Abstract
Assessing the impact of temperature changes on insect population and overwintering on nontarget hosts is important for prediction of nontarget effects in weed biological control. Agasicles hygrophila is a beetle used for biological control of the invasive plant alligator weed, Alternanthera philoxeroides, with nontarget damage to a native plant, Alternanthera sessilis. In this study, we monitored plant growth and phenology along with beetle population and overwintering on these two hosts along a latitudinal gradient from subtropical to temperate climates (Guilin, Wuhan and Kaifeng) in China. We found only annual A. sessilis seedlings in temperate Kaifeng, but both annual seedlings and perennial ramets of A. sessilis were found in Guilin and Wuhan. However, in winter, living A. sessilis plants were found in only subtropical Guilin. Beetles at the Guilin site could successfully maintain their populations and overwinter on A. sessilis. Although the beetle could sustain its populations on A. sessilis at the other two higher-latitude sites during the growing season, it failed to overwinter on either species, indicating that the temperatures in different climate zones may directly and/or indirectly affect the development of insect biological control agents and their population sizes on nontarget hosts. Therefore, it is important to consider the shifting plant–insect interactions induced by climate when assessing potential nontarget effects of species introduced as biological control agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
Assessing the impact of climate on insect on host plants is important for accurate prediction of nontarget effects in weed biological control.
We hypothesized that the population size and overwintering ability of insects used for weed biocontrol vary with latitudes.
Insects successfully maintained populations and overwintered on their target weed and nontarget host at low-latitude sites but failed to overwinter at higher-latitude sites.
Consideration of the shifting interactions among weeds, biocontrol insects and native plants induced by climate is critical for biological control programs.
Introduction
Classical biological control of exotic weeds, based on the introduction of specialist natural enemies from the weed’s native range, is often a long-term solution for regulating exotic weed populations in their introduced ranges (Müller-Schärer and Schaffner 2008). However, insect host specificity and potential nontarget effects on native plants remain important concerns (McEvoy 1996; Myers and Cory 2017; Raghu et al. 2007). Many factors, especially climate and host plant availability, may affect insect populations and their damage to host plants and even result in host shifts from target plants to surrounding plant species (Ismail et al. 2017; Jang et al. 2015; Posledovich et al. 2015; Rasmann et al. 2014). Studies of the effects of spatial and temporal variations in climate on the performance of biological control agents may help to predict their nontarget effects after release (Lu et al. 2015).
Temperature plays an important role in the biology of insects; thus, the variations of temperature in different climatic zones may directly affect insect survival, population and distribution (Ismail and Brooks 2016; Lencioni 2004; Ma et al. 2006; Robinet and Roques 2010; Stoeckli et al. 2012). Temperature may also indirectly determine insect performance by affecting host plant development, availability and quality (Jamieson et al. 2015; Rasmann et al. 2014; Uelmen Jr et al. 2016; Yang and Rudolf 2010). As a consequence, insects tend to adapt to unfavorable conditions by overwintering, i.e., they pass the winter season by almost completely ceasing activity until climate or food conditions become more favorable. Overwintering ability is a key criterion in determining insect population dynamics, especially at high latitudes, where average temperatures in winter are usually low (Bale and Hayward 2010). Overwintering is particularly important when assessing whether insect biological control agents can sustain populations on hosts across years. For weed biological control programs, if the target weeds, insect biological control agents and nontarget hosts co-occur across different climate zones, then insect populations, overwintering abilities and nontarget effects may vary with average temperature where climates differ (Lu et al. 2015, 2016). Thus, monitoring insect biological control agent populations and overwintering on nontarget hosts at different latitudes is important for assessing nontarget effects in different climate zones.
Native to South America, Agasicles hygrophila Selman and Vogt (Coleoptera: Chrysomelidae), is a specialized herbivorous beetle that has been introduced for biological control of alligator weed, Alternanthera philoxeroides (Mart.) Griseb. (Amaranthaceae), in many countries, such as the USA, Australia and parts of Asia (Coulson 1977; Julien et al. 1979; Ma 2001). Alternanthera philoxeroides can grow in both aquatic and terrestrial habitats. Agasicles hygrophila has been found to prefer the aquatic form of the plant to the terrestrial ones, thus providing effective control of aquatic A. philoxeroides at lower latitudes in the USA, Australia and China (but not of the terrestrial and plants in higher latitudes) (Coulson 1977; Ma 2001). However, winter temperatures could restrict the beetle’s range at higher latitudes (Coulson 1977; Julien et al. 1995; Lu et al. 2013). Alternanthera sessilis (L.) R.Br. ex DC., the only native congener of A. philoxeroides in China, is widely distributed from tropical to temperate regions. Its geographical distribution largely overlaps with the current distribution of the invasive A. philoxeroides and A. hygrophila (Lu et al. 2015). Unlike A. philoxeroides, which grows in both terrestrial and aquatic habitats, A. sessilis occurs mostly in terrestrial habitats (Lu et al. 2015; Ma 2001).
Earlier laboratory studies of host specificity showed that A. hygrophila has a narrow host range, being able to complete its development on only A. philoxeroides (Maddox et al. 1971; Wang et al. 1988; Wu et al. 1994). As A. hygrophila lays its eggs on leaves, and mature larvae pupate in stems, previous studies have also suggested that A. hygrophila might not be able to complete its life cycle on the terrestrial forms of A. philoxeroides, due to the susceptibility of the eggs to desiccation and the restrictions in physical structure, such as stem diameter and tissue mass density, of terrestrial plants, which were thought to prevent pupation (Coulson 1977; Ma et al. 2003; Pan et al. 2011). Recent works, however, have demonstrated that A. hygrophila can also feed on a co-occurring nontarget host plant, A. sessilis (Lu et al. 2012, 2015). Furthermore, Lu et al. (2015) proved that the beetle could occur in terrestrial habitats and could pupate inside the stems of both A. philoxeroides and A. sessilis and that increasing temperature could increase A. hygrophila damage to A. sessilis along latitudes. As differing climate across latitudes can dramatically affect plant performance, climate-induced variations in A. sessilis phenology and food availability may affect A. hygrophila development and population dynamics. However, no studies have yet reported how geographical and climatic variation might affect the population and overwintering of this beetle on A. sessilis.
In this study, we conducted field experiments at three different latitudes representing different climates across China (Fig. 1) to assess the effects of climate on beetle interactions with A. sessilis. Specifically, we asked how these different climates directly and/or indirectly (via the phenology and growth of the host plants) affect A. hygrophila development and population dynamics and, in particular, the beetle’s ability to overwinter on A. sessilis. We predict that A. hygrophila performs better on A. sessilis in warmer climates in low-latitude regions than in colder and higher-latitude regions.
Locations of the field experiment sites in China (a) and temperatures at each site in January 2017 (b). The geographical coordinates of each site are 25.0804°N, 110.3004°E (Guilin), 30.5476°N, 114.4182°E (Wuhan) and 34.8185°N, 114.3025°E (Kaifeng), respectively. Meteorological data for each site was obtained from www.tianqi.com
Materials and methods
Study organisms
Alternanthera philoxeroides is a perennial herbaceous plant able to asexually propagate from stem and root buds (i.e., clonal ramets), while its seeds are not viable in China (Zhu et al. 2015). Alternanthera sessilis is an annual or perennial herbaceous plant able to reproduce both sexually and asexually in southern China but only via seeds in the temperate northern regions (Lu et al. 2015). Adults and larvae of A. hygrophila feed on the leaves of A. philoxeroides and A. sessilis, causing severe damage to the aboveground parts, and adults also lay eggs on the leaves. Mature larvae bore into the stems and pupate there. Upon emerging, adults chew out of the stems, leaving emergence holes (Maddox et al. 1971). In tropical southern China, A. hygrophila may have 8–9 generations/year, and each generation may last 1–2 months (Ma 2001).
Study sites
We conducted the experiments at three sites along a latitudinal gradient: Guilin Botanical Garden in Guangxi Province (25.0804°N, 110.3004°E) (hereafter referred to as Guilin), Wuhan Botanical Garden in Hubei Province (30.5476°N, 114.4182°E) (hereafter referred to as Wuhan) and Kaifeng, in Henan Province (34.8185°N, 114.3025°E) (hereafter referred to as Kaifeng). The climate in Guilin is typically subtropical, with warm winters (minimum temperatures in January 2017 ranged from 3 to 15 °C) and hot, humid summers. The climate in Wuhan is between subtropical and temperate, while Kaifeng is temperate and has a cold winter (see Fig. 1). These three sites represent the geographical range of A. philoxeroides and A. sessilis in China. While A. hygrophila is commonly found in Guilin and may be able to overwinter in Wuhan if the winter is warm, it has never been found in Kaifeng (Lu et al. 2015). All experimental sites were in open fields that were mowed before the experiment.
Experimental design
In October 2015, we collected A. sessilis seeds in Xianning, Hubei Province, and cultured the plants in March 2016. Alternanthera philoxeroides was collected as whole plants in Wuhan in May 2016. To obtain similar-sized plants of these two species, we cut their stems and propagated them in a greenhouse. Similar-sized plants of both species were then transferred to the experimental fields at each of the three sites on the July 5, 10 and 15, 2016, at Guilin, Wuhan and Kaifeng, respectively.
To determine whether A. hygrophila could overwinter with only A. sessilis available and to monitor its population dynamics, we established 15 1.0 × 1.0 m plots (1.0 m apart) at each site in July 2016. As the soils at each of the three sites are of different quality, we removed the native soil in each plot to a depth of 30 cm and replaced it with identical peat moss. Plots were randomly assigned to one of three plant composition treatments, (1) mixed culture (A. philoxeroides + A. sessilis), (2) A. philoxeroides monoculture and (3) A. sessilis monoculture, with five replicates (plots) for each treatment. For the monocultures, ten similar-sized stems of A. sessilis or A. philoxeroides were planted in each plot. In the mixed cultures, five A. philoxeroides and five A. sessilis stems were planted in each plot, with two of the same species planted in opposing quadrat corners, and the remaining single A. philoxeroides and A. sessilis stems planted in the center of the plot. All plots were caged using nylon mesh to exclude outside herbivores or natural enemies of the beetle. Prior to releasing the insects, we randomly sampled three individuals of each plant species from each plot at all three sites and measured the main stem diameter. The stem diameter of A. philoxeroides was bigger than that of A. sessilis, regardless of site and plant combination. The diameter of monoculture A. sessilis was similar among the three sites (Supplementary Table 1).
On August 26 and 30 and September 2, 2016, we released four pairs of newly mated A. hygrophila into each plot in Guilin, Wuhan and Kaifeng. The beetles were collected in a field in Wuhan. After releasing the beetles, we counted A. hygrophila adults in each plot every 2 weeks until early November, after which we counted insects every 4 weeks between November 2016 and February 2017 corresponding with plant depletion and insect removal (see details below); then, we counted insects every 3 weeks after February 2017 corresponding with the growth of plants. At the Guilin site, to avoid heavy defoliation that could kill all the plants and cause the insect population to collapse, we removed all adults and mature larvae from the A. philoxeroides monoculture and mixed culture plots after each survey between October 23, 2016 and February 18, 2017. Similarly, all adults and mature larvae were removed from the monoculture A. sessilis plots in Guilin on December 26, 2016 and February 18, 2017. We also removed half of all adults after each survey in the monoculture A. philoxeroides and mixed culture plots between March 10 and April 19, 2017. A previous study had shown that such removal is an effective approach for long-term monitoring of insect populations in host preference/performance tests (Ding et al. 2007). We removed insects at only the Guilin site; no insects were removed at other two sites due to very low populations.
To examine the effects of host plants on insect development, we used the fresh weight of the beetles as an index of the body size of adults, as it was easily and rapidly measured during the frequent experimental periods (Knapp and Knappová 2013), and data were collected from Guilin, which had the longest survey period. At each survey date, female and male adults were randomly collected from each monoculture plot of A. philoxeroides and A. sessilis, and their sizes were measured. The number of adults varied among the plant composition treatments over time (5–74 females and 9–54 males on A. sessilis, 15–81 females and 16–81 males on A. philoxeroides) depending on insect availability during the experiment, with low numbers at earlier stages and high numbers at later stages. All weight measurements were taken using an electronic balance (Sartorius BS 110 S, Germany).
To investigate the effects of the winter and spring seasons on plant growth and/or food availability, which might affect insect development, we made phenology measurements of the plants. We began noting greening of stem buds (perennial or biennial ramets) in both species and seed germination (annual seedlings) of A. sessilis in January 2017. We counted the number of perennial or biennial ramets of each species and the annual seedlings of A. sessilis. We randomly selected ten ramets from each monoculture plot for both plants and ten seedlings of A. sessilis from its monoculture plots to measure ramet length and annual seedling height.
Statistical analysis
To examine the impacts of latitude and host on A. hygrophila overwintering, the number of adults was analyzed using the generalized linear model (GLM) with a Poisson distribution. The model included experiment site (Guilin, Wuhan and Kaifeng) as a fixed factor and time (n = 12) as a covariate. Each host plant combination (A. philoxeroides monoculture, A. sessilis monoculture, and A. philoxeroides and A. sessilis mixture) was analyzed separately. To examine the impact of host on the performance of A. hygrophila in Guilin, adult weight was analyzed using a linear model (LM) with plant species (A. philoxeroides and A. sessilis monocultures) as a fixed factor and time (n = 12) as a covariate. Females and males were analyzed separately. To examine the growth of the two plant species in their monoculture treatments, the number of ramets and ramet length were analyzed using GLM and LM, respectively. The model included experiment site and plant species as fixed factors and time as a covariate. To examine the growth of A. sessilis annual seedlings in the monoculture treatment, the number of seedlings and their heights were analyzed using GLM and LM, respectively. The model included experimental site as a fixed factor and time as a covariate. All data were analyzed using R 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria). Multiple comparisons were carried out using least square mean post hoc tests (LSM), and p values were adjusted using false discovery rate (Benjamini and Hochberg 1995).
Results
Population dynamics and overwintering of A. hygrophila
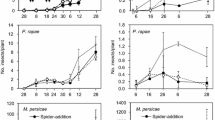
Beetle numbers decreased with increasing latitude (from Guilin to Kaifeng) in all combinations (A. philoxeroides monoculture: \(\chi_{2}^{2}\) = 9345.3, P < 0.001; A. sessilis monoculture: \(\chi_{2}^{2}\) = 5207.4, P < 0.001; mixture: \(\chi_{2}^{2}\) = 7509.8, P < 0.001). There were also significant effects of time and its interaction with site on beetle numbers (time in A. philoxeroides monoculture: \(\chi_{1}^{2}\) = 529.8, P < 0.001; time in A. sessilis monoculture: \(\chi_{1}^{2}\) = 139.3, P < 0.001; time in mixture: \(\chi_{1}^{2}\) = 157.6, P < 0.001; site × time in A. philoxeroides monoculture: \(\chi_{2}^{2}\) = 490.5, P < 0.001; site × time in A. sessilis monoculture: \(\chi_{2}^{2}\) = 323.2, P < 0.001; site × time in mixture: \(\chi_{2}^{2}\) = 163.2, P < 0.001). From late September to mid-November, there were always more adults in Guilin and fewer adults in Kaifeng in every host combination, despite the removal of insects from the cages in Guilin to prevent insect outbreaks from causing plant collapse (see Supplementary Table 2). Beetle numbers decreased to zero in Kaifeng and Wuhan in mid-November and early December 2016, respectively (Fig. 2). However, A. hygrophila successfully overwintered on A. sessilis in Guilin, although the populations in the monoculture A. sessilis plots decreased from January to April 2017 (Fig. 2).
Population of Agasicles hygrophila adults in monoculture Alternanthera philoxeroides plots (a), monoculture Alternanthera sessilis plots (b) and mixed plots (c) in three sites from September 2016 to April 2017. M, L and E labels on the X-axis refer to ‘Middle’, ‘Late’ and ‘Early’, respectively. Arrows indicate the dates of insect removal at Guilin. Data are the mean ± SE
The performance of A. hygrophila
Plant species had no significant effect on the weight of female or male adults (female: F1, 838 = 1.862, P = 0.173; male: F1, 791 = 2.614, P = 0. 106) at the Guilin site, but time and its interaction with host species had significant effects on beetle weight (time in female: F1, 838 = 3.857, P = 0.050; time in male: F1, 791 = 11.657, P < 0.001; species × time in female: F1, 838 = 19.280, P < 0.001; species × time in male: F1, 791 = 8.910, P = 0.003) (Fig. 3). Adult weights varied significantly with time. Females that fed on A. philoxeroides were significantly larger than those that fed on A. sessilis on November 7, 2016 and significantly smaller on March 10 and 30 and April 19, 2017. Males that fed on A. philoxeroides were significantly larger than those that fed on A. sessilis on September 25 and November 7, 2016 and significantly smaller on October 23, 2016 and February 18 and April 19, 2017.
Plant phenology
Annual A. sessilis seedlings (seed origin) were found in Kaifeng, but both annual seedlings and ramets of A. sessilis were found in Guilin and Wuhan (Figs. 4, 5). The annual seedlings of A. sessilis started to grow on October 23, 2016 and April 12 and 18, 2017 in Guilin, Wuhan and Kaifeng, respectively. Meanwhile, the ramets of A. sessilis started to grow on February 27 and March 15, 2017 in Guilin and Wuhan, respectively, and the ramets of A. philoxeroides started to grow on the March 1 and 9, 2017 in Wuhan and Kaifeng, respectively. Both A. philoxeroides and A. sessilis survived above ground in Guilin during winter, and new seeds of A. sessilis and stem buds of A. philoxeroides were sprouting during winter 2016.
Ramet number and ramet length were significantly affected by site (ramet number: \(\chi_{2}^{2}\) = 1633, P < 0.001; ramet length: F2, 138 = 33. 831, P < 0.001), species (ramet number: \(\chi_{1}^{2}\) = 6175, P < 0.001; ramet length: F1, 138 = 37.241, P < 0.001), and time (ramet number: \(\chi_{1}^{2}\) = 75,984, P < 0.001; ramet length: F1, 138 = 96.207, P < 0.001) (Fig. 4). The interactions among site, species and time significantly affected ramet number (site × species: \(\chi_{2}^{2}\) = 7204, P < 0.001; site × time: \(\chi_{2}^{2}\) = 11, P = 0.004; species × time: \(\chi_{1}^{2}\) = 199, P < 0.001; site × species × time: \(\chi_{2}^{2}\) = 219, P < 0.001). The interaction between site and time (F2, 138 = 4.249, P = 0.016) and the interaction between species and time (F1, 138 = 16.083, P < 0.001) significantly affected ramet length.
Both the number and the height of annual A. sessilis plants were significantly affected by site and time (number by site: \(\chi_{2}^{2}\) = 126,717, P < 0.001; number by time: \(\chi_{1}^{2}\) = 59,335, P < 0.001; height by site: F2, 69 = 291.152, P < 0.001; height by time: F1, 69 = 27.507, P < 0.001), and the interaction between site and time had a significant effect on seedling number (\(\chi_{2}^{2}\) = 7197, P < 0.001) and height (F2, 69 = 10.531, P < 0.001) (Fig. 5).
Discussion
Monitoring insect populations and examining the effects of climate on insect performance are critical for assessing nontarget effects in current and future weed biological control programs (Lu et al. 2015). Many previous studies have examined insect nontarget effects by assessing host specificity in laboratory and common garden experiments (Myers and Cory 2017; Raghu et al. 2007); however, studies examining how climate affects nontarget effects are rare (but see Lu et al. 2015). To the best of our knowledge, our study is the first to monitor the populations and overwintering of insect biological control agents on nontarget hosts at different latitudes simultaneously. We found that A. hygrophila could successfully sustain populations on native A. sessilis during the growing season at all three latitudinal sites with different climates, but it was able to overwinter on A. sessilis only in subtropical Guilin, not in the colder northern areas of Wuhan and Kaifeng.
Previous studies have shown that low temperature can restrict the distribution of A. hygrophila at high latitudes (Julien et al. 1995) and that low temperatures in winter can directly reduce the activity of A. hygrophila, leading to less feeding and lower fecundity (Guo et al. 2012; Zhao et al. 2015). In this study, we found that A. hygrophila successfully overwintered in Guilin, but all beetles died in December in Wuhan and Kaifeng, suggesting that the lack of cold resistance of A. hygrophila could lead to the mortality of populations in cold regions. Thus, low overwintering ability is a determining factor that restricts the distribution of A. hygrophila. Furthermore, our monoculture experiments showed that A. sessilis could support the beetle’s development under natural conditions through the winter in Guilin (Fig. 2), and this result was similar with that of a previous study (Lu et al. 2015).
Temperature also affects A. hygrophila populations indirectly, through its impact on the phenology and growth of host plants. As the beetle does not undergo winter diapause, A. hygrophila was unable to survive in the absence of hosts at low temperatures (Julien et al. 1995). Lu et al. (2015) found that increasing temperature favored the overwintering of the beetle on A. philoxeroides. In this study, both A. philoxeroides and A. sessilis in Guilin survived aboveground all year, including ramets of A. philoxeroides and seedlings of A. sessilis, providing the beetle with food and refuge against cold weather. Agasicles hygrophila could successfully overwinter in Guilin by taking advantage of A. sessilis, a suitable host at low latitudes. In contrast, the low temperatures in winter in Wuhan and Kaifeng could directly restrict the distribution of A. hygrophila and indirectly suppress these insects by affecting the survival of the two host plants.
Our results showed that the fresh weight of A. hygrophila was similar when feeding on A. philoxeroides or A. sessilis, suggesting that the variations in plant resources between these two hosts may not affect the adult growth of A. hygrophila. Ma et al. (2003) argued that plant physical structures such as stem diameter, rather than nutrients, are key factors affecting beetle development. Moreover, it has been demonstrated that A. sessilis had a negative impact on the preoviposition period and fecundity of A. hygrophila (Lu et al. 2010), which might explain the low populations in the A. sessilis monoculture plots relative to those in the A. philoxeroides monoculture plots and the mixed plots in our study. Thus, our experiments with mixed plots further confirmed that the beetle prefers the invasive A. philoxeroides over the native host, A. sessilis. Furthermore, the changes in adult size over time in Guilin (Fig. 3) might partly depend on the availability of suitable stems for pupation. Due to the gradual withering and death of plant aboveground portions with increasing feeding by the beetle population, a lack of suitable stems might account for the smaller sizes of some adults.
Although A. hygrophila prefers the aquatic form of A. philoxeroides to the terrestrial ones (Julien et al. 1995; Maddox et al. 1971), recent experimental studies and field surveys have found that the beetle can also thrive on the terrestrial forms of both the invasive A. philoxeroides and the native A. sessilis (Lu et al. 2015, 2016). In this study, we conducted our experiment in terrestrial habitats, where this beetle could sustain populations and overwinter on native A. sessilis in the subtropical Guilin area. The lack of hollow stems in the terrestrial forms of A. philoxeroides was previously thought to explain the pupation failure of A. hygrophila (Coulson 1977; Ma et al. 2003). However, our results did not support this assertion. Since environmental heterogeneity can affect plant morphology and physical structure, and since A. philoxeroides and A. sessilis occur over large geographical regions, variations in stem diameter and tissue mass density among terrestrial plant populations may affect beetle pupation on these hosts. In this study, we focused on the effects of climate on A. hygrophila population and its overwintering on A. sessilis at three latitude sites; however, we only measured the stem diameter before releasing the beetle but did not compare the diameter and tissue densities of plants at these three sites during the experiment, and these variables may deserve further study. We also acknowledge that our tests were conducted in cages and lasted for only 1 year; thus, we cannot rule out the effects of other biotic and abiotic factors on the hosts and beetles. Under natural condition, the beetle may disperse among latitudes during summer. However, if they disperse to higher latitudes where the winter is too cold, then they may die during the winter, as indicated by our findings. The results of a recent warming experiment showed that increasing temperature increased the overwintering of A. hygrophila on A. sessilis, and living pupae have been found in field A. sessilis in subtropical areas but not in temperate areas in China (Lu et al. 2015, 2016), supporting our conclusions that A. hygrophila can sustain populations on terrestrial A. sessilis and that whether it can overwinter on this native host depends on climate.
Our results also have important implications for understanding the effects of climate on insect host performance and assessing nontarget effects in weed biological control. Differences in A. hygrophila overwintering among latitudinal sites suggest that testing the safety of biological control agents should consider variations in climatic and geographical factors, which influence the key life history period that restricts insect development and population. Such studies might be more critical when assessing nontarget effects under the scenario of climate change (Lu et al. 2015, 2016), which may alter plant physiology. Importantly, the results of our study may also extend to other weed biological control programs for risk assessment of nontarget effects. Many weeds, potential nontarget plants and insect biological control agents overlap in varying climate zones, likely leading to different effects of the insects on nontarget hosts. Furthermore, global climate warming may expand the geographical distributions of many invasive plants, biological control agents and potential nontarget native species (Lu et al. 2015; McEvoy et al. 2012), creating novel biotic interactions.
In summary, we found that A. hygrophila, a biological control agent of A. philoxeroides, could sustain populations on the nontarget native plant A. sessilis during the growing season. When A. sessilis was the only host, the beetle could overwinter in subtropical regions but not in colder areas. While previous laboratory tests and field surveys have reported only its ability to damage A. sessilis, our study reveals how variations in climate may affect beetle use of this nontarget plant under natural conditions. We recommend considering the shifting interactions of invasive plants, biological control insects and native plants induced by climate when assessing the nontarget effects of biocontrol agents.
Author contribution statement
YW and JD designed the experiment; YW conducted the experiment; WH and MI analyzed the data; YW wrote the manuscript; all authors revised and approved the manuscript.
References
Bale JS, Hayward SAL (2010) Insect overwintering in a climate change. J Exp Biol 213:980–994. https://doi.org/10.1242/jeb.037911
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B (Stat Method) 57:289–300. https://doi.org/10.2307/2346101
Coulson JR (1977) Biological control of alligatorweed, 1959–1972. A review and evaluation. Agricultural Research Service, United States Department of Agriculture, p 1547
Ding J, Wang Y, Jin X (2007) Monitoring populations Galerucella birmanica (Coleoptera: Chrysomelidae) on Brasenia schreberi and Trapa natans (Lythraceae): implications for biological control. Biol Control 43:71–77. https://doi.org/10.1016/j.biocontrol.2007.06.010
Guo JY, Fu JW, Xian XQ, Ma MY, Wan FH (2012) Performance of Agasicles hygrophila (Coleoptera: Chrysomelidae), a biological control agent of invasive alligator weed, at low non-freezing temperatures. Biol Invasions 14:1597–1608. https://doi.org/10.1890/14-1092.1
Ismail M, Brooks M (2016) The impact of geographical origin of two strains of the herbivore, Eccritotarsis catarinensis, on several fitness traits in response to temperature. J Therm Biol 60:222–230. https://doi.org/10.1016/j.jtherbio.2016.07.008
Ismail M, Compton SG, Brooks M (2017) Interaction between temperature and water nutrient levels on the fitness of Eccritotarsus catarinensis (Hemiptera: Miridae), a biological control agent of water hyacinth. Biol Control 106:83–88. https://doi.org/10.1016/j.biocontrol.2017.01.001
Jamieson MA, Schwartzberg EG, Raffa KF, Reich PB, Lindroth RL (2015) Experimental climate warming alters aspen and birch phytochemistry and performance traits for an outbreak insect herbivore. Global Change Biol 21:2698–2710. https://doi.org/10.1111/gcb.12842
Jang T, Rho MS, Koh SH, Lee KP (2015) Host-plant quality alters herbivore responses to temperature: a case study using the generalist Hyphantria cunea. Entomol Exp Appl 154:120–130. https://doi.org/10.1111/eea.12261
Julien MH, Broadbent JE, Harley KLS, Medd RW, Auld BA (1979) The current status of biological control of Alternanthera philoxeroides in Australia. In: Proceedings of the 7th Asian-Pacific weed science society conference, pp 231–233
Julien MH, Skarratt B, Maywald GF (1995) Potential geographical-distribution of alligator weed and its biological-control by Agasicles Hygrophila. J Aquat Plant Manage 33:55–60
Knapp M, Knappová J (2013) Measurement of body condition in a common carabid beetle, Poecilus cupreus: a comparison of fresh weight, dry weight, and fat content. J Insect Sci 13:1–10. https://doi.org/10.1673/031.013.0601
Lencioni V (2004) Survival strategies of freshwater insects in cold environments. J Limnol 63:45–55. https://doi.org/10.4081/jlimnol.2004.s1.45
Lu JJ, Zhao LL, Ma RY, Zhang PP, Fan RJ, Zhang JT (2010) Performance of the biological control agent flea beetle Agasicles hygrophila (Coleoptera: Chrysomelidae), on two plant species Alternanthera philoxeroides (alligatorweed) and A. sessilis (joyweed). Biol Control 54:9–13. https://doi.org/10.1016/j.biocontrol.2010.02.012
Lu JJ, Zhao LL, Ma RY, Fan RJ, Zhang JT, Wang R (2012) Non-target plant testing of the flea beetle Agasicles hygrophila, a biological control agent for Alternanthera philoxeroides (alligatorweed) in China. Biocontrol Sci Techn 22:1093–1097. https://doi.org/10.1080/09583157.2012.708019
Lu X, Siemann E, Shao X, Wei H, Ding J (2013) Climate warming affects biological invasions by shifting interactions of plants and herbivores. Global Change Biol 19:2339–2347. https://doi.org/10.1111/gcb.12244
Lu X, Siemann E, He M, Wei H, Shao X, Ding J (2015) Climate warming increases biological control agent impact on a non-target species. Ecol Lett 18:48–56. https://doi.org/10.1111/ele.12391
Lu X, Siemann E, He M, Wei H, Shao X, Ding J (2016) Warming benefits a native species competing with an invasive congener in the presence of a biocontrol beetle. New Phytol 211:1371–1381. https://doi.org/10.1111/nph.13976
Ma RY (2001) Ecological adaptation for the intruduced biocontrol agent, Agasicles hygropghila, for Alligatorweed, Alternanthera philoxeroides, in China. Dissertation, Chinese Academy of Agricultural Sciences, Beijing
Ma RY, Ding JQ, Li BT, Wu ZQ, Wang R (2003) The pupation adaptability of Agasicles hygrophial on different ecotypes alligator weed. Chin J Biol Control 19:54–58
Ma RY, Hao SG, Kong WN, Sun JH, Kang L (2006) Cold hardiness as a factor for assessing the potential distribution of the Japanese pine sawyer Monochamus alternatus (Coleoptera: Cerambycidae) in China. Ann For Sci 63:449–456. https://doi.org/10.1051/forest:2006025
Maddox DM, Andres LA, Hennessey RD, Blackburn RD, Spencer NR (1971) Insects to control alligatorweed: an invader of aquatic ecosystems in the United States. Bioscience 21:985–991. https://doi.org/10.2307/1296135
McEvoy PB (1996) Host specificity and biological pest control—how well is research on host specificity addressing the potential risks of biological control. Bioscience 46:401–405. https://doi.org/10.2307/1312873
McEvoy PB, Higgs KM, Coombs EM, Karaçetin E, Starcevich LA (2012) Evolving while invading: rapid adaptive evolution in juvenile development time for a biological control organism colonizing a high-elevation environment. Evol Appl 5:524–536. https://doi.org/10.1111/j.1752-4571.2012.00278.x
Müller-Schärer H, Schaffner U (2008) Classical biological control: exploiting enemy escape to manage plant invasions. Biol Invasions 10:859–874. https://doi.org/10.1007/s10530-008-9238-x
Myers JH, Cory JS (2017) Biological control agents: invasive species or valuable solutions? In: Vilà M, Hulme PE (eds) Impact of biological invasions on ecosystem services. Springer International Publishing, Berlin, pp 191–202. https://doi.org/10.1007/978-3-319-45121-3_12
Pan XY, Jia X, Zeng J, Sosa A, Li B, Chen JK (2011) Stem tissue mass density is linked to growth and resistance to a stem-boring insect in Alternanthera philoxeroides. Plant Spec Biol 26:58–65. https://doi.org/10.1111/j.1442-1984.2010.00307.x
Posledovich D, Toftegaard T, Wiklund C, Ehrlén J, Gotthard K (2015) The developmental race between maturing host plants and their butterfly herbivore—the influence of phenological matching and temperature. J Anim Ecol 84:1690–1699. https://doi.org/10.1111/1365-2656.12417
Raghu S, Dhileepan K, Scanlan JC (2007) Predicting risk and benefit a priori in biological control of invasive plant species: a systems modelling approach. Ecol Model 208:247–262. https://doi.org/10.1016/j.ecolmodel.2007.05.022
Rasmann S, Pellissier L, Defossez E, Jactel H, Kunstler G (2014) Climate-driven change in plant–insect interactions along elevation gradients. Funct Ecol 28:46–54. https://doi.org/10.1111/1365-2435.12135
Robinet C, Roques A (2010) Direct impacts of recent climate warming on insect populations. Integr Zool 5:132–142. https://doi.org/10.1111/j.1749-4877.2010.00196.x
Stoeckli S, Hirschi M, Spirig C, Calanca P, Rotach MW, Samietz J (2012) Impact of climate change on voltinism and prospective diapause induction of a global pest insect—Cydia pomonella (L.). PLoS ONE 7:e35723. https://doi.org/10.1371/journal.pone.0035723
Uelmen JA Jr, Lindroth RL, Tobin PC, Reich PB, Schwartzberg EG, Raffa KF (2016) Effects of winter temperatures, spring degree-day accumulation, and insect population source on phenological synchrony between forest tent caterpillar and host trees. For Ecol Manag 362:241–250. https://doi.org/10.1016/j.foreco.2015.11.045
Wang R, Wang Y, Zhang GC, Li JX (1988) The host specificity test of Agasicles hygrophila. Chin J Biol Control 4:14–17. https://doi.org/10.16409/j.cnki.2095-039x.1988.01.006
Wu ZQ, Cai YC, Guo ZX, Wang TB (1994) Host specificity tests for Agasicles hygrophila (Coleoptera: Chrysomelidae), a biological control agent of alligator weed. J Biosafety 3:98–100
Yang LH, Rudolf VHW (2010) Phenology, ontogeny and the effects of climate change on the timing of species interactions. Ecol Lett 13:1–10. https://doi.org/10.1111/j.1461-0248.2009.01402.x
Zhao LL, Jia D, Yuan XS, Guo YQ, Zhou WW, Ma RY (2015) Cold hardiness of the biological control agent, Agasicles hygrophila, and implications for its potential distribution. Biol Control 87:1–5. https://doi.org/10.1016/j.biocontrol.2015.02.007
Zhu Z, Zhou CC, Yang J (2015) Molecular phenotypes associated with anomalous stamen development in Alternanthera philoxeroides. Front Plant Sci 6:1–10. https://doi.org/10.3389/fpls.2015.00242
Acknowledgements
We would like to thank Saichun Tang, Yumei Pan, Chunqiang Wei, Baoliang Tian, Xiao Sun, Xiangqin Li, Li Xiao, Jia Liu, Zhenzhen Yu, Zhen Liu and Shunliang Feng for field and laboratory assistance. Edits and comments by Roy Van Driesche, Emmet Van Driesche, Springer Nature Author Services as well as the journal editor and reviewers improved earlier versions of this manuscript. This work was supported by the National Key Research and Development Program (2017 YFC 1200100).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by M. Traugott.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Ismail, M., Huang, W. et al. Population dynamics and overwintering of a biological control beetle, Agasicles hygrophila, on a nontarget plant Alternanthera sessilis, along a latitudinal gradient. J Pest Sci 92, 835–845 (2019). https://doi.org/10.1007/s10340-018-1031-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-018-1031-8