Abstract
The flea beetle, Agasicles hygrophila, was introduced to control the alligator weed, Alternanthera philoxeroides, in southern China and redistributed for over 20 years. The beetle has succeeded in establishing local field populations. Temperature, especially extreme low temperature in winter, is hypothesized to be a key factor determining the distribution of A. hygrophila. We studied the adult reproduction and leaf consumption, egg hatching rate, larval and pupal survival and development of A. hygrophila in response to low non-freezing temperatures. Female and male adults of A. hygrophila survived at 4°C for 11.4 and 14.2 days, respectively, and adult longevity increased with increasing temperature from 4 to 12°C. Adult longevity was significantly longer at 12°C than at 25°C, and the fecundity at 12°C was approximately 10% of that at 25°C. When A. hygrophila eggs, first instar larvae and pupae were kept at 4–12°C for 1, 4, 7 or 10 days, respectively, and then transferred to 25°C, over one third of eggs hatched after cold treatment at 4°C for 7 days, with an average egg development duration of 3.6 days excluding the cold treatment period. Egg hatching rate increased as temperature during the cold treatment increased and the cold treatment duration reduced. Eggs pretreated at 12°C and those kept constantly at 25°C did not differ in their hatching rates. The first instar larvae of A. hygrophila could not survive 12°C or lower, and exposure to these low temperatures resulted in 100% mortality and a lifespan shorter than 1 day. Eclosion rate of A. hygrophila pupae was 71, 60, 24 and 15% after cold treatment at 4–12°C for 1, 4, 7 and 10 days, respectively, which was lower than that at constant 25°C (over 93%) but did not differ among the cold treatments. Comparing with the prediction in 1980s that A. hygrophila could not overwinter further north than the approximate position of the 9°C isotherm, our recent survey showed that A. hygrophila has now distributed in the regions with January isotherms of 0–9°C in China. These results indicated that A. hygrophila has the capacity to stand relatively low non-freezing temperatures for short durations, which would help it to overwinter and establish natural populations in some areas, especially in areas where protected cultivations are extensive and ambient temperatures are not as low as those in the open field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alligator weed Alternanthera philoxeroides, a herbaceous amphibious weed of the family Amaranthaceae, is native to South America (Sosa et al. 2004). As one of the worst invasive alien weed species, it has caused economic and ecological problems in America, New Zealand, Australia, Thailand and China (Maddox and Rhyne 1975; Hruska et al. 1985; Julien et al. 1979; Wang et al. 1988). Alternanthera philoxeroides was introduced into suburban Shanghai from Japan as a forage crop in the late 1930s, and spread intentionally to eastern China in 1950s and to southern China in 1960s–1970s (Ye et al. 2003). Now it occurs across 18 provinces and autonomous regions in China (Zhang et al. 1993; Wan et al. 2005).
As a highly competitive plant, A. philoxeroides can displace natural aquatic and terrestrial vegetation, change local habitats and result in some significant hazards. For example, its infestations can block drainage and irrigation canals, and thus increases the risk of flooding (Coulson 1977). It can also restrict traffic in navigable waterways and limit fishing, swimming and other recreational uses of lakes, rivers and streams (Spencer and Coulson 1976).
Agasicles hygrophila, a chrysomelid which originates from South America, is considered a potential agent for the control of A. philoxeroides (Buckingham 1996). The beetle was introduced into USA, New Zealand, Australia and China for the biological control of A. philoxeroides (Coulson 1977; Stewart et al. 1996; Julien and Chan 1992; Wang et al. 1988). The optimal temperature range of A. hygrophila is from 22 to 28°C (Stewart et al. 1996, 2000). Its tolerance to extreme low and high temperatures is poor and the population of the beetle is therefore likely to fluctuate (Spencer and Coulson 1976). Its population density declines sharply under extreme temperature conditions (Julien et al. 1979). Distribution of A. hygrophila was restricted to areas without extreme winter temperature in USA (Spencer and Coulson 1975).
Agasicles hygrophila was introduced into China for the control of A. philoxeroides in 1987 from Florida, USA, and was released in Chongqing municipality (29.32 N, 106.26 E), Fuzhou, Fujian province (26.05 N, 119.10 E), and Changsha, Hunan province (28.12 N, 113.04 E), in 1988 (Wang et al. 1988; Ma et al. 2003). Agasicles hygrophila established populations in some release sites and spread gradually to other places in southern China. It causes the collapse of alligator weed populations from May to June and from September to October in aquatic habitats in Fujian and Hunan provinces, when the density of A. philoxeroides reaches over 600 individuals/m2. However, the beetle’s density declines to less than 2 individuals/m2 in summer and winter, apparently due to the extreme high and low temperatures in summer and winter respectively which limit the beetle’s population development.
Agasicles hygrophila failed to overwinter at the release sites in Chongqing and Changsha in the initial years, and it became necessary to have the insect protected indoors or covered with plastic sheets in the field to help it successfully overwinter (Li and Wang 1994). But after its naturalization for about 20 years, it established year-round field population in Chongqing and Changsha and has spread to new places over 400 km away. The most northern record of the establishment of A. hygrophila in China was in Chaohu, Anhui province (31.35 N, 117.71 E) north of the Yangtze River (Huang 2007). Thus, A. hygrophila in China has been exposed to lower temperatures than those in its original introduction site Florida, USA, where the average temperature in January is 20°C. Zhang et al. (1997) reported that A. hygrophila in China could tolerate a short period of extreme low temperature of −7°C.
We hypothesize that temperature is a key abiotic factor affecting the population collapse and re-establishment of A. hygrophila in the field. In southern China, the population of A. hygrophila usually drops to a very low density in the winter from November to December. To determine the effects of low non-freezing temperatures on the population establishment and collapse of A. hygrophila, and to predict its expansion to new overwinter areas under current weather conditions, especially under global warming, we studied the effects of low non-freezing temperature on the adult survival and reproduction of A. hygrophila. The data are discussed in relation to the threshold temperature for overwintering in this insect.
Materials and methods
Plants and insects
Seedlings of A. philoxeroides were collected from a pond in the Institute of Plant Protection, Hunan Academy of Agricultural Sciences (IPP, HAAS), Changsha, Hunan province in 2007. They were transplanted into plastic pots (45 cm × 35 cm × 15 cm) with a humus soil at a density of 45–50 plants per pot. The pots were placed in the greenhouse in IPP, HAAS, and were watered twice a week to keep the soil moist. Thirty days after transplanting, when the plants were 20–30 cm in height, the stems with leaves were used for experiments.
Agasicles hygrophila were collected from the pond in IPP, HAAS in 2007 and maintained in an insectary in IPP, HAAS, under the conditions of 25 ± 2°C, 85% ± 5%RH, and a photoperiod of 12L:12D. They were reared for 3 generations with potted A. philoxeroides before the experiments.
Reproduction and feeding of A. hygrophila adults at low temperature
Ten pairs of newly eclosed A. hygrophila adults (≤24 h) from the culture were randomly selected and transferred to a plastic box (18 cm × 11 cm × 7 cm, with a piece of moistened filter paper at the bottom to keep moisture) with fresh A. philoxeroides stems collected from the greenhouse. The plastic box with the insects was transferred to a climate chamber at the same humidity and photoperiod conditions as those in the insectary, and temperature was set at 4, 6, 8, 10, or 12°C (± 1°C), respectively, with 25 ± 1°C as the control. Survival and oviposition of A. hygrophila adults were assessed every 24 h. At each of the daily observations, the eggs deposited were removed using a fine brush and plant stems were replaced with fresh ones, until all females had died. The leaf area eaten by A. philoxeroides was recorded by marking on a transparent plotting paper. Thirty unaffected fresh leaves were randomly selected and weighed using an electronic balance (METTLER TOLEDO AB204-S, Switzerland, with an accuracy of 0.01 mg) and their leaf areas were measured using the plotting paper. The average biomass of A. philoxeroides leaf was estimated in mg per square centimeter, and the consumption of A. hygrophila was calculated as mg per pair of adults. Each treatment was repeated 3 times. In total, 10 pairs × 6 treatments × 3 replications = 180 pairs of adults were used for this experiment.
Survival of A. hygrophila adults at low temperature
Using the method as described above for the reproduction and feeding experiment, survival of ten pairs of newly eclosed A. hygrophila adults (≤24 h) was studied at the temperature 4, 6, 8, 10, and 12°C (±1°C), respectively, with 25 ± 1°C as control. Observations here were observed every 5d and the host plants were changed at each observation. Host plants in the control group were changed daily because of greater feeding. Because leaf consumption by A. hygrophila was very low and plants kept fresh within the 5d observation interval in the five low-temperature (4–12°C) treatments, plant exchange frequency was not considered a key factor affecting beetle performance. Each treatment was repeated 3 times. In total, 10 pairs × 6 treatments × 3 replications = 180 pairs of adults were used for this experiment.
Development of A. hygrophila eggs at low temperature
Leaves of A. philoxeroides with newly laid A. hygrophila egg masses (≤12 h, 27–34 eggs, one egg mass per leaf) were randomly selected and cut from the culture. Ten such leaves were maintained in a plastic box as described above. The plastic boxes with the eggs were transferred to a climate chamber at 4, 6, 8, 10, or 12°C (± 1°C), and at each of the temperatures for 1, 4, 7, or 10 days, respectively. After the low temperature exposure, the eggs were transferred to another climate chamber at 25 ± 1°C. All the chambers were at 85% ± 5% RH and a photoperiod of 12L:12D conditions. The number of hatchlings in each of the egg masses was then recorded every 12 h for 5 days. Each treatment was repeated 3 times. A control treatment was conducted at 25 ± 1°C. In total, 10 egg masses × 24 treatments × 3 replications = 720 egg masses were used for this experiment.
Development of A. hygrophila larvae at low temperature
Thirty newly hatched A. hygrophila larvae (≤12 h) were transferred to a plastic box as described above and provided with fresh A. philoxeroides stems for feeding. The boxes with A. hygrophila larvae were held at 4, 6, 8, 10, or 12°C (± 1°C) and at each of the temperatures for 1, 4, 7, or 10 days, respectively, and then transferred to 25 ± 1°C. The larvae were observed every 12 h to record mortality and moulting. To do so, the first section on the abdomen of the 1st and 2nd instar larvae were marked with red paint (SZ51: Olympus (China) Co., Ltd.) at a similar quantity before treatment (our preliminary observations indicated that the marking did not affect survival of the larvae and was persistent before the larva moulted). A larva was judged as having moulted when the red mark disappeared and its body grew larger. When a 2nd instar larva had moulted and bored into an A. philoxeroides stem, it was regarded as a 3rd instar. The plants were replaced every 24 h. Each treatment was repeated 5 times. Again a control treatment was conducted at 25 ± 1°C. In total, 30 larvae × 24 treatments × 5 replications = 3600 newly hatched larvae were used for this experiment.
Development of A. hygrophila pupae at low temperature
The top 5–6 nodes of A. philoxeroides stems were cut from plants in the greenhouse and transplanted into pieces of soaked floral foam (17 cm × 10 cm × 2 cm), at a density of 45 stems per foam. The foam with the young stems was placed into a plastic box and 30 later 3rd instar larvae were added. After the larvae had bored into the stems, the boxes with A. hygrophila larvae (pupae) were held at 4, 6, 8, 10, or 12°C (± 1°C) and at each of the temperatures for 1, 4, 7, or 10 days, respectively, and then transferred to 25 ± 1°C. The boxes were then observed every 12 h for 10 days and the eclosed adults were counted and removed at each observation. Each treatment was repeated 3 times. A control treatment was conducted at 25 ± 1°C. In total, 30 larvae × 24 treatments × 5 replications = 3600 later 3rd instar larvae were used for this experiment.
Data analysis
All data were tested for homogeneity of variance by Bartlett’s test and for outliers by Dixon’s test (Sokal and Rohlf 1995). Data on egg hatching rate and pupal eclosion rate were arcsine-transformed. Data on development time and longevity were log-transformed. Data on the number of eggs were square root-transformed (Sokal and Rohlf 1995). Data on the egg hatching rate, pupal eclosion rate, egg and pupal development times were compared by two-way analysis of variance (ANOVA) among treatments (type III), with temperature and pretreatment period as factors. Data on the adult longevity and fecundity, egg hatching rate, pupal eclosion rate, egg development time, larval development time, leaf consumption by larvae and adults were also compared by the least significant difference (LSD) test after one-way analysis of variance (ANOVA) among treatments, with either temperature and pretreatment period as factor. The comparison of a mean value with that of the control was accomplished with the Student’s t-test. All calculations were done using the SAS 6.12 statistical package (SAS Institute Inc. USA 1996). For uncensored cohorts, the adult survivorship data were analyzed using a Kolmogorov–Smirnov test (Pyke and Thompson 1986; Sokal and Rohlf 1995). The map of isotherms was made based on the grid data of Jra25 (Japanese 25-year Reanalysis Project generated meteorological dataset) 1979–2008 (downloaded from the website http://www.jreap.org/). Climate data were extracted by the software Grads2.0 (downloaded from the website: http://www.iges.org/grads), and imported into GIS (ArcInfo8.3, ESRI). Data of overwintering sites were obtained from previous reports as well as our own data, and the dataset was overlaid with climate data.
Results
Reproduction and feeding of A. hygrophila adults at low temperature
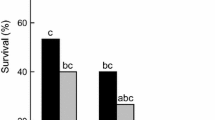
Female and male adults of A. hygrophila survived at 4°C for 11.4 and 14.2 days, respectively. Longevity of female and male adults of A. hygrophila significantly increased as the temperature increased from 4°C to 12°C (ANOVA: LSD, F ♀ = 103.52, df = 4, 145, P < 0.0001; F ♂ = 50.05, df = 4, 154, P < 0.0001). The average longevity of females (mean = 53.45 days) and males (mean = 44.2 days) was significantly longer at 12°C than at 25°C (F ♀ = 7.31, df = 1, 58, P = 0.0090; F ♂ = 10.20, df = 1, 58, P = 0.0023).
Among the ten females tested per temperature treatment, all laid eggs at 25°C and at 12°C, but none oviposited at temperatures from 4 to 10°C. Mean fecundity at 12°C (69.9 eggs per female) was significantly lower than that at 25°C (739.7 eggs per female) (Student’s t-test, t = 11.847, df = 4, P < 0.0001). Leaf consumption by adults increased significantly with increased temperature (F = 453.93, df = 4, 10, P < 0.0001). The mean leaf consumption of 132.19 mg per pair at 12°C was only one fourth of that at 25°C (Table 1).
Survival of A. hygrophila adults at low temperature
The survivorship curves of A. hygrophila female adults differed significantly between temperatures. The survival of females ranked from high to low at the following series of temperature: 12°C > 25°C > 10°C > 8°C, 6°C > 4°C (Kolmogorov–Smirnov test, n1 = n2 = 30, D12°C&25°C = 0.367, D25°C&10°C = 0.800, D10°C&8°C = 0.533, D10°C&6°C = 0.700, D6°C&4°C = 0.667, all > D0.05 = 0.351; D6°C&8°C = 0.167 < D0.05 = 0.351) (Fig. 1).
The survivorship curves of male adults also differed between temperatures. The survival of males ranked from high to low at the following series of temperatures: 12°C > 25°C > 10°C, 8°C > 6°C, 4°C (Kolmogorov–Smirnov test, n1 = n2 = 30, D12°C&25°C = 0.433, D12°C&10°C = 0.597, D25°C&8°C = 0.400, D8°C&6°C = 0.567, D8°C&4°C = 0.700, all > D0.05 = 0.351; D25°C&10°C = 0.333, D10°C&8°C = 0.200, D6°C &4°C = 0.133, all < D0.05 = 0.351) (Fig. 1).
Development of A. hygrophila eggs at low temperature
Both cold treatment temperature and period significantly affected egg hatching rate of A. hygrophila (two-way ANOVA; F temperature = 44.873, df = 4, P < 0.0001; F period = 609.481, df = 3, P < 0.0001). The interaction between cold treatment temperature and period also significantly affected egg hatching rate (F = 11.214, df = 12, P < 0.0001).
Agasicles hygrophila eggs had the capacity to stand relatively low temperatures for short durations. About one third of eggs hatched after cold treatment at 4°C for 7 days, with an average duration of 3.6 days excluding the cold treatment period. When cold treated at 4–12°C for certain period, egg hatching rate of A. hygrophila increased as temperature during the cold treatment increased (F 1 day = 64.735, df = 4, 10, P < 0.0001; F 4 days = 12.051, df = 4, 10, P = 0.0001; F 7 days = 34.447, df = 4,10, P < 0.0001). Egg hatching rate also increased as the cold treatment duration reduced (F 4°C = 36.167, df = 2, 6, P < 0.0001; F 6°C = 7.717, df = 2,6, P = 0.0022; F 8°C = 74.566, df = 2, 6, P < 0.0001; F 10°C = 38.986, df = 2, 6, P < 0.0001). Eggs pretreated at 12°C for 1–7 days and those kept constantly at 25°C did not differ in their hatching rates (Student’s t-test, t 1 day = 1.242, df = 4, P = 0.282; t 4 days = 1.478, df = 4, P = 0.213; t 7 days = 0.108, df = 4, P = 0.919) (Table 2).
Both cold treatment temperature and period significantly affected egg development duration of A. hygrophila (two-way ANOVA; F temperature = 16.406, df = 4, P < 0.0001; F period = 469.794, df = 2, P < 0.0001). The interaction between cold treatment temperature and period also significantly affected egg development duration (F = 6.671, df = 8, P < 0.0001).
Egg stages of A. hygrophila were significantly shortened when treated at 4–12°C for 4 and 7 days than those at constant 25°C (F 4 days = 35.63, df = 5, 48, P < 0.0001; F 7 days = 179.935, df = 5,48, P < 0.0001), and so did it at 8 or 12°C for 1 day than at constant 25°C (F 1 day = 7.14, df = 5,48, P < 0.0001). Duration of egg development decreased following extension of cold treatment at each of the low temperatures (F 4°C = 51.53, df = 2,24, P < 0.0001; F 6°C = 139.76, df = 2, 24, P < 0.0001; F 8°C = 223.91, df = 2, 24, P < 0.0001; F 10°C = 127.03, df = 2, 24, P < 0.0001; F 12°C = 153.16, df = 2,24, P < 0.0001) (Table 2).
Development of A. hygrophila larvae at low temperature
The first instar larvae of A. hygrophila could not survive temperatures from 4 to 12°C and all died within 1 day, whereas the mean duration of development at constant 25°C was 2.9 days (F longevity = 213.98, df = 5,162, P < 0.0001). At constant 25°C, the second and third instar larvae had a significantly higher survival rate (mean = 100%) than the first instar (mean = 92.7%) (F = 34.56, df = 2, 12, P < 0.0001).
Development of A. hygrophila pupae at low temperature
Cold treatment temperature did not affect pupal eclosion rate of A. hygrophila (two-way ANOVA; F = 0.496, df = 4, P = 0.739). But cold treatment period significantly affected pupal eclosion rate (F = 230.454, df = 3, P < 0.0001). There was no interaction between cold treatment temperature and period on pupal eclosion rate (F = 0.515, df = 12, P = 0.892).
Agasicles hygrophila pupae could stand relatively low temperatures for short durations. Eclosion rate of A. hygrophila pupae was 71, 60, 24 and 15% after cold treatment at 4–12°C for 1, 4, 7 and 10 days, respectively, which was significantly lower than that at constant 25°C (over 93%) (F 1 day = 3.96, df = 5, 12, P = 0.0235; F 4 day = 12.19, df = 5, 12, P < 0.0001; F 7 days = 40.52, df = 5, 12, P < 0.0001; F 10 days = 184.12, df = 5, 12, P < 0.0001). But it did not differ among the cold treatments at 4–12°C for certain period (F 1 day = 0.477, df = 4, 10, P = 0.752; F 4 days = 0.450, df = 4, 10, P = 0.770; F 7 days = 0.156, df = 4, 10, P = 0.956; F 10 days = 1.952, df = 4, 10, P = 0.178). Pupae eclosion rates were also significantly increased with an shortened cold treatment duration (F 4°C = 32.23, df = 3,8, P < 0.0001; F 6°C = 31.19, df = 3,8, P < 0.0001; F 8°C = 66.99, df = 3,8, P < 0.0001; F 10°C = 75.35, df = 3,8, P < 0.0001; F 12°C = 288.34, df = 3,8, P < 0.0001) (Table 3).
Both cold treatment temperature and period significantly affected pupal development duration of A. hygrophila (two-way ANOVA; F temperature = 592.380, df = 4, P < 0.0001; F period = 745.839, df = 3, P < 0.0001). The interaction between cold treatment temperature and period also significantly affected pupal development duration (F = 99.242, df = 12, P < 0.0001).
Pupal stages were significantly prolonged when treated at 4–12°C for 1 day than those at constant 25°C (F 1 day = 113.26, df = 5,424, P < 0.0001). After pre-treated at 4–12°C for 4 and 7 days, durations of pupal development decreased following the increased temperatures, which differed significantly with those at constant 25°C (F 4 days = 11171.57, df = 5, 356, P < 0.0001; F 7 days = 2314.71, df = 5, 205, P < 0.0001) (Table 3). Duration of pupal development decreased following extension of cold treatment at each of the low temperatures (F 4°C = 74.87, df = 3, 158, P < 0.0001; F 6°C = 74.44, df = 3, 160, P < 0.0001; F 8°C = 133.87, df = 3, 166, P < 0.0001; F 10°C = 448.76, df = 3, 163, P < 0.0001; F 12°C = 573.60, df = 3, 171, P < 0.0001) (Table 3).
Discussion
After the introduction of A. hygrophila into China in 1987 and release in Chongqing (Fig. 2A), Changsha (Fig. 2B), and Fuzhou (Fig. 2C) in 1988 (Wang et al. 1988), they were found fail to overwinter during 1988 to 1991 in Chongqing and Changsha, with average January temperatures of 5–11.1°C and 0.1–6.3°C, respectively (temperature data from http://cdc.cma.gov.cn/, air temperature in a Stephenson screen, same for the following temperature and isotherm description). Overwintering adults were found in Chongqing in 1992 after repeated releases for 4 years (Zhang et al. 1997). The results suggested that the tolerance of A. hygrophila to a range of low non-freezing temperatures could be achieved after naturalizing for some years.
Insect females may preserve biological potential by laying fewer eggs and increasing longevity when suffering unfavorable biotic and abiotic conditions, and thus, extending the chance to find and lay eggs at preferred conditions (Dernovici et al. 2006; Bale et al. 2002; Speight et al. 2008). This strategy is summarized as bet-hedging strategy when the insect is in the face of uncertainty about conditions during the breeding season (Stearns 1976). In the case of A. hygrophila, it was found that adult longevity was longer and eggs laid per female were less at 15°C than those at 20°C (Stewart et al. 1999a). Our results showed that the longevity of A. hygrophila adults was longer at 12°C than at 25°C, with a lower fecundity of about one tenth of that at 25°C, indicating that A. hygrophila adults had a potential to tolerate lower, normally unfavorable, temperature. We assume that when encountering a sudden cold wave in autumn or cool conditions of a late spring, A. hygrophila has the potential to prolong its lifespan into subsequent periods of favorable temperature. Females can lay some eggs to allow at least some surviving progeny, although the fecundity at these temperatures was much lower. Such a bet-hedging strategy of A. hygrophila adults under low temperatures appears to be important for its population maintenance and development.
The threshold temperature for the development of A. hygrophila eggs is 13.3°C (Stewart et al. 1996; Zhang et al. 1997). Our results showed that the egg stages of A. hygrophila (excluding cold treatment periods) were significantly shorter after exposure to temperatures at 4–12°C for 4 or 7 days, compared with those at constant 25°C. As Liu and Meng (1999) pointed out that development thresholds, produced by the linear regression technique or by nonlinear models, do not model the processes that control development at low temperatures. They are simply notional estimates of thresholds derived by extrapolation from development rates at moderate and high temperatures, and thus are not points with any intrinsic relationship to a true development threshold. Development rate at the notional threshold temperature, as defined by the linear model, is usually 8% of the maximum development rate at the temperature optimum for development (Lamb 1992; Liu and Meng 1999). We speculated that A. hygrophila eggs could develop under the low non-freezing temperature treatments at 4–12°C to some extent, which explained why the development of A. hygrophila eggs after the cold exposure was a little bit shorter than the untreated control. We need anatomical evidence to check whether the eggs did not develop at all or did not complete development to hatch at 12°C or lower. The threshold temperature for the developmental of A. hygrophila pupae is 12°C (Wu 1997). Results for the pupal development after short durations of low non-freezing temperature treatments are analogous to those for egg development.
Though the adults and eggs of A. hygrophila showed tolerance to low non-freezing temperature to some extent, the first instar larvae were very sensitive to low non-freezing temperatures. We conclude that when encountering adverse weather, such as late spring cold, the first instar larvae would not tolerate it and would die.
Stewart et al. (1999b) concluded that no viable eggs of A. hygrophila were laid at 15°C and larval survival was poor. Considering late spring cold is a common phenomenon in most provinces in southern China, such a cold periods may lead to lower reproduction, lower egg hatching rate and higher larval mortality of A. hygrophila, and thus may have impact on its population establishment and development and weaken the control efficacy of A. hygrophila against A. philoxeroides. The peak of the A. hygrophila population was observed to occur during late summer but the density of A. hygrophila was too low or the damage too late in the growing season to provide control. Therefore, A. hygrophila is unlikely to cause a reduction in alligator weed in New Zealand (Stewart et al. 2000).
Coulson (1977) reported that the most suitable area for the growth of A. hygrophila is the regions with the average January isotherms of 9–15.5°C, and the overwintering region of A. hygrophila in northern China is restricted to the area with the average January isotherm of 9°C. According to our recent field surveys, A. hygrophila is not only distributed in the South part of the region with the average January isotherm of 9°C, but also in areas with the average January isotherms of 4–9°C. Some populations even spread to the region with the average January isotherms of 0–4°C, such as Jingzhou and Wuhan, Hubei province and Chaohu, Anhui province (Fig. 2F, G, D) (Wu and Jin 1999; Huang 2007). Julien et al. (1995) predicted that the potential distribution area of A. hygrophila in China by using the CLIMEX System. Their prediction showed that A. hygrophila can establish field populations in Southeast coast of China, but not in Changsha, Hunan province. Actually, A. hygrophila has been observed in Changsha, Hunan province in January and February since 1993 (Li et al. 2000; Zhao et al. 2009) (Fig. 2B). Yang et al. (2002) also reported the naturalized population of A. hygrophila in Shanghai, where the average January temperature is 1.9°C (Fig. 2E). So the distribution of A. hygrophila in China is much wider than the prediction by Julien et al. (1995). This beetle has displayed encouraging control efficacy against A. philoxeroides in aquatic environments not only in southern China but also in some areas north of Yangtze River (Huang 2007). We speculated that the capacity of low non-freezing temperature tolerance of A. hygrophila may have improved after it has lived in these regions with low winter temperatures for some years, but this speculation warrants future investigation with a comparison of the temperature tolerance of native and introduced populations.
Our data showed that A. hygrophila has the capacity to stand relatively low non-freezing temperatures for short durations, and this capacity would help the insect to overwinter in some areas, especially in areas where protected cultivations are extensive because these areas may provide some microhabitats for the insect in which ambient temperatures are not as low as those in the open field. Its capacity to tolerate relatively low non-freezing temperature could assist the insect to survive and expand its distribution in the regions with relative low temperature. This may explain why its distribution in China expanded greatly after establishment for 20 years. In 2008, it encountered extremely cold conditions in southern China, such as a minimum temperature in Changsha, Hunan province of −1.9 to −5.2°C, which lasted for 27 days, and in Wuhan, Hubei province of 0 to −4.7°C for 32 days. Even after suffering such extreme weather conditions, overwintered A. hygrophila adults were found in Changsha in May, 2009 (authors’ observation, unpublished data), showing a relatively high capacity of low non-freezing temperature tolerance of the beetle. On the other hand, microhabitat that the insect actually lives in can buffer changes in the general air temperature (Jones 1992). Microclimate reflects interactions between ambient environmental characteristics (e.g. air temperature, solar radiation, wind speed) and leaf morphology and physiology (e.g. leaf size, color, pubescence, transpiration) (Potter et al. 2009). So even though a location suffered extreme cold snap, the microhabitats A. hygrophila hides in provided better conditions to survive. Further work should be carried out to develop a sub-model that relates microclimate temperature to microhabitat as has been done for other insects (Potter et al. 2009).
Predicting the climatic limits of an insect is critical in biological control programs. Insects can develop a gradual change in climate tolerance to expand its range. For instance, critical daylengths for diapause induction of the leaf beetle, Diorhabda elongata Brullé deserticola Chen, a classical biological control agent for Tamarix spp., differ among different populations. D. elongata populations collected from Fukang (China) and Chilik (Kazakhstan) could not overwinter at sites south of the 38°N latitude in the western United States where summer daylengths are below the critical photoperiod. Other populations collected from more southern latitudes, e.g. Turpan (China), Tunisia, Crete, and, Uzbekistan, have shorter critical photoperiods for diapause induction and are promising for control at more southern latitudes in North America (Bean et al. 2007; DeLoach et al. 2004). Alligator weed occurs across middle and southern China, including Hunan, Hubei, Sichuan, Chongqing, Fujian provinces and autonomous regions, etc. (Fig. 2, shading part), and its infestations are more serious along the middle and lower reaches of Yangtze River (Zhang et al. 1993; Wan et al. 2005). The potential of A. hygrophila to expand further north has been restricted by climatic conditions unfavorable for brood development. Our survey indicated that the distribution A. hygrophila achieved further north after naturalizing for many years was much broader than the predicted range by Coulson (1977). The Intergovernmental Panel on Climate Change (IPCC) assessed that the global mean temperature is projected to increase by 1.4–5.8°C over the period 1990–2100, in the absence of policies to limit climate change, and that the frequency of extreme weather conditions will also increase (Houghton et al. 2001). From our current experiments and survey, we predict that the distribution of A. hygrophila will extend further north under global warming because the increased temperature will allow a better overwintering of the beetle, which will make it an effective biological control agent for alligator weed further north. Further research should be focused on the development and reproduction of A. hygrophila in response to elevated high temperature.
References
Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK, Butterfield J, Buse A, Coulson JC, Farrar J, Good JEG, Harrington R, Hartley S, Jones TH, Lindroth RL, Press MC, Symrnioudis I, Watt AD, Whittaker JB (2002) Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob Change Biol 8:1–16
Bean DW, Dudley TL, Keller JC (2007) Seasonal timing of diapause induction limits the effective range of Diorhabda elongata deserticola (Coleoptera: Chrysomelidae) as a biological control agent for Tamarisk (Tamarix spp.). Environ Entomol 36(1):15–25
Buckingham GR (1996) Biological control of alligatorweed, Alternanthera philoxeroides, the world’s first aquatic weed success story. Castanea 61(3):232–243
Coulson JR (1977) Biological control of alligatorweed, 1959–1972. A review and evaluation. Technical Bulletin, Agricultural Research Service, United States Department of Agriculture 1547, pp 98–100
DeLoach CJ, Carruthers RI, Dudley TL, Eberts D, Kazmer DJ, Knutson AE, Bean DW, Knight J, Lewis PA, Milbrath LR, Tracy JL, Tomic-Carruthers N, Herr JC, Abbott G, Prestwich S, Harru VG, Everitt JH, Thompson DC, Mityaev I, Jashenko R, Li B, Sobhian R, Kirk A, Robbins TO, Delfosse ES (2004) First results for control of saltcedar (Tamarix spp.) in the open Weld in the Western United States. In: Proceedings of the XI international symposium on biological control weeds, 27 April–2 May 2003, Canberra, Australia. CSIRO Entomology, Australia, pp 505–513
Dernovici SA, Teshler MP, Watson AK (2006) Is sunflower (Helianthus annuus) at risk to damage from Ophraella communa, a natural enemy of common ragweed (Ambrosia artemisiifolia)? Biocontrol Sci Technol 16(7):669–686
Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linden PJ, Xiaosu D (2001) Climate change 2001: the scientific basis. Cambridge University Press, Cambridge
Hruska AJ, Gladstone SM, Wilson KG (1985) Expanded range of the alligatorweed flea beetle (Agasicles hygrophila Selman and Vogt) in South Carolina. J Aquat Plant Manag 23:92–93
Huang WX (2007) Research on the over-winter protection technology and evaluation of control effect of Agasicles hygrophila against alligator weed, Alternanthera philoxeroides. Agro Environ Dev (2):61–62
Jones GH (1992) Plants and microclimate: a quantitative approach to environmental plant physiology. Cambridge University Press, Cambridge
Julien MH, Chan RR (1992) Biological control of alligator weed: unsuccessful attempts to control terrestrial growth using the flea beetle Disonycha argentinensis (Col., Chrysomelidae). Entomophaga 37(2):215–221
Julien MH, Broadbent JE, Harley KLS (1979) The current status of biological control of Alternanthera philoxeroides in Australia. In: Proceedings of the 7th Asian-Pacific weed science society conference, Sydney, Australia, pp 231–235
Julien MH, Skarratt B, Maywald GF (1995) Potential geographical distribution of alligator weed and its biological control by Agasicles hygrophila. J Aquat Plant Manag 33:55–60
Lamb RJ (1992) Developmental rate of Acyrthosiphon pisum (Homoptera: Aphididae) at low temperatures: implications for estimating rate parameters for insects. Environ Entomol 21:10–19
Li HK, Wang R (1994) Biological control of alligatorweed Alternanthera philoxeroides, in central China by inoculative releases of Agasicles hygrophila with artificical overwintering protection. Chin J Biol Control 10(1):11–14
Li HK, Li M, Li D (2000) Alligator weed and its biological control. Word Agric 2:36
Liu SS, Meng XD (1999) Modelling development time of Myzus persicae (Hemiptera: Aphididae) at constant and natural temperatures. Bull Entomol Res 89:53–63
Ma RY, Ding JQ, Li BT, Wu ZQ, Wang R (2003) The pupation adaptability of Agasicles hygrophila on different ecotypes alligatorweed. Chin J Biol Control 19(2):54–58
Maddox DM, Rhyne M (1975) Effects of induced host-plant mineral deficiencies on attraction, feeding and fecundity of the alligator weed flea beetle. Environ Entomol 4(5):682–686
Potter K, Davidowitz G, Woods HA (2009) Insect eggs protected from high temperatures by limited homeothermy of plant leaves. J Exp Biol 212:3448–3454
Pyke DA, Thompson JN (1986) Statistical analysis of survival and removal experiments. Ecology 67:240–245
SAS Institute Inc. (1996) SAS/STAT software: changes and enhancements through release 6.12. SAS Institute Inc, Cary
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. W.H. Freeman and Company, New York
Sosa AJ, Julien MH, Cordo HA (2004) New research on Alternanthera philoxeroides (alligator weed) in its South American native range. In: Proceedings of the XI international symposium on biological control of weeds, pp 180–185
Speight MR, Hunter MD, Watt AD (2008) Ecology of insect: concepts and applications, 2nd edn. Watt Wiley-Blackwell, Hoboken, p 37
Spencer NR, Coulson JR (1975) The biological control of alligator weed. In: Proceedings of a symposium on water quality and management through biological control, Gainesville, pp 36–43
Spencer NR, Coulson JR (1976) The biological control of alligatorweed, Alternanthera philoxeroides, in the United States of America. Aquat Bot 2(3):177–190
Stearns SC (1976) Life-history tactics—a review of the ideas. Q Rev Biol 51:3–47
Stewart CA, Emberson RM, Syrett P (1996) Temperature effects on the alligator weed flea-beetle, Agasicles hygrophila (Coleoptera: Chrysomelidae): implications for biological control in New Zealand. In: Proceedings of the 9th international symposium on biological control of weeds, pp 393–398
Stewart CA, Chapman RB, Barrington AM, Frampton CMA (1999a) Influence of temperature on adult longevity, oviposition and fertility of Agasicles hygrophila Selman & Vogt (Coleoptera: Chrysomelidae). NZ J Zool 26(3):191–197
Stewart CA, Chapman RB, Emberson RM, Syrett P, Frampton CMA (1999b) The effect of temperature on the development and survival of Agasicles hygrophila Selman & Vogt (Coleoptera: Chrysomelidae), a biological control agent for alligator weed (Alternanthera philoxeroides). NZ J Zool 26(1):11–20
Stewart CA, Chapman RB, Frampton CMA (2000) Growth of alligator weed (Alternanthera philoxeroides (Mart.) Griseb. (Amaranthaceae)) and population development of Agasicles hygrophila Selman & Vogt (Coleoptera: Chrysomelidae) in northern New Zealand. Plant Prot Q 15(3):95–101
Wan FH, Zheng XB, Guo JY (2005) Biology and management of invasive alien species in agriculture and forestry. Science Press, Beijing, pp 715–739
Wang R, Wang Y, Zhang GC, Li JX (1988) Host specificity tests for Agasicles hygrophila. Chin J Biol Control 4(1):14–17
Wu ZQ (1997) Effect of temperature on the development of Agasicles hygrophila. Chin J Appl Ecol 8(2):181–184
Wu DC, Jin XL (1999) Occurrence and biological characteristics of Agasicles hygrophila in Jingzhou, Wuhan Province. Hubei Plant Prot (6):4–5
Yang ZH, He CJ, Qian DM (2002) A preliminary observation for population dynamics of Agasicles hygropila Selman et Vogt and its control effect on Alligator altenanthera in the suburb of Shanghai. Acta Agric Shanghai 18(4):79–83
Ye WH, Li J, Cao HL, Ge XJ (2003) Genetic uniformity of Alternanthera philoxeroides in South China. Weed Res 43:297–302
Zhang GC, Li JX, Chen XH (1993) Biology of Alternanthera philoxeroides. J Weed Sci (2):10–12
Zhang GC, Li JX, Chen XH (1997) Applications of Agasicles hygropila to control alligator weed (Alternanthera philoxeroides (Mart.)). China South Fruit 26(5):47–49
Zhao X, Fu JW, Wan FH, Guo JY, Wang JJ (2009) Effects of brief high temperature expose on reproductive character of Agasicles hygropila. Acta Entomol Sin 52(8):867–874
Acknowledgments
We thank Mr. Yan-Ning Li and Prof. Yuan-Hua Luo (Institute of Plant Protection, Hunan Academy of Agricultural Sciences) for their help during the experiment; Prof. Shu-Sheng Liu (Zhejiang University, Hangzhou, China), Dr. Gang Wu (Huazhong Agricultural University, Wuhan, China), Prof. L.A.P. (Bert) Lotz (Plant Research International B.V., Wageningen UR, Netherlands) and Prof. Dan Johnson (Lethbridge University, Alberta, Canada) for suggestions and linguistic revision of the manuscript. This work was funded by the National Basic Research and Development Program of China (grant No. 2009CB119200); and the National Key Technologies Research and Development Program of China (grant No. 2006BAD08A18).
Author information
Authors and Affiliations
Corresponding author
Additional information
The first authors Jian-Ying Guo and Jian-Wei Fu, contributed equally to this article.
Rights and permissions
About this article
Cite this article
Guo, JY., Fu, JW., Xian, XQ. et al. Performance of Agasicles hygrophila (Coleoptera: Chrysomelidae), a biological control agent of invasive alligator weed, at low non-freezing temperatures. Biol Invasions 14, 1597–1608 (2012). https://doi.org/10.1007/s10530-010-9932-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-010-9932-3