops through five larval instars, and the emigrant adults leave the reproduction hosts from May until the beginning of July (Thébaud et al. 2009). With migration between such divergent host plant species, the challenge of long-range host location comes along. While plant volatiles are exploited by psyllids to find their reproduction or overwintering host plants during migration (Gross 2016; Mayer and Gross 2007; Mayer et al. 2011), the full set of factors triggering psyllids’ migratory behavior still remain unknown (Gallinger and Gross 2018; Jarausch et al. 2019a).
The migrating psyllid C. pruni is the vector of ‘Candidatus Phytoplasma prunorum’, a cell wall-lacking bacteria, causing European Stone Fruit Yellows (ESFY) in various cultivated species of Prunus (Marcone et al. 2010). Today, ESFY is of considerable economic significance in European agriculture, particularly in peach (Prunus persica) and apricot (Prunus armeniaca) cultivation (Marcone et al. 2010, 2011). Diverse crossbreedings of different Prunus spp. offer a wide range of available rootstocks and cultivars. Various stone fruit species show different susceptibilities to ESFY infections in targeted grafting experiments (Carraro et al. 2002, 2004; Kison and Seemüller 2001) and varying infestation rates in field surveys (Jarausch et al. 1998, 2000, 2019b; Richter 2002). Differences in the natural occurrence of infection rates of Prunus spp. could be linked to different infestation levels with C. pruni (Carraro et al. 2002; Mergenthaler et al. 2017) due to their host plant preferences. Prunus spinosa is the preferred reproduction host in natural habitats and is asymptomatically infected with ‘Ca. P. prunorum’ (Jarausch et al. 2019a, b).
Former studies with another European migrating psyllid species, Cacopsylla melanoneura, revealed differences in host plant preferences between emigrants and remigrants (Mayer and Gross 2007). The carrot psyllid Trioza apicalis was repelled by sawdust made from evergreen conifers, their overwintering hosts (Nehlin et al. 1994). Furthermore, repellent effects of VOCs from winter hosts were found in migrating aphids (Pettersson et al. 1994). Based on these findings, we hypothesize that C. pruni remigrants are attracted to volatiles emitted from Prunus spp. plants, during their spring migration period (reproduction host), and repelled by volatiles typically released from conifers (overwintering hosts). Potentially, this preference is reversed during their summer migration period and induces the migration of C. pruni emigrants from Prunus spp. to conifers located in higher altitudes.
In the present study, the occurrence of C. pruni in the field was monitored to determine psyllid preference for specific host species and varieties over three years. The results of this survey lead to the identification of attractive and non-attractive Prunus cultivars. Emitted volatile organic compounds (VOCs) of one cultivar from a non-attractive species (P. persica) and one from an attractive species (Prunus insititia) were analyzed at different phenological stages. Additionally, the volatile blends from an overwintering host (Abies alba) were firstly sampled during the migration period of C. pruni from conifers to Prunus spp. and secondly during the migration period of the new generation from Prunus spp. to conifers. Species-specific and developmental stage-specific compounds were identified, and the electrophysiological response of C. pruni toward (+)-limonene, α-pinene, terpinolene, myrcene, camphene, bornylacetate, hexanal, octanal, nonanal, decanal, dodecanal, (Z)-3-hexenyl acetate, (Z)-3-hexene-1-ol, 1-phenylethane-1-one and tridecane was investigated to identify compounds, which may drive psyllid preferences for different hosts. Identification of attractant and repellent VOCs could improve the development of alternative plant protection measurements like push–pull or attract-and-kill strategies against C. pruni.
Materials and methods
Insects
In early spring (March/April), C. pruni remigrants (overwintered adults) were caught using beating tray method according to Weintraub and Gross (2013). Remigrants used for olfactometer trails were sampled from P. spinosa and Prunus besseyi x Prunus cerasifera (cv. Ferlenain) trees at an experimental orchard of Dienstleistungszentrum Ländlicher Raum (DLR) Rheinpfalz, Neustadt an der Weinstrasse, Germany, and from P. spinosa, P. cerasifera, P. insititia (cv. GF 655-2) and P. domestica (cv. Wavit) at the experimental field of the Julius Kühn-Institut (JKI) in Dossenheim, Germany (40 km linear distance to Neustadt an der Weinstrasse). Caught psyllids from both sampling sides were reared in cages (BugDorm, MegaView Science Co, Taiwan 47.5 × 47.5 × 93 cm) with four-year-old (one year nursery and three years potted) potted P. spinosa plants. The rearing was housed in a climate chamber at 20 °C (photophase) and 16 °C (scotophase) (L16:D8). Newly emerged emigrants were separated by transferring them to new cages with a three-year-old (1 year nursery and 2 years potted) potted plant of P. domestica (cv. Wavit). To prevent that older emigrants die because they may reject feeding on Prunus trees, a five-year-old (3 years nursery and 2 years potted) potted silver fir (A. alba) was placed in each cage, after 3 weeks. Prunus plants were potted in 1.8-L pots with clay substrate (Klasmann-Deilmann GmbH, Geeste, Germany); Abies alba trees were potted in 1.8-L pots with clay substrate (80%) and sand (20%). All plants were fertilized with ~ 500 ml Triabon (COMPO Expert GmbH, Münster, Germany, 2 g/l) once (in March) and were housed in an insect save environment until they were used for the rearing. Prunus trees were once treated with paraffin oil in March to prevent infestations with spider mites.
Population monitoring in the field
The population dynamics and preferences for different Prunus genotypes of C. pruni were monitored by beating tray method in the experimental orchard of DLR Rheinpfalz in Neustadt an der Weinstrasse, Germany, where no chemical plant protection products for pest control were applied. Table 1 shows the genetic background of the ten different Prunus genotypes. Trees of apricot, peach and blackthorn cultivars were of 2–3 m height without rootstock suckers (shoots that grew up from the rootstocks of a tree, which were removed regularly). Rootstock varieties were grown to trees of approximately 2 m height. Detailed information on the setting of the Prunus orchard is provided in Fig. S1 (Supplementary material 1). All Prunus genotypes were sampled weekly, starting with the arrival of the first C. pruni remigrants in March until migration of the new generation (emigrants) in July, for 3 years (2010, 2011, and 2012). At the beginning of the monitoring period, up to 100 branches of P. spinosa trees were sampled, to evaluate whether the migration of C. pruni to Prunus trees had begun. Up to 50 branches of the Prunus genotypes listed in Table 1 were sampled weekly (Table S1 with no. of samples: Supplementary material 1). Two beats on one branch were defined as a sample unit. C. pruni individuals were captured with a beating tray measuring 40 × 60 cm. Psyllids were directly aspirated with an exhaustor to avoid loss of insects. Captured psyllids were frozen at − 20 °C, and species determination was done under a stereomicroscope.
Volatile analysis
Headspace sampling
Headspace samples from stone fruit P. insititia cv. GF 655-2 (rootstock), P. persica cv. South Haven and white fir A. alba were sampled at the experimental field of the JKI, Dossenheim, Germany. No chemical plant protection products for pest control were applied, and the pruning of trees was omitted during the time of the experiment. Samples were taken with a mobile 6-channel headspace sampling device composed of vacuum pumps (KNF Neuberger GmbH, Freiburg, Germany) connected to mass flow controllers (M + W Instruments GmbH, Leonhardsbuch, Germany) as described by Rid et al. (2016) and modified as described in Gross et al. (2019). Single branches of the Prunus and white fir trees were carefully wrapped in polyethylene terephthalate oven plastic bags (Toppits, Melitta, Minden, Germany). Ambient air was filtered through inline clean air filter cartridges (ICAF 2X6, Sigma Scientific, Micanopy, USA) and pumped through the oven bags with a flow of 1000 mL/min. Volatiles were trapped on stainless steel, prepacked sample tubes with 200 mg Tenax TA60/80 sorbent (PerkinElmer, Rodgau, Germany; Markes, Neu-Isenburg, Germany). After a final volume of 50 L had passed through the matrix, sampling tubes were closed with Teflon-coated brass compression caps (Swagelok, PerkinElmer) and stored until analysis. The headspace of eleven A. alba trees was sampled in spring (beginning of March), when C. pruni starts to migrate to Prunus trees for reproduction and in fall at the end of the season, when all C. pruni emigrants had left the Prunus orchards (October). Volatile sampling of five P. persica and five P. insititia trees was repeated weekly from beginning of March until end of May. Samples were assigned to the phenological growth stages of stone fruit trees, classified following the BBCH identification keys of stone fruit by principal growth stages according to Meier (2018): Principal stages include: 0: sprouting/bud development; 1: leaf development; 5–6: inflorescence emergence and flowering; 7: development of fruit. Secondary stages include: 00: dormancy; 01: bud swelling; 03: end of leaf bud swelling; 09: green leaf tips; 10 first leaves emerging; 11: first leaves unfolded; 19: first leaves fully expanded; 71: ovary growing and fruit fall, 72: sepals beginning to fall; 73: second fruit fall; 75: fruit 50% of final size; 76: fruit 60% of final size; 77: fruit 70% of final size; 78: fruit 80% of final size, 79: fruit 90% of final size.
Thermodesorption–GC–MS
Samples were analyzed using an automated thermal desorber (TurboMatrix™ ATD 650, PerkinElmer) connected to a gas chromatograph coupled with mass spectrometer (GC–MS). Sample tubes were desorbed for 10 min at 250 °C. The cold trap (Tenax TA) was held at − 20 °C throughout the tube desorption process, then heated at a rate of 99 K/s to 250 °C and desorbed for 1 min. The thermal desorbed compounds were separated using a PerkinElmer Clarus R 680 GC system equipped with a nonpolar Elite-5 (crossbond 5% diphenyl–95% dimethyl polysiloxane, PerkinElmer) capillary column (30 × 0.25 mm id × 0.25 µm film thickness) and coupled to a PerkinElmer quadrupole inert mass selective detector. Injection was employed using helium as carrier gas (Helium 6.0, Linde, Munich, Germany) at 130 kPa column head pressure. The GC temperature program was as follows: Initial oven temperature of 40 °C was held for 1 min, increased at a rate of 5 K/min to 180 °C, followed by a rate of 20 K/min to the final temperature of 280 °C and held for 6 min. The GC inlet line temperature was 250 °C, and the ion source temperature was 180 °C. The quadrupole mass detector was operated in the electron impact (EI) mode at 70 eV. All data were obtained by collecting the full-scan mass spectra within the range of 35–350 m/z.
Identification and quantification with AMDIS
GC–MS chromatograms were analyzed using “Automated Mass spectral Deconvolution and Identification System” (AMDIS, V. 2.71; National Institute of Standards and Technology NIST, Boulder, CO) following the protocol in Gross et al. (2019). Detected compounds were identified by comparing characteristic ion fragmentation patterns, retention times and retention indices (RI) with available standard compounds introduced in the same system according to Weintraub and Gross (2013). For non-available compounds, the RI values were obtained from NIST Chemistry WebBook (Linstrom and Mallard 1997), corresponding to the used column diameter and film thickness as well as a comparable stationary phase material. Compounds for which proper identification was not possible were defined as “known unknown” and incorporated to the AMDIS library. Identification criteria for AMDIS were applied as follows: minimum match factor was set to 80% and the relative retention index deviation to 5 with an increase by multiplication of the RI reference value with 0.01; level: strong; maximum penalty: 20; and “no RI in library”: 20. For quantification, the peak areas were integrated after deconvolution with AMDIS. The settings for deconvolution were: component width: 32; adjacent peak subtraction: one; resolution: low; sensitivity: medium; shape requirements: low. Only peaks with a signal-to-noise ratio higher than 50 were considered in the analysis. Additionally, compounds which occurred in less than 8% of all samples were excluded from the analysis. Compounds exclusive for one treatment and occurring in at least 50% of the samples of this treatment were not excluded. Relative proportions of volatile organic compounds were calculated by setting the sum of all selected compounds to 100%.
Electroantennography
Female C. pruni adults (2–4 weeks old) were placed in pipette tips (200 µL) as described in George et al. (2016). The head and the left antennae were exposed. Backward movement of psyllids was prevented using a piece of cotton wool. The psyllid in the pipette tip was mounted 1 cm in front of a filtered and humidified airstream. The air was passed through the antennae with a continuous flow of 1.23 Ln/min. Glass capillaries (0.58 mm I.D., Science Products, Hofheim, Germany) were pulled with a glass Micro-Electrode Pipette Puller (PN-3, Narishige, Japan) to prepare thin and sharp glass electrodes. Two glass electrodes were filled with Ringer solution (NaCl 7.5 g, KCl 0.35 g, CaCl2 0.21 g, 1 L H2O). Pieces of silver wire were inserted through glass electrode holders into the saline solution. The indifferent electrode was inserted through the mesonotum into the back of the psyllid. The distal end of the left antenna (half of last segment) was placed in the second glass electrode connected to a Combi Probe (INR-II, Ockenfels Syntech®). Substances were diluted in methylene chloride (DCM) to a concentration of 10 µg/µL. 10 μL of the dilutions was pipetted on a piece of filter paper (Type 413, VWR Collection) and inserted in glass Pasteur pipettes (23 cm). 10 μL of DCM on filter paper was used as negative control. Cartridges were prepared fresh, and the solvent was evaporated for 3 min before puffing over the antenna. The order of compounds was changed randomly for every replicate. The pipette was connected with a tube to the data acquisition controller (IDAC-2, Ockenfels Syntech®), and the stimulus was passed through the antenna for 1 s with a flow of 1.46 Ln/min by activating the stimulus air puff via the pedal switch. Refractory period was 60 s allowing the antenna to recover. A negative control was applied before each test compound. The reactivity of the antenna was verified by puffing 1000 µg hexanal (10 µL of 100 µg/µL) after every fifth compound. Proper preparations remained functional for several hours. All compounds were tested randomly on each antenna. The signals from ten female emigrants were recorded with EAGPro software (version 1.1, Ockenfels Syntech®) and extracted for further analysis.
Behavioral experiments
Plant material
Twigs were excised from P. persica cv. South Haven, P. insititia cv. GF 655-2 and A. alba plants in the field at the experimental area of the JKI in Dossenheim. As monitoring studies showed a low abundance of C. pruni in P. persica cultivars, we chose P. persica cv. South Haven as an example for a non-attractive cultivar. In contrast, P. spinosa, P. cerasifera and P. insititia showed high abundances of C. pruni in field studies. We decided to include P. insititia cv. GF 655-2 in our experiments, as this cultivar is a commonly used rootstock for stone fruit cultivars and tends to produce suckers. The cut ends of the twigs were placed in plastic tubes filled with water and sealed with plastic paraffin film. Twigs were always freshly cut right before the experiments.
Olfactometer
A dynamic Y-shaped olfactometer was used with the following specifications: glass tube: entrance arm length: 12.5 cm, test arm length: 8 cm, inner diameter: 1 cm, angle: 75°, mounted on a board in an angle of 40° from the horizontal plane. All experiments were conducted in a darkroom with a light source (LED-Lupenleuchte, Purelite, UK) mounted 45 cm (280 lx) above the middle of the olfactometer. The olfactometer experiments were conducted between 10:00 a.m. and 6:00 p.m. at room temperature (20–26 °C and 30–35% RH) on bright days, since psyllid motivation is low on cloudy and rainy days with low air pressure. For each experiment, one fresh twig of each host plant was carefully wrapped in oven plastic bags (TopHits, Melitta, Minden, Germany, 31 × 50 cm) and connected to the test arms. A charcoal-filtered and humidified airflow of 75 ml/min (with a max. difference of 1 ml/min) was pumped through the odor source into the test arms. The system was equilibrated for 30 min. To ensure an equal airstream, the flow of each arm was adjusted with plastic valves and controlled by a flowmeter (MASS-STREAM, M + W Instruments, Allershausen, Germany) at the outlet of the oven bags. Remigrants were kept on potted P. spinosa trees in a rearing chamber. The odors of P. insititia and P. persica (n = 20) or P. insititia and A. alba (n = 41) were offered simultaneously to C. pruni. The preferences of one-week-old female (n = 36) and male (n = 42) remigrants and of 6-week-old female (n = 49) emigrants for P. insititia or A. alba were tested. Single psyllids were collected in small plastic vials about 60 min before the olfactometer tests and were kept in the dark until experiments were conducted. Each psyllid was released at the base of the entrance arm of the olfactometer and was allowed to walk out from the plastic vial. To prevent side effects, the entire system was twisted after five replicates. The number of psyllids that entered one of the test arms (1 cm) and stayed there for at least 30 s was counted. Psyllids that did not reach one of the test arms within 10 min were recorded as “no choice.” Females and males were tested separately. After the bioassays all tubes, valves and glass olfactometers were cleaned with ethanol (70%) and heated at 230 °C (except plastic valves: 60 °C) for 2 h.
Statistics
All statistical analyses were done in R version 3.4.3 “Kite-Eating Tree” (R Core Team 2017). Graphics were produced using the ggplot2 package (Wickham 2009).
Volatile profiles
Random Forest (RF) approach was used to compare the volatile composition of Prunus spp. and A. alba trees at different developmental stages. Ntree = 10.000 bootstrap trees were drawn with myrt = 11 random variables at each node. Accuracy of the assignment of the groups (plant species + BBCH) was investigated by the confusion matrix of the RF model with the corresponding classification error. Additional RF models were calculated to identify specific compounds of each plant species at the several principal growth stages. Therefore, each group was tested against all other samples, which were defined as one group (“one-versus-the-rest approach”) (Ranganathan and Borges 2010). Each model was calculated with the same settings (Ntree = 10.000 and myrt = 11). The average mean decrease in accuracy (MDA) of each model was calculated with the “importance” function from the randomForest package (Liaw and Wiener 2002), to identify the most important compounds that distinguish the volatile profile of one group from all other samples (“the rest”).
EAG recordings
Wilcoxon matched pairs signed-rank test was used to calculate significant differences between EAG signals toward the tested volatiles and their respective DCM controls. For visualization of the signal strength toward the volatiles, the response elicited by each respective DCM control was subtracted.
Olfactometer assays
Preference for a host plant volatile profile was evaluated with the binominal test.
Results
Population monitoring
Population dynamics
The first C. pruni remigrants were captured at mid-March in the examined stone fruit orchard (Fig. 1). The growing season of the investigated Prunus species is just beginning, at this time of the year (BBCH 01-09). The population peak was reached 3 weeks after arrival (calendar weeks 14 to 15). Remigrants were detected for up to 8 weeks, until no further adults of the last year generation were observed (BBCH 19; 71-79). The first adult emigrants were captured three to 4 weeks after remigrants died. Newly hatched adults remained in the Prunus orchards for several days before they started migrating to overwintering hosts. Emigrants were captured on Prunus spp. over a period of five to 6 weeks. No further emigrants were caught after the end of July (Fig. 1). The mean sex ratio of males to females of C. pruni remigrants was 1:3.3 for all years. In contrast, the mean sex ratio males to females of emigrants was around 1:1.1 for each year.
Stacked area graph of captured individuals of C. pruni per branch in different Prunus cultivars. Cultivars are ordered by the quantity of caught remigrants. Population dynamics of C. pruni were monitored weekly from March to July in the years of 2010 to 2012 at the experimental field of the DLR (Dienstleistungszentrum Ländlicher Raum Rheinpfalz, Neustadt an der Weinstrasse, Germany). *not sampled
Cultivar preferences
The distribution of the psyllid captures on the different Prunus genotypes was constant during the three monitoring years. The lowest number of C. pruni individuals was observed in the apricot and peach cultivars (Fig. 2). Only 0.03 C. pruni remigrants per branch (corresponding to 17 psyllids in total) were caught in the cultivar Vineyard Peach (P. persica) and 0.04 remigrants per branch (corresponding to 24 psyllids) in P. armeniaca cv. Bergeron (apricot), over 3 years. Likewise, low numbers of remigrants were sampled in the rootstock varieties Citation (Prunus salicina × P. persica, 0.1 psyllids per branch) and St. Julien A. (P. insititia, 0.13 psyllids per branch) (Fig. 2). The highest captures of remigrants were recorded on P. spinosa (613 individuals, 0.7 psyllids per branch) and two rootstock varieties: GF 655-2 belonging to the species P. insititia (336 individuals on 570 branches, 0.59 psyllids per branch) and Ferlenain, a hybrid of P. besseyi × P. cerasifera (405 individuals on 550 branches, 0.74 psyllids per branch) (Fig. 2). Intermediate numbers of remigrants were observed on the P. domestica rootstocks Torinel, Wavit and Ferdor with, respectively, 0.35, 0.17 and 0.19 psyllids per branch (Fig. 2). The low abundance of remigrants in the cultivars Vineyard Peach and Bergeron was reflected by low numbers of individuals of the new generation, as only few emigrants were caught over the 3 years in these varieties (0.02 and 0.03 emigrants per branch, resp.). While there were fewer numbers of emigrants than remigrants captured in these two cultivars, the number of emigrants caught in all other varieties was higher. Most C. pruni emigrants were observed on P. spinosa, GF 655-2 and Ferlenain. Captures in P. spinosa, GF 655-2 and Ferlenain increased, respectively, to 1.01, 0.80 and 1.00 emigrants per branch. The greatest difference in the number of remigrants and emigrants was detected in Ferdor: 3.7 times more emigrants than remigrants per branch were caught on this rootstock variety.
Box–Whisker plots with numbers of collected C. pruni remigrants (A) and emigrants (B) per branch in different Prunus spp. and cultivars from 2010 to 2012. Each dot represents the annually collected number of C. pruni per branch. Medians are shown as lines, and whiskers extend to 1.5 times of the interquartile ranges
Volatile analysis
Treatment classification
The confusion matrix of the Random Forest (RF) model showed that the different host plant species were clearly separable by their volatile profiles. The RF model achieved 93.2% accuracy for the classification of volatile samples (Table 2). Misclassifications occurred between the phenological stages within the plant species and Prunus species at BBCH 0 (Table 2). The volatile composition emitted from A. alba trees was clearly separated from volatile profiles of Prunus trees (Fig. 3). In total, 10 out of 11 volatile samples from A. alba trees in spring and fall were correctly assigned (Table 2). In both cases, one of the samples was classified as A. alba at the other time point (Table 2). Clustering of the samples from Prunus trees at different growth stages illustrated the shift in the volatile composition from growth stage to growth stage (Fig. 3). All samples for P. persica were correctly assigned with a good accuracy to the respective growth stage. Whereas all volatile profiles from P. insititia at BBCH 1 were assigned correctly, two out of 21 samples of P. insititia BBCH 0 were misclassified: one sample is classified as P. insititia at BBCH 1 and one sample as P. persica at bud development (BBCH 0) (Table 2).
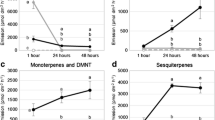
Phenological and interspecific variations in host volatiles
The calculation of the MDA values revealed that predominantly aldehydes and alkanes were responsible for the differentiation of P. insititia as well as for P. persica volatile profiles at the principal growth stage BBCH 0 from the other plants and growth stages (Table 3). Additionally, the ketone 1-phenylethane-1-one was the characteristic component of the volatile profiles from P. insititia during bud development (BBCH 0) (Fig. 4). The relative amounts of octanal, nonanal and decanal in Prunus samples were decreasing with increasing plant development (Fig. 4). Prunus insititia samples at growth stage 1 were mainly characterized by green leaf volatiles (Table 3), which occur only in Prunus samples collected during leaf development and later growth stages (Fig. 4). For differentiation of P. insititia at BBCH 1 from all other samples, also terpenes were important (Table 3); they were less abundant than in A. alba samples (Fig. 4). A. alba volatile profiles sampled in spring were mainly characterized by high abundance of terpenes and bornylacetate (acetate ester of the terpene borneol) and the low abundance of hexanal (Table 3 and Fig. 4). Abies alba profiles in fall were characterized by additional terpenes and low abundance of nonanal and decanal (Table 3 and Fig. 4). The relative amount of camphene, myrcene, alpha-pinene and limonene was higher in A. alba profiles in spring, whereas terpinolene was increasing in fall (Fig. 4). The mean relative amounts of all compounds detected in the headspace analysis are presented in Table S2 (Supplementary material 2).
Electroantennography
The most important compounds according to calculation with the “importance” function from the randomForest package (Liaw and Wiener 2002) were tested for antennal responses in electrophysiological experiments. A puff of 1000 µg hexanal was identified to elicit a reliable and reproducible response of C. pruni antenna significantly greater than DCM puffs (Fig. 5, Wilcoxon matched pairs signed-rank test, V = 780, p < 0.001) and was used as positive control for EAG recordings. For C. pruni female emigrants, terpenes typically emitted by A. alba, (+)-limonene, α-pinene, terpinolene, myrcene and camphene elicited significant antennal responses compared to negative DCM controls (Fig. 5, Wilcoxon matched pairs signed-rank test, (+)-limonene: V = 55, p < 0.01; α-pinene: V = 55, p < 0.01, V = 55; terpinolene: p < 0.01, V = 66; myrcene: p < 0.001; camphene: V = 53, p < 0.01). The aldehydes octanal, nonanal and decanal also elicited a significant response compared to negative controls (Fig. 5, Wilcoxon matched pairs signed-rank test, octanal: V = 55, p < 0.01; nonanal: V = 55, p < 0.01; decanal: V = 66, p < 0.001), whereas dodecanal was not detected by C. pruni antenna (Fig. 5, Wilcoxon matched pairs signed-rank test, V = 34, p > 0.05). While the green leaf volatile (Z)-3-hexenyl acetate and the ketone 1-phenylethane-1-one elicited significant female antennal responses (Fig. 5, Wilcoxon matched pairs signed-rank test, (Z)-3-hexenyl acetate: V = 49, p < 0.05; 1-phenylethane-1-one: V = 52, p < 0.01, resp.), no antennal response was detectable from (Z)-3-hexene-1-ol, tridecane and bornylacetate puffs (Fig. 5, Wilcoxon matched pairs signed-rank test, (Z)-3-hexene-1-ol: V = 28, p > 0,05; tridecane: V = 42, p > 0,05; bornylacetate: V = 12, p > 0,05).
Mean ± standard deviation of summated receptor potentials (n = 10) elicited by odor puffs of 100 µg (except the positive control hexanal: 1.000 µg) of synthetic volatiles on the antenna of female C. pruni emigrants. Responses to respective negative controls (DCM) were subtracted. Responses that differ significantly from negative controls are marked with asterisks (Wilcoxon matched pairs signed-rank test, p < 0.05)
Olfactometer assay
Female C. pruni remigrants did not prefer odor blends from P. insititia over blends from P. persica or A. alba (Fig. 6, binominal test, p > 0.05). Also, young (~ 7 days old) female and male emigrants did not show a preference for P. insititia rather than A. alba when offered simultaneously in olfactometer assays (Fig. 6, binominal test, p > 0.05). Older female emigrants (~ 6 weeks) still did not show any preference nor avoidance to the volatile profile of one of these host plants (Fig. 6, binominal test, p > 0.05). Motivation was higher in remigrants (90% and 83%) than in emigrants (females: 81%, males: 71%) and further decreased with increasing age of the emigrants (females: 63%) (Fig. 6).
Choice of C. pruni (remigrants and emigrants) for odor profiles of different host plants in a Y-tube olfactometer. Amount of psyllids that made a choice (dark gray) and that did not (light gray) is presented as pie charts on the right (n.s. = not significant, binominal test). N gives the number of tested psyllids per treatment
Discussion
Cacopsylla pruni is an univoltine psyllid species which migrates over long distances in early spring from its overwintering places to its reproduction hosts. The first choice of migrating insects is mediated mainly by olfactory and visual cues over distance (Deletre et al. 2016). Therefore, we hypothesized that apart from wind drifts and visual cues in particular olfactory cues might be responsible for host plant finding of C. pruni. The monitoring data demonstrated that C. pruni individuals preferred some Prunus spp. to others in their natural environment. These host plant preferences remained stable over the vegetation period as well as over the three consecutive years of monitoring. In our study, the highest population densities were found on P. spinosa and P. cerasifera, which are autochthonous European Prunus species. These findings concur with other studies by Labonne and Lichou (2004) and Mergenthaler et al. (2017). As C. pruni is a European and Central Asian species (Lauterer 1999), these plants are considered as natural reproduction host for C. pruni. Accordingly, C. pruni was caught on every P. spinosa bush sampled in wild habitats in Germany (Jarausch et al. 2019b). P. domestica genotypes and genotypes of P. insititia exhibit a varying attractiveness to C. pruni ranging from the highly populated rootstock variety GF 655-2 to low population densities on St. Julien A. The highest economic damage of ESFY is caused on apricot and peach. However, these Prunus species of Asian origin are the least attractive to C. pruni. This contradiction might be explained by a hypersensitive reaction to ‘Ca. P. prunorum’, which is considered as being autochthonous to Europe (Jarausch et al. 2019b).
The results of the presented olfactometer trials could not confirm our hypothesis and rather reveal that these host plant preferences were not or not only due to olfactory signals. In accordance with this, we found that Prunus volatiles, which can be perceived by the psyllids antenna, were more specific for phenological growth stages rather than for Prunus species. Feeding and oviposition preferences of insects for different species, varieties or cultivars in the field may be often influenced by other plant cues like nonvolatile chemicals, leaf texture, trichomes, color and more (Markheiser et al. 2018; Rid et al. 2018; Visser 1988). Indeed, the use of olfactory cues for host and mate location was shown for several psyllid species (Alquézar et al. 2017; Gross and Mekonen 2005; Horton and Landolt 2007; Martini et al. 2014; Mas et al. 2014; Mayer et al. 2008a, b, 2011; Mayer and Gross 2007; Soroker et al. 2004), but further studies also highlight the impact of visual cues (Farnier et al. 2014, 2015) and the interplay of olfactory and visual cues (Wenninger et al. 2009) on psyllid behavior. Moreover, the results of the field monitoring may also reflect contact cues or probing behavior after landing on the plant. This had been shown for the migrating black bean aphid Aphis fabae as settling behavior, whereby oviposition was promoted after landing on potential host plants by gustatory cues perceived during stylet penetration (Powell and Hardie 2001). Patt and Sétamou (2010) found no attraction of Asian citrus psyllid (ACP) Diaphorina citri to the grapefruit cv. Rio Red (Citrus paradisi) in olfactometer trials; nonetheless, D. citri is abundant in cv. Rio Red orchards. They, therefore, concluded that also textual cues could play a role in host acceptance of ACP. Additional cues may also influence the host preferences of C. pruni. Further studies should investigate whether the host odors and single detectable VOCs in general exhibit a chemotactic response in olfactometer trails. Recent studies revealed that olfaction in some psyllid species plays a greater role in short-range host recognition and feeding in post-landing behavior (Farnier et al. 2018, Lapointe et al. 2016).
Nevertheless, we identified eleven volatiles from different host plants perceptible by the olfactory system of C. pruni. As not only the most abundant volatiles were responsible for attraction to hosts but rather minor compounds and odor blends (Bruce and Pickett 2011; Clavijo McCormick et al. 2014), we used RF approach to identify characteristic volatiles from the different host plants of C. pruni. Interestingly, we found components emitted from conifers (e.g., myrcene and terpinolene) and deciduous trees (aldehydes, 1-phenylethane-1-one and (Z)-3-hexenyl acetate) to be electrophysiologically active. Single sensillum recordings identified that among others terpinolene and nonanal were also perceptible by T. apicalis (Kristoffersen et al. 2008) and responses to terpenoids and aldehydes were also recorded from ACP in SSR studies (Coutinho-Abreu et al. 2014). The perception of specific host plant VOCs could enable C. pruni to distinguish the chemical profiles of overwintering and reproduction hosts and locate desired plants. Because we were not able to find a repellent effect of the reproduction host on C. pruni emigrants at different ages (Fig. 6), the perceptible volatiles were possibly always attractants. Cacopsylla pruni females showed antennal reaction to the green leaf volatile cis-3-hexenyl acetate, which is commonly found within different plants. This component is characteristic for the preferred rootstock (GF 655-2) at BBCH 1 and might be signaling leaf development to females which need foliage for oviposition. Yuvaraj et al. (2013) revealed the electrophysiological activity of neurons of the blue gum psyllid Ctenarytaina eucalypti by GLVs, terpenes and alcohols. Furthermore, the perception of common GLVs was shown for several aphid species (Han et al. 2012; Hardie et al. 1994; Visser et al. 1996). Additionally, some of the identified compounds could also act as phagostimulants as shown for a blend of three EAG active volatiles for D. citri (George et al. 2016; Lapointe et al. 2016). Therefore, the impact of the identified components on psyllid behavior should be examined in further bioassays, investigating their olfactory and gustatory effects.
We expected a change of preference for volatile blends of reproduction and overwintering host plants due to the developmental stage of C. pruni, as reported for related European psyllid species C. melanoneura (Mayer and Gross 2007) and T. apicalis (Nehlin et al. 1994). Contrastingly, there was no repellent effect of volatiles emitted from A. alba on C. pruni remigrants. If remigration is not initiated by repellence, it could be triggered by temperature difference and day length (Wolda 1988) or other meteorological factors (Drake 1988). The fact that female remigrants did not prefer the odor of their reproduction host over the odor of conifers also supports the idea that additional factors, such as gustatory cues, affect the location of suitable oviposition sites. This is in accordance with the findings of Gallinger and Gross (2018) that C. pruni nymphs cannot develop on coniferous trees due to their nutritional composition. Emigrants leave Prunus spp. trees early after development, at a time when deciduous plants are still providing food and no detectable changes in abiotic factors like day length and temperature are obvious. Therefore, we hypothesized that the migration to overwintering hosts might by induced by repellent volatile cues released from reproduction hosts. We could neither show a repellent effect of Prunus spp. volatile profiles on emigrants nor preference for overwintering hosts with olfactometer experiments. This indicates that emigration behavior might be triggered by other parameters, such as gustatory resp. nutritional factors due to changes in phloem composition related to leaf ontogeny. Sandström (2000) detected changes in the amino acid composition in the phloem of Prunus padus during growing season from May to September. Such a change could trigger the migration of phloem feeding insects. Our results rather support the hypothesis that genetically determined factors of the psyllids regulate their migration behavior, such as metabolic changes in their energy budget, or concentration of juvenile or adipokinetic hormones. The latter are known to be involved in energy mobilization for migration flight of monarch butterfly (Dingle 2009; Rankin 1992), and juvenile hormone titers are influencing the migration behavior of lepidopterans and other insect species like the large milkweed bug (Rankin 1992; Reppert et al. 2016). In the black cutworm Agrotis ipsilon, juvenile hormone influences the responsiveness toward sex pheromones (Anton and Gadenne 1999; Gadenne et al. 1993), indicating that the hormonal balance can influence the olfactory sensitivity and preferences of insects. Thus, the parameters regulating the psyllid preference for overwintering or reproduction host plants still remain to be elucidated and their analysis could give new insights into how migration behavior in C. pruni is controlled.
ESFY is one of the economically most severe diseases in European fruit production. Application of chemical pesticides against its vector C. pruni is, in the context of global insect decline, a controversial issue, and in some countries like Germany insecticides for plum psyllid control are no longer approved (Bundesamt für Verbraucherschutz und Lebensmittelsicherheit 2018). Paleskić et al. (2017) demonstrated a high efficiency of thiacloprid, a neonicotinoid that negatively affects honey bees and bumblebees (Brandt et al. 2016; Ellis et al. 2017) and cypermethrin, a non-selective pyrethroid affecting natural enemies, too (Loch 2005; Michaud and Grant 2003; Tooming et al. 2014). In this regard, and on the basis of the presented monitoring results, we recommend to remove plant rootstock suckers of attractive Prunus species like P. insititia (GF 655-2) in apricot and peach orchards. The high populations of C. pruni on these suckers dramatically increase the chance of “Ca. P. prunorum” infection of the highly susceptible P. armeniaca and P. persica cultivars. Beyond that, the presented results on the perception of plant volatiles by C. pruni could be used to develop an environmentally friendly and selective control strategy based on the use of semiochemicals. Introducing pest control with semiochemicals in integrated pest management strategies will be the future of sustainable and eco-friendly agriculture (Gross and Gündermann 2016). The application of repellents, for example, could prevent the invasion and distribution of migrating psyllids, whereas attractive volatiles could be used to lure and trap the insects. Both components can be applied artificially in the field and combined to a push–pull system with synthetic kairomones instead of intercropping (Cook et al. 2007; Xu et al. 2018). The behavioral response of C. pruni toward two blends of repellent compounds was proved in olfactometer bioassays recently (Gallinger et al. 2019, in press). In this study, both genders of C. pruni remigrants preferred the pure odor of a Prunus tree over the odor of a Prunus tree equipped with different repellent mixtures incorporated in polypropylene plastic card dispensers. Detectable volatiles and mixtures of further components shall be investigated in future studies, to identify both repellent and attractive volatiles. The identification of electrophysiologically active compounds was the initial step for the development of an alternative control strategy for C. pruni with plant volatiles. We had to refuse our hypothesis that the preference of C. pruni for reproduction and overwintering hosts changes during their life span. This offers the possibility to identify behavior manipulating volatile blends that are effective for emigrants as well as remigrants. Such an innovative control method against C. pruni remigrants and emigrants could help to reduce the number of new ESFY infections.
Authors contribution
JGa and JGr designed the study. JGa, JGr, BJ and WJ contributed to the interpretation of the data, approved the final version of the manuscript and ensured the accuracy and integrity of the work. BJ and WJ conducted the field monitoring. JGa conducted the EAG and olfactometer experiments, headspace analysis and wrote the first draft of the manuscript. The manuscript was revisited and edited by BJ, WJ and JGr. JGr supervised the project.
References
Alquézar B, Volpe HXL, Magnani RF, de Miranda MP, Santos MA, Wulff NA, Bento JMS, Parra JRP, Bouwmeester H, Peña L (2017) β-caryophyllene emitted from a transgenic Arabidopsis or chemical dispenser repels Diaphorina citri, vector of Candidatus Liberibacters. Sci Rep 7:5639. https://doi.org/10.1038/s41598-017-06119-w
Anton S, Gadenne C (1999) Effect of juvenile hormone on the central nervous processing of sex pheromone in an insect. PNAS 96:5764–5767. https://doi.org/10.1073/pnas.96.10.5764
Brandt A, Gorenflo A, Siede R, Meixner M, Büchler R (2016) The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J Insect Physiol 86:40–47. https://doi.org/10.1016/j.jinsphys.2016.01.001
Bruce TJA, Pickett JA (2011) Perception of plant volatile blends by herbivorous insects-finding the right mix. Phytochemistry 72:1605–1611. https://doi.org/10.1016/j.phytochem.2011.04.011
Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (2018) Pflanzenschutzmittel-Verzeichnis 2018—Teil 2: Gemüsebau—Obstbau—Zierpflanzenbau, Braunschweig. www.bvl.bund.de/infopsm. Accessed 18 Feb 2019
Carraro L, Ferrini F, Ermacora P, Loi N (2002) Role of wild Prunus species in the epidemiology of European stone fruit yellows. Plant Pathol 51:513–517. https://doi.org/10.1046/j.1365-3059.2002.00732.x
Carraro L, Ferrini F, Ermacora P, Loi N (2004) Transmission of European stone fruit yellows phytoplasma to prunus species by using vector and graft transmission. Acta Hortic 657:449–453. https://doi.org/10.17660/ActaHortic.2004.657.72
Clavijo McCormick A, Gershenzon J, Unsicker SB (2014) Little peaks with big effects: establishing the role of minor plant volatiles in plant-insect interactions. Plant, Cell Environ 37:1836–1844. https://doi.org/10.1111/pce.12357
Cook SM, Khan ZR, Pickett JA (2007) The use of push-pull strategies in integrated pest management. Annu Rev Entomol 52:375–400. https://doi.org/10.1146/annurev.ento.52.110405.091407
Coutinho-Abreu IV, McInally S, Forster L, Luck R, Ray A (2014) Odor coding in a disease-transmitting herbivorous insect, the asian citrus psyllid. Chem Senses 39:539–549. https://doi.org/10.1093/chemse/bju023
Deletre E, Schatz B, Bourguet D, Chandre F, Williams L, Ratnadass A, Martin T (2016) Prospects for repellent in pest control: current developments and future challenges. Chemoecology 26:127–142. https://doi.org/10.1007/s00049-016-0214-0
Dingle H (2009) Migration. In: Resh VH, Cardé RT (eds) Encyclopedia of insects. Elsevier/Academic Press, Amsterdamm, pp 628–633. https://doi.org/10.1016/b978-0-12-374144-8.00176-4
Drake V (1988) The influence of atmospheric structure and motions on insect migration. Annu Rev Entomol 33:183–210. https://doi.org/10.1146/annurev.ento.33.1.183
Ellis C, Park KJ, Whitehorn P, David A, Goulson D (2017) The neonicotinoid insecticide thiacloprid impacts upon bumblebee colony development under field conditions. Environ Sci Technol 51:1727–1732. https://doi.org/10.1021/acs.est.6b04791
Farnier K, Dyer AG, Steinbauer MJ (2014) Related but not alike: not all Hemiptera are attracted to yellow. Front Ecol Evol 2:263. https://doi.org/10.3389/fevo.2014.00067
Farnier K, Dyer AG, Taylor GS, Peters RA, Steinbauer MJ (2015) Visual acuity trade-offs and microhabitat-driven adaptation of searching behaviour in psyllids (Hemiptera: Psylloidea: Aphalaridae). J Exp Biol 218:2660. https://doi.org/10.1242/jeb.128967
Farnier K, Davies NW, Steinbauer MJ (2018) Not led by the nose: volatiles from undamaged eucalyptus hosts do not influence psyllid orientation. Insects 9:166–179. https://doi.org/10.3390/insects9040166
Gadenne C, Renou M, Sreng L (1993) Hormonal control of pheromone responsiveness in the male black cutworm Agrotis ipsilon. Experientia 49:721–724. https://doi.org/10.1007/BF01923960
Gadenne C, Barrozo RB, Anton S (2016) Plasticity in Insect olfaction: to smell or not to smell? Annu Rev Entomol 61:317–333. https://doi.org/10.1146/annurev-ento-010715-023523
Gallinger J, Gross J (2018) Unraveling the host plant alternation of Cacopsylla pruni—adults but not nymphs can survive on conifers due to phloem/xylem composition. Front Plant Sci 9:484. https://doi.org/10.3389/fpls.2018.00484
Gallinger J, Dippel C, Gross J (2019) Interfering host location of Cacopsylla pruni with repellent plant volatiles. IOBC-WPRS Bulletin (in press)
George J, Robbins PS, Alessandro RT, Stelinski LL, Lapointe SL (2016) Formic and acetic acids in degradation products of plant volatiles elicit olfactory and behavioral responses from an insect vector. Chem Senses 41:325–338. https://doi.org/10.1093/chemse/bjw005
Gross J (2016) Chemical communication between phytopathogens, their host plants and vector insects and eavesdropping by natural enemies. Front Ecol Evol 4:104. https://doi.org/10.3389/fevo.2016.00104
Gross J, Gündermann G (2016) Principles of IPM in cultivated crops and implementation of innovative strategies for sustainable plant protection. In: Horowitz AR (ed) Advances in insect control and resistance management. Springer, Cham, pp 9–26
Gross J, Mekonen N (2005) Plant odours influence the host finding behaviour of apple psyllids (Cacopsylla picta; C. melanoneura). IOBC/WPRS Bull 28:351–355
Gross J, Gallinger J, Rid M (2019) Collection, identification, and statistical analysis of volatile organic compound patterns emitted by phytoplasma infected plants. In: Musetti R, Pagliari L (eds) Phytoplasmas. Springer, New York, pp 333–343
Han B, Zhang Q-H, Byers JA (2012) Attraction of the tea aphid, Toxoptera aurantii, to combinations of volatiles and colors related to tea plants. Entomol Exp Appl 144:258–269. https://doi.org/10.1111/j.1570-7458.2012.01303.x
Hardie J, Visser JH, Piron PGM (1994) Perception of volatiles associated with sex and food by different adult forms of the black bean aphid, Aphis fabae. Physiol Entomol 19:278–284
Hodkinson ID (2009) Life cycle variation and adaptation in jumping plant lice (Insecta: Hemiptera: Psylloidea): a global synthesis. J Nat Hist 43:65–179. https://doi.org/10.1080/00222930802354167
Homberg U (2015) Sky compass orientation in desert locusts-evidence from field and laboratory studies. Front Behav Neurosci 9:346. https://doi.org/10.3389/fnbeh.2015.00346
Horton DR, Landolt PJ (2007) Attraction of male pear psylla, Cacopsylla pyricola, to female-infested pear shoots. Entomol Exp Appl 123:177–183. https://doi.org/10.1111/j.1570-7458.2007.00537.x
Jarausch W, Jarausch B (2016) A permanent rearing system for Cacopsylla pruni, the vector of ‘Candidatus Phytoplasma prunorum’. Entomol Exp Appl 159:112–116. https://doi.org/10.1111/eea.12427
Jarausch W, Lansac M, Saillard C, Broquaire JM, Dosba F (1998) PCR Assay for specific detection of European stone fruit yellows phytoplasmas and its use for epidemiological studies in France. Eur J Plant Pathol 104:17–27
Jarausch W, Saillard C, Broquaire JM, Garnier M, Dosba F (2000) PCR-RFLP and sequence analysis of a non-ribosomal fragment for genetic characterization of European stone fruit yellows phytoplasmas infecting various Prunus species. Mol Cell Probes 14:171–179. https://doi.org/10.1006/mcpr.2000.0304
Jarausch W, Jarausch B, Fritz M, Runne M, Etropolska A, Pfeilstetter E (2019a) Epidemiology of European stone fruit yellows in Germany: the role of wild Prunus spinosa. Eur J Plant Pathol 50:185. https://doi.org/10.1007/s10658-019-01669-3
Jarausch B, Tedeschi R, Sauvion N, Gross J, Jarausch W (2019b) Psyllid vectors. In: Bertaccini A, Weintraub P, Rao GP, Mori N (eds) Phytoplasmas: Plant Pathogenic Bacteria - II. Springer, Singapore, pp 53–78. https://doi.org/10.1007/978-981-13-2832-9_3
Kison H, Seemüller E (2001) Differences in Strain virulence of the European stone fruit yellows phytoplasma and susceptibility of stone fruit trees on various rootstocks to this pathogen. J Phytopathol 149:533–541. https://doi.org/10.1046/j.1439-0434.2001.00671.x
Kristoffersen L, Larsson MC, Anderbrant O (2008) Functional characteristics of a tiny but specialized olfactory system: olfactory receptor neurons of carrot psyllids (Homoptera: Triozidae). Chem Senses 33:759–769. https://doi.org/10.1093/chemse/bjn034
Labonne G, Lichou J (2004) Data on the life cycle of cacopsylla pruni, psyllidae vector of European stone fruit yellows (ESFY) phytoplasma, in France. Acta Hortic 657:465–470
Lapointe SL, Hall DG, George J (2016) A phagostimulant blend for the Asian citrus psyllid. J Chem Ecol 42:941–951. https://doi.org/10.1007/s10886-016-0745-4
Lauterer P (1999) Results of the investigations on Hemiptera in Moravia, made by the Moravian museum (Psylloidea 2). Acta Musei Moraviae Sci Biol (Brno) 84:71–151
Liaw A, Wiener M (2002) Classification and regression by randomforest. R News 2:18–22
Loch AD (2005) Mortality and recovery of eucalypt beetle pest and beneficial arthropod populations after commercial application of the insecticide alpha-cypermethrin. For Ecol Manage 217:255–265. https://doi.org/10.1016/j.foreco.2005.06.006
Linstrom P, Mallard, WG (1997) NIST Chemistry WebBook, NIST standard reference database 69. Accessed 16 Feb 2019
Marcone C, Jarausch B, Jarausch W (2010) Candidatus Phytoplasma prunorum, the causal agent of European stone fruit yellows: an overview. J Plant Pathol 92:19–34
Marcone C, Jarausch B, Jarausch W, Dosba F (2011) CHAPTER 43: European stone fruit yellows phytoplasma. In: Hadidi A, Barba M, Candresse T, Jelkmann W (eds) Virus and virus-like diseases of pome and stone fruits. APS Press/American Phytopathological Society, St. Paul, pp 233–241
Markheiser A, Rid M, Biancu S, Gross J, Hoffmann C (2018) Physical factors influencing the oviposition behaviour of European grapevine moths Lobesia botrana and Eupoecilia ambiguella. J Appl Entomol 142:201–210. https://doi.org/10.1111/jen.12423
Martini X, Kuhns EH, Hoyte A, Stelinski LL (2014) Plant volatiles and density-dependent conspecific female odors are used by Asian citrus psyllid to evaluate host suitability on a spatial scale. Arthropod Plant Interact 8:453–460. https://doi.org/10.1007/s11829-014-9326-z
Mas F, Vereijssen J, Suckling DM (2014) Influence of the pathogen Candidatus Liberibacter solanacearum on tomato host plant volatiles and psyllid vector settlement. J Chem Ecol 40:1197–1202. https://doi.org/10.1007/s10886-014-0518-x
Mayer CJ, Gross J (2007) Different host plant odours influence migration behaviour of Cacopsylla melanoneura (Förster), an insect vector of the apple proliferation phytoplasma. IOBC/WPRS Bull 30:177–184
Mayer CJ, Vilcinskas A, Gross J (2008a) Pathogen-induced release of plant allomone manipulates vector insect behavior. J Chem Ecol 34:1518–1522. https://doi.org/10.1007/s10886-008-9564-6
Mayer CJ, Vilcinskas A, Gross J (2008b) Phytopathogen lures its insect vector by altering host plant odor. J Chem Ecol 34:1045–1049. https://doi.org/10.1007/s10886-008-9516-1
Mayer CJ, Vilcinskas A, Gross J (2011) Chemically mediated multitrophic interactions in a plant-insect vector-phytoplasma system compared with a partially nonvector species. Agric For Entomol 13:25–35. https://doi.org/10.1111/j.1461-9563.2010.00495.x
Meier U (2018) Growth stages of mono- and dicotyledonous plants: BBCH Monograph. https://doi.org/10.5073/20180906-074619
Mergenthaler E, Kiss B, Kiss E, Viczián O (2017) Survey on the occurrence and infection status of Cacopsylla pruni, vector of European stone fruit yellows in Hungary. Bull Insectol 70:171–176
Michaud JP, Grant AK (2003) IPM-compatibility of foliar insecticides for citrus: Indices derived from toxicity to beneficial insects from four orders. J Insect Sci 3:265. https://doi.org/10.1093/jis/3.1.18
Nehlin G, Valterová I, Borg-Karlson AK (1994) Use of conifer volatiles to reduce injury caused by carrot psyllid, Trioza apicalis, Förster (Homoptera, Psylloidea). J Chem Ecol 20:771–783. https://doi.org/10.1007/BF02059612
Ossiannilsson F (ed) (1992) The Psylloidea (Homoptera) of Fennoscandia and Denmark. Fauna entomologica Scandinavica, vol 26. Brill, Leiden, New York, Köln
Paleskić C, Bachinger K, Brader G, Kickenweiz M, Engel C, Wurm L, Czipin L, Riedle-Bauer M (2017) Cage and field experiments as basis for the development of control strategies against Cacopsylla pruni, the vector of European stone fruit yellows. Ann Appl Biol 170:357–368. https://doi.org/10.1111/aab.12340
Patt JM, Sétamou M (2010) Responses of the Asian citrus psyllid to volatiles emitted by the flushing shoots of its rutaceous host plants. Environ Entomol 39:618–624. https://doi.org/10.1603/EN09216
Pettersson J, Pickett JA, Pye BJ, Quiroz A, Smart LE, Wadhams LJ, Woodcock CM (1994) Winter host component reduces colonization by bird-cherry-oat aphid, Rhopalosiphum padi (L.) (homoptera, aphididae), and other aphids in cereal fields. J Chem Ecol 20:2565–2574. https://doi.org/10.1007/BF02036192
Powell G, Hardie J (2001) The chemical ecology of aphid host alternation: How do return migrants find the primary host plant? Appl Entomol Zool 36:259–267
Ranganathan Y, Borges RM (2010) Reducing the babel in plant volatile communication: using the forest to see the trees. Plant Biol 12:735–742. https://doi.org/10.1111/j.1438-8677.2009.00278.x
Rankin M (1992) The cost of migration in insects. Annu Rev Entomol 37:533–559. https://doi.org/10.1146/annurev.ento.37.1.533
R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 21 Oct 2018
Reppert SM, Guerra PA, Merlin C (2016) Neurobiology of monarch butterfly migration. Annu Rev Entomol 61:25–42. https://doi.org/10.1146/annurev-ento-010814-020855
Richter S (2002) Susceptibility of Austrian apricot and peach cultivars to ESFY. Plant Protect Sci 38:281–284
Rid M, Mesca C, Ayasse M, Gross J (2016) Apple proliferation phytoplasma influences the pattern of plant volatiles emitted depending on pathogen virulence. Front Ecol Evol 3:271. https://doi.org/10.3389/fevo.2015.00152
Rid M, Markheiser A, Hoffmann C, Gross J (2018) Waxy bloom on grape berry surface is one important factor for oviposition of European grapevine moths. J Pest Sci 91:1225–1239. https://doi.org/10.1007/s10340-018-0988-7
Rosenberg J, Burt PJA (1999) Windborne displacements of desert locusts from Africa to the Caribbean and South America. Aerobiologia 15:167–175
Sandström J (2000) Nutritional quality of phloem sap in relation to host plant-alternation in the bird cherry-oat aphid. Chemoecology 10:17–24. https://doi.org/10.1007/s000490050003
Soroker V, Talebaev S, Harari A, Wesley SD (2004) The role of chemical cues in host and mate location in the pear psylla Cacopsylla bidens (Homoptera: Psyllidae). J Insect Behav 17:613–626. https://doi.org/10.1023/B:JOIR.0000042544.35561.1c
Thébaud G, Yvon M, Alary R, Sauvion N, Labonne G (2009) Efficient transmission of ‘Candidatus phytoplasma prunorum’ Is delayed by eight months due to a long latency in its host-alternating vector. Phytopathology 99:265–273. https://doi.org/10.1094/PHYTO-99-3-0265
Tooming E, Merivee E, Must A, Sibul I, Williams I (2014) Sub-lethal effects of the neurotoxic pyrethroid insecticide Fastac 50EC on the general motor and locomotor activities of the non-targeted beneficial carabid beetle Platynus assimilis (Coleoptera: Carabidae). Pest Manag Sci 70:959–966. https://doi.org/10.1002/ps.3636
Visser JH (1988) Host-plant finding by insects: orientation, sensory input and search patterns. J Insect Physiol 34:259–268
Visser JH, Piron PGM, Hardie J (1996) The aphids’ peripheral perception of plant volatiles. Entomol Exp Appl 80:35–38. https://doi.org/10.1111/j.1570-7458.1996.tb00880.x
Weintraub P, Gross J (2013) Capturing insect vectors of phytoplasmas. In: Dickinson M, Hodgetts J (eds) Phytoplasma, vol 938. Humana Press, Totowa, pp 61–72
Wenninger EJ, Stelinski LL, Hall DG (2009) Roles of olfactory cues, visual cues, and mating status in orientation of Diaphorina citri Kuwayama (Hemiptera: Psyllidae) to four different host plants. Environ Entomol 36:225–234. https://doi.org/10.1603/022.038.0128
Wickham H (2009) ggplot2: elegant graphics for data analysis. Use R. Springer, New York
Wolda H (1988) Insect seasonality: Why? Ann Rev Ecol Syst 19:1–18
Xu Q, Hatt S, Lopes T, Zhang Y, Bodson B, Chen J, Francis F (2018) A push–pull strategy to control aphids combines intercropping with semiochemical releases. J Pest Sci 91:93–103. https://doi.org/10.1007/s10340-017-0888-2
Yuvaraj JK, Andersson MN, Steinbauer MJ, Farnier K, Anderbrant O (2013) Specificity and sensitivity of plant odor-detecting olfactory sensory neurons in Ctenarytaina eucalypti (Sternorrhyncha: Psyllidae). J Insect Physiol 59:542–551. https://doi.org/10.1016/j.jinsphys.2013.03.004
Acknowledgements
We thank Svenja Stein, Sabine Wetzel, Sebastian Faus and Kai Lukat (Dossenheim, Germany) for experimental assistance. We are particularly grateful to Uwe Harzer (DLR Rheinpfalz, Neustadt, Germany) for the permission to conduct experiments and sampling in institute’s orchards. We thank Eva Gross (Schriesheim, Germany) for language editing. We are grateful to Stephen Lapointe, Justin George and Paul S. Robbins (USDA, Fort Pierce, USA) for helpful advices for conducting EAG with psyllids.
Funding
JGa was supported by a fund of the “Landwirtschaftliche Rentenbank” number 28RF4IP008. WJ was supported by the ZIM project KF2248403 MD9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by M. Traugott.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gallinger, J., Jarausch, B., Jarausch, W. et al. Host plant preferences and detection of host plant volatiles of the migrating psyllid species Cacopsylla pruni, the vector of European Stone Fruit Yellows. J Pest Sci 93, 461–475 (2020). https://doi.org/10.1007/s10340-019-01135-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-019-01135-3