Abstract
Adult white-spotted flower chafers (WSFC), Protaetia brevitarsis, feed on developing sweet corn ears in China causing extensive losses in quality and quantity. Considering these losses, the lack of natural enemies caused by excessive past chemical pesticide use and a complete lack of present insecticides for acceptable use against WSFC on developing sweet corn, it is important to find novel measures for controlling this serious pest. The attractiveness of eight compounds, mainly phenolic compounds, fruit esters, acetoin, and some aliphatic alcohols, was established in a specially designed olfactometer. Subsequently, rubber dispensers containing 40-mg of those attractive compounds were hung in field plots on 12 sweet corn ears on the borders and down the center of 11 × 9.6-m plots comprised of 615 plants. One day later, the same ears were sprayed with Malathion. These attract-and-kill plots were maintained in 2006, 2007, and 2008, and provided substantial protection of non-treated ears in the same field. The mean damage value (DV from 0: none to 3: >5% eaten ears) and percent of damaged ears in the treated fields (DV: 0.8 ± 0.3, 0.8 ± 0.3, and 0.7 ± 0.3; % damage: 19.4, 19.4, and 19.0%) was significantly lower than that in control plots (DV: 1.8 ± 0.2, 2.0 ± 0.1, and 1.7 ± 0.1; % damage: 92.1, 89.7, and 95.0%) for 2006–2008. This attract-and-kill method gives sweet corn growers a method to protect their crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Corn is one of China’s most important crops, covering an area of about 2.38 × 107 hectares, representing 20% of the total cropped area. The main varieties are field corn (93%), sweet corn (5.1%), and other (1.9%).
The white-spotted flower chafer (WSFC) Protaetia brevitarsis, also published as Potosia brevitarsis or brevitaris (Coleoptera: Scarabaeidae: Cetoniinae) is a native species in China and is distributed all over the country, especially in the Xinjiang district (Zhang and Wang 1955; Forest Health Technology Enterprise Team 2004; Australian Government 2004; Chen et al. 2006, 2010; Chen and Zhao 2008). In addition, it is found on the Korea Peninsula, and in Japan and Thailand (Park et al. 1994; Forest Health Technology Enterprise Team 2004; Kim et al. 2008a, b).
Within the last decade, feeding by WSFC caused extensive damage, especially on developing corn ears, that has led to a ring-like formation of a thick scar tissue layer on the ear surface. This occurred at the top of the ear because the pest prefers to feed there in sheltered areas where the bracts touch the corn kernels. In serious cases, the scar extended over half the ear (Park et al. 1994; Australian Government 2004). This damage reduced kernel mass (~5% productivity loss on average) in field corn, and the marketable value in sweet corn. Chen et al. (2006) and Chen and Zhao (2008) found that ~10% of sweet corn could not be sold because of the presence of WSFC, or reduced ear quality caused by WSFC, and noted that such losses are critical to farmers. In addition, the beetles can infect corn with rot diseases, significantly damaging the quality and quantity of corn.
Because of a lack of natural enemies of WSFC, due to excessive insecticide use in the past, and no reliable control methods (biological, cultural, mechanical, or chemical), WSFC population have increased many fold within the last three decades, causing a critical problem to corn growers in China (Chen et al. 2006; Chen and Zhao 2008). Although synthetic insecticides were used against WSFC on vegetables, and in other situations, in the past, they can no longer be applied to developing sweet corn ears. Government regulations on “Standards for the safe application of pesticides” and “Regulations on the control of agricultural chemicals” promulgated by Decree No. 216 of the State Council of the People's Republic of China (May 8, 1997), outlawed the use of over 200 chemical pesticides for vegetables. Although Malathion, and a few other chemicals, can still be used on vegetables, the interval of time between needed applications to harvest is too short for effective WSFC control on sweet corn ears. Therefore, it is necessary to establish a non-pesticide, or non-traditional, solution for the WSFC problem.
For this purpose, an attract-and-kill, environment friendly, pest control method was developed. Treated sweet corn ears (with attractant rubber dispenser and insecticide) diverted WSFC beetles from non-treated corn ears in field plots. The actual efficacy of the attract-and-kill method depends largely on the attraction capability of the chemical dispenser. Therefore, it was necessary to select the most attractive synthetic compounds to WSFC to get the best results from this method.
A series of synthetic compounds have been shown to be attractants for scarabs in China (Li et al. 1995; Meng et al. 1999; Chen et al. 2001), and others have been reported as attractive to WSFC (Hao et al. 2005a, b; Wang and Zhang 2005, 2008). Wang and Zhang (2005) noted that ethyl acetate, phenol, hexylenic aldehyde, and butyl acetate appeared attractive to WSFC in preliminary field tests. In other preliminary tests, Hao et al. (2005a) reported methanol, ethanol, and propanol as potential attractants. Wang and Zhang (2008) and Hao et al. (2005b) suggested that methyl benzoate, ethyl benzoate, phenol, anethole, and diphenyl ketone, may be involved in WSFC sex attraction. Information on volatiles from WSFC hosts is also available (Vallat et al. 2005; Robertson et al. 1995; Matich et al. 2003; He et al. 2005). Potential scarab attractants in general, and those for Cetoniinae like the WSFC in particular (Chen et al. 2006; Chen and Zhao 2008), can be grouped into: (1) “flowery” or “fruity” compounds like aromatics and esters; (2) simple esters commonly found in flower or fruit aromas, but also in other plant tissues; (3) simple carboxylic acids commonly found as microbial degradation or fermentation products; and (4) certain six-carbon alcohols, aldehydes and their derivatives, commonly known as “green leaf volatiles”, which are released from all plant tissues as by-products of plant metabolism. Geraniol and anethole, often along with eugenol, are attractive to several Cetoniinae in Europe (Toth et al. 2003, 2004, 2009; Vuts et al. 2010a, b) the USA (Cherry et al. 1996; Crocker et al. 1999), and China (Li et al. 1995; Chen et al. 2001). In addition, Toth et al. (1994), Tolasch et al. (2003), Ruther (2004), and Ruther and Mayer (2005) and have reported leaf alcohols, plant volatiles and acetoin as attractants for Anomala spp., Phyllopertha horticola, Melolontha spp., and Amphimallon solstitiale in Europe.
From the above information, we selected and assessed the attraction of 20 synthetic compounds to mature WSFC in an olfactometer using a blank as a control. Subsequently, during 2006, 2007, and 2008, using the most attractive screened synthetic compounds, we evaluated the attract-and-kill method in a cornfield to suppress WSFC populations and to protect the non-treated ears.
Materials and methods
Olfactometer experiment
Experimental insects
Adult chafers were obtained by shaking the host crop plants or trees during the night and collecting the fallen beetles. Collected adults were kept in 45 × 20 cm Plexiglas cages with a 15 cm bottom layer of soft soil (treated at 150°C), under controlled environmental conditions (temperature 25 ± 1°C, 65 ± 5% relative humidity (RH), 12 h light/12 h dark). After ~15 days, the soil was examined for eggs and larvae. Larvae were fed for about 280 days on organic soil from the forest (Chen and Zhao 2008). As soon as pupae appeared, they were removed from the soil on a daily basis, placed in Petri plates containing moistened vermiculite and kept under the conditions described above. Each Petri plate contained ~15 pupae and was checked twice daily for adult eclosion which occurred after about 1 month. Five- to ten-day-old WSFC, which had fed on fresh immature corn ears daily, were used for behavioral tests. Preliminary tests indicated that newly emerged adult WSFC can survive for at least 1 week without any food, and during that week, no change in the attraction of unfed beetles to immature sweet corn or a specific lure was observed. For the olfactometer tests, beetles were starved for 2 days prior to experiments. The number of beetles “tested” (= put in the central chamber) are listed in Table 1 for each of the compounds.
Olfactometer apparatus
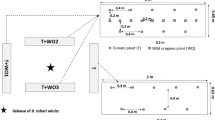
The olfactometer, or wind tunnel, was designed to assess the response of mature WSFC to various chemical stimuli upwind (Fig. 1), and had a central chamber consisting of a transparent plastic box (140 × 80 × 35 cm) covered with a white opaque lid. Two exit tubes (inner diameter 10 cm, length 12 cm) were made of an opaque plastic material, and protruded 3 cm into the box through the short side, thus connecting the central chamber with collection containers, which had either a test attractant or the empty control. WSFC adults placed into the central chamber could take short flights of 0.3–0.5 m to reach the test attractant through the exit tubes. To facilitate this movement, a 2 cm styrofoam sheet covered the bottom of the box and raised the floor to the level of the exit tubes. The styrofoam was covered with a protective paper, lined with plastic foil on the bottom, and held tightly by adhesive tape. The protective paper was changed after each compound to avoid contamination. The collection jars were screwed into the plastic box and were easily removed and switched to avoid a position effect.
Schematic drawing of two-choice olfactometer used for behavioral tests (not drawn to scale). Air was evacuated through the exhaust chamber, creating an air stream through the empty control, and odorized chambers into the central chamber. White-spotted flower chafer adults were placed in the central chamber and could exit through either of two tubes leading to the odorized/collection chambers
The olfactometer had airflow (30 cm s−1) through holes, to the collection jars and into the central chamber. Each entrance hole was covered with a 3 cm thick charcoal filter sheet. The wind tunnel was evacuated by an exhaust fan inside the exhaust chamber, and was operated in a climate chamber at 28°C, 65 ± 1–5% RH, and with illumination from two greenhouse lamps (36 W, >1 kHz, Philips).
Olfactometer experiments
Experiments were conducted between 8 AM and 4 PM during April, May, and June, 2005 and 2006. Small groups of beetles were placed into the central chamber to reduce interactions between adults, and allowed to acclimate for several minutes before the lure was placed in a collection jar.
Either three or four beetles were used in each replicate (91% cases), and in the other cases 2 or 3, or 4 or 5 according to the availability of the chafers. Nothing was placed in the control jar, and the exhaust was activated just after the lures were added. Adults were allowed to walk freely in the central chamber and exit through either of the two tubes during replicates. Compounds were tested in random order, and each treatment was replicated eight times. The number of beetles in the collection jars was noted after 15 min. All beetles were removed from the system before the next replicate was started. The positions of the collection jars were switched after every two replicates (30 min) to avoid position effects.
Test stimuli
Initial evaluations were performed on developing sweet corn ears, weighing approximately 300 g each. The fresh ears were put into one of the collection jars, and were invisible from the central chamber, while the control jar was empty. Once the validity of the system was demonstrated by movement to the know attractant (sweet corn ears), the 20 synthetic compounds (Table 1) were tested separately against a blank control. These compounds represented Chinese scarab attractants (Li et al. 1995; Meng et al. 1999; Chen et al. 2001: Hao et al. 2005a, b; Wang and Zhang 2005, 2008), WSFC host volatiles (Vallat et al. 2005; Robertson et al. 1995; Matich et al. 2003; He et al. 2005), or were among the most attractive lures to the Japanese beetle (McGovern and Ladd 1984).
Cotton dental wicks were loaded with 10 mg of a test compounds dissolved in 100 µl of solvent. We used 100 ml of hexane or acetone as a solvent, depending on chemical solubility (H or A in Table 1). The cotton wick absorbed the solutions, and 3–5 min were allowed for the solvent to evaporate before testing. This worked well for 17 of the compounds, but the evaporation of the other materials was too high and resulted in complete dispersal before the end of the test. To slow down evaporation the wick was placed in an open glass tube for, cinnamyl alcohol, methyl salicylate, and phenyl ethanol.
Field trials
Attractant dispenser preparation
Eight synthetic compounds which had a higher capture than the sweet corn ear in our olfactometer assay: geraniol, citronellol, propenol, methyl salicylate, acetoin, benzyl alcohol, butanol, and benzyl carbinol were purchased from Sigma Inc., Aldrich Inc. or Fluka Inc. The purity of the samples was >96% by GC. A dispenser was prepared using a piece of rubber tubing (1.5 cm o.d. × 2 cm, with a wall thickness of 1.5 mm) which was put into boiling 98% ethanol for 10 min (three times), and then into 98% methylene chloride overnight (three times). To make the dispensers, 40 mg of a selected compound, in a hexane solution, was placed on the surface of the rubber dispenser. After the solvent evaporated, dispensers were wrapped separately with pieces of aluminum foil and were stored at −10°C until use as described by Donaldson et al. (1986).
Treated corn ears
The attractant dispensers were attached with a thread to the top end of each treated ear. One day later, 15 ml of a Malathion solution—3 µg/µl (Dow Agro Sciences, Indianapolis, IN USA) were sprayed onto the same developing corn ears. Malathion has been used as a chemical control for WSFC and other insects in China (Wei et al. 1989; Wu et al. 2004) and was chosen based on its previous usage. To determine the most suitable time for these applications, three basic factors were taken into consideration: (a) large numbers of adult WSFC, (b) the time of the kernel formation, and (c) the absence of rain. The most crucial period of damage to corn is the first 15–29 days after kernel formation. The first application of the insecticide and the attractant dispensers was during the time of massive adult appearance that coincides with kernel formation (10–11 July 2006, 5–6 July 2007, and 12–13 July 2008). The second application was approximately 2 weeks later (24–25 July 2006, 19–20 July 2007, and 26–27 July 2008).
Field tests
A 3-year experiment (2006–2008) was carried out in a sweet corn field of ~20 ha at the Jilin Agricultural University Experimental Station (The northeast plain area of China). Sweet corn had been grown in this field for many years for research purposes, and adult WSFC were very numerous. Adjacent farms producing chickens and deer may have provided near-by, high organic matter ideal for the development of WSFC larvae.
The experiment was designed with a standard of randomness and complete blindness, with the eight attractants listed at “Attractant dispenser preparation” section (treatments) and one control without an attractant. Each treatment was replicated three times and was associated with three non-adjacent control plots. The layout of ears in the treatment plots is shown in Fig. 2. In contrast, there were neither insecticides nor attractants in control plots. The plot size was 11.0 × 9.6 m and contained 13 rows. Each row had 53 sweet corn plants (variety “Titian 6”). Adjacent plots were separated by non-planted buffer areas (4.0 × 11.0 m or 4.0 × 9.6 m) to diminish interactions between treatments. The distance between treated crops was approximately 3.2–5.5 m.
Field observations
The damage to ears from by WSFC was assessed in late September 2006, 2007, and 2008, by randomly selecting 100 corn ears from a box containing treated or non-treated ears from each attractant plot, and 100 ears from the control plots. The damage values for each group were assessed using the following damage criterion: 0 (no damage), 1 (up to 1% kernels eaten), 2 (2–6% eaten), and 3 (6–10% eaten). The percent damage was the number of ears with any damage out of the 100 randomly selected. Protection values were based on the percent damage in the control minus the percent damage in the treatments.
Data analysis
Since the olfactometer experiments showed no sexual dimorphism in odor preference (data not presented) here or in previous studies (Chen et al. 2006), the male and female numbers were pooled for analyses. Each treatment was replicated eight times. A Wilcoxon sign test was used as a non-parametric measure of preference for the odorant versus the blank control.
Results
Olfactometer
Out of 543 beetles used, 314 entered the collection chambers and were utilized for statistical comparisons. The numbers of beetles tested for each compound are shown in Table 1. Initial results with the olfactometer showed that the developing sweet corn ear attracted 22 of the 33 beetles released, thus validating the use of this instrument to test attractants. Without adjustment for different release rates, the stimuli had significant differences in attraction (Table 1), as indicated by the percent of beetles choosing the lure. Eight out of the 19 synthetic compounds were more attractive than the sweet corn ear in our olfactometer assay. The Wilcoxon sign test found 7 of the compounds and the corn ears had significant attraction (Table 1). The attraction of benzyl carbinol was not significant. There was significant avoidance of the jars with propanol and phenyl ethanol based on the Wilcoxon sign tests.
Control in field trials
Significant differences (P < 0.05) were found between the mean damage values for non-treated sweet corn ears in the treatment groups and for ears in the controls for the 3 year totals (Table 2). The reduced damage value and improved protection of non-treated ears in the treatment group from the applied synthetic compounds varied year-to-year, though their effects were all statistically significant. In 2007, significantly lower effectiveness of the lure and kill method was observed, which coincided with unexpected bad weather conditions (heavy rain, low night temperatures, and strong winds) during the fruiting period. These factors negatively affected the population densities. According to previous observations, treatments should have been done 3 weeks after the sweet corn kernels begin to form (Chen et al. 2006; Chen and Zhao 2008). However, during this period in 2007, very strong winds and heavy rains took place, and postponed the applications. Therefore, the WSFC beetles had already caused serious damage to the developing sweet corn before the compounds were applied. The ratios between the mean damage value for treated sweet corn ears and that for non-treated ears was 2.6:1, 2.5:1, and 3:1 for 2006, 2007, and 2008, respectively, thus demonstrating the beetles were going to, and staying on, or being killed by the treated ears.
Discussion
The most important factor in getting positive results from an olfactometer is the choice biological extracts or synthetic compounds as attractants. Polyphagous species would be expected to feed opportunistically on available leaves, fruits or flowers, utilizing alcohols and other odors commonly found in many plant parts (Donaldson et al. 1990; Lin and Phelan 1991; Phelan and Lin 1991; Reinecke et al. 2002; Ruther and Hilker 2003; Ruther 2004; Ruther and Mayer 2005). Chen et al. (2006) previously identified a wide selection of locally available fruit which could produce numerous volatiles attractive to WSFC. In addition, several compounds were tentatively identified as WSFC attractants (Hao et al. 2005a). The attractive compounds from the references above, and our observations, resulted in the immature sweet corn ears and 20 synthetic compounds utilized here. The olfactometer tests showed that eight chemicals not previously known to be attractive to WSFC caused more upwind movement to containers than did the blank controls. The attractive compounds are phenylic and hydroxylic compounds, including several short chain aliphatic alcohols, and some phenylic fruit alcohols and terpenes. The only exception is acetoin. This matches the profile of potential WSFC attractants proposed by Chen et al. (2006) and Chen and Zhao (2008). Five compounds previously indicated as WSFC attractants (Wang and Zhang 2005, 2008; Hao et al. 2005a, b) did no better than the controls. This could be due to differences in conditions between the tests, or doses which did not trigger behavioral responses under our conditions.
Of these eight synthetic compounds, geraniol was only one of the Japanese beetle attractants to show activity. The importance of combinations of chemicals as scarab attractants throughout the world cannot be overlooked (Ladd et al. 1981, Klein and Edwards 1989; Reed et al. 1991; Leal 1998; Ruther 2004; Ruther and Mayer 2005). It is possible that combinations of attractants would also prove superior for WSFC. This avenue of research will be examined in the future.
Although there was an overlap of attractive substances between WSFC and other scarabs, the degree of the attractiveness was different. For example, methyl salicylate was highly attractive to WSFC, but exhibited low attraction to the Japanese beetle (Langford et al., 1943). However, it is part of the lure combination for green June beetle, Cotinus nitida, in the USA (Loppez et al. 2002) and Cetonia aurota aurota and Potosia cuprea in Hungary (Vuts et al. 2010a, b). In addition, Landolt (1990) found isopropanol to be highly attractive to the green June beetle, but here, propenol was the most attractive alcohol. Among the attractive compounds, acetoin is a novel scarab kairomone, although it was identified as the pheromone for Amphimallon solstitiale (Tolasch et al. 2003) and a component of male released aggregation pheromones of two dynastine scarab beetles (Rochat et al. 2000a, b).
No sexual or behavioral attraction between beetles was observed here or in previous experiments with WSFC (Chen et al. 2006). With this olfactometer, WSFC were removed from the collection jars during each trial to minimize any interaction between beetles. Concerns about erratic and random escape attempts, leading to high control captures (Syed and Guerin 2004; Chen et al. 2006) were circumvented by forcing beetles walk through a tube with lower illumination than the central chamber. Since beetles were not counted until they were in the collection jars, we were sure they were drawn by attractants, not light, and random control encounters were suppressed.
Field observations indicated the number of WSFC on lure treated ears was much greater than on non-treated ears. Using specific key compounds to locate food appears to be a strategy of oligophagous insects when characteristic attractants have been identified (Renwick 1989). Since WSFC probably utilize a similar strategy to find food, their preference for, and extensive feeding on treated ears was probably due to the attractant dispenser. The results from the field were fairly consistent with order of attraction from the olfactometer except that geraniol and benzyl carbinol switched places. Thus, using benzyl carbinol or propenol as attractants to protect non-treated ears in the same field gives growers a tool to use, and reduces insecticide usage about 99% over that of conventional insecticide sprays that were previously available.
The efficacy of this control method would be negatively affected by attraction of WSFC to early arriving beetles on non-treated ears (Chen et al. 2006). Although, no interactions between WSFC were observed in the olfactometer, feeding by early arriving beetles could change this dynamic. The interactions within WSFC populations are not well understood, but effective control of WSFC in an actual cornfield was possible.
The low efficacy with acetoin or butanol treated ears suggests that only compounds with the highest attraction are effective under field conditions. The four best attractants from the olfactometer provided very good results, with mean protection values between 78 and 86.8%.
In an olfactometer, mature WSFC exhibited very limited dispersal and would aggregate on an immature sweet corn ear with an attractant for a considerable time (Chen et al. 2006). This showed that treated ears could arrest the movement of WSFC after an initial contact. Thus, relatively long durations of feeding on treated ears, and high death rates, would be expected if the numbers of ear kernels are adequate and the insecticide is poisonous enough. It may be that WSFC moving to attractive ears, and an increase in attraction with the release of corn volatiles, is the major factor in the success of this technique. Overall, the damage values showed that treated ears had a significantly negative effect on WSFC populations, although we do not know where the poisoned adults went. Another possible limitation on the efficacy of this control method in the field would be the dispersal of mature WSFC following their aggregation on treated ears. However, we saw no indication that the mass occurrence of WSFC might cause competition and higher migration of beetles to the neighboring non-treated ears.
Protection with an insecticide other than Malathion may be more effective due to the relatively short field life of the insecticide (a few days). The possible low level of control with Malathion suggests that the attract-and-kill scheme may be even more effective with a more potent, long-lasting, insecticide. However, despite this limitation, the mean damage value and damage percentage of non-treated corn in the treatment group was significantly lower than in the control group or treated ears. Clearly, our attract-and-kill method resulted in reduced damage and good protection to the sweet corn that is not available from any other technique.
These results demonstrate that propenol or benzyl carbinol could be used now, and will also serve as a basis for future development of attract-and-kill methods. Since the synthetic compounds and insecticide used in this study are not specific for WSFC, additional work will be needed to evaluate the potential negative impact on beneficials and other non-target insects, the manner in which these synthetic compounds travel, combinations of chemical attractants, longer lasting insecticides, and optimum timing for application. In particular, a better understanding of how far a volatile cloud extends under variable field conditions, how long it persists and the shape of its decay with distance will be key issue in the continued development of this technology.
References
Australian Government Department of Agriculture, Fishers and Forestry (2004) Longan and lychee fruit from the People's Republic of China and Thailand. Final Import Risk Analysis Report Part A
Chen R-z, Zhao R-G (2008) General characteristics of White-spotted flower chafer, Protaetia brevitaris in China. China Plant Protect 28:10–20
Chen S-B, Yang H-W, Cui J-Y, Klein MG, Deng C-S, Zhang Y (2001) Preliminary observations on trapping anthophaous scarab beetle with attractant. Chin J Biol Control 17:143–145
Chen R-z, He K-l, Yin J (2006) Preliminary study on propensity of Potosia brevitaris Lewis and its behavior law in endangering maize by formicating. J Jilin Agric Univ 28:240–243
Chen R-z, Shao D-x, Zhang Yu, Gao Z-x, Chang T-n, Li yu (2010) Evaluation of the role of sweet corn plant as cropping trap against Potosia brevitaris for common corn. J Jilin Agric Univ 32:622–625
Cherry RH, Klein MG, Leal WS (1996) Attraction of adult Anomala marginata (Coleoptera: Scarabaeidae) to anethole. J Agric Entomol 13:359–364
Crocker RL, Klein MG, Wei X, Jailon WT (1999) Attraction of Phyllophaga congrua, P. crassissima, P. crinite, and Cyclocephala lurida (Coleoptera: Scarabaeidae) adults to selected volatile compounds. SW Entomol 24:315–319
Donaldson JMI, McGovern TP, Ladd TL (1986) Trapping techniques and attractants for Cetoniinae and Rutelinae (Coleoptera: Scarabaeidae). J Econ Entomol 79:374–377
Donaldson JMI, McGovern TP, Ladd TL (1990) Floral attractants for Cetoniinae and Rutelinae (Coleoptera: Scarabaeidae). J Econ Entomol 83:1298–1305
Forest Health Technology Enterprise Team (2004) Invasive plants established in the United States that are found in Asia, and their associated natural enemies. 2:30
Hao Sh, Chen Al, Zhou Yw, Wang Jl, Ma Zq, Zhang X (2005a) Attraction of short chainalcohols to scarab beetle, Potosia brevitarsis. Chin J Pesticide Sci 7(2):181–184
Hao Sh, Li Gz, Zhang T, Zhang Q, Feng Jt, Zhang X (2005b) Behavior of the scarab beetle, Potosia brevitarsis, and pheromone attraction effect. Chin J Biol Control 21(2):124–126
He P-g, Lin X-z, Shen J-h, Huang X-h, Chen K-s, Li G-g (2005) Induction of volatile organic Lycopersicon esculentum compound in the leaves chitosan oligomer. High Technol Lett 11:95–100
Kim BY, Lee KS, Choo YM, Kim I, Hwang JS, Sohn HD, Jin BR (2008a) Molecular cloning and characterization of a transferrin cDNA from the white-spotted flower chafer, Protaetia brevitarsis. Mitocondrial DNA 19:148–150
Kim BY, Kim HJ, Lee KS, Sook JS, Jin BR (2008b) Catalase from the white-spotted flower chafer, Protaetia brevitarsis: cDNA sequence, expression, and functional characterization. Comp Biochem Physiol 149:134–142
Klein MG, Edwards DC (1989) Captures of Popillia lewisi (Coleoptera:Scarabaeidae) and other scarabs on Okinawa with Japanese beetle lures. J Econ Entomol 82:101–103
Ladd T, Klein MG, Tumlinson JH (1981) Phenethyl propionate + eugenol + geraniol (3:7:3) and Japonilure: a highly effective joint lure for Japanese beetles. J Econ Entomol 74:665–667
Landolt PJ (1990) Trapping the green June beetle (Coleoptera: Scarabaeidae) with isopropanol. Fl Entomol 73:328–330
Langford GS, Muma MH, Cory EN (1943) Attractiveness of certain plant constituents to the Japanese beetle. J Econ Entomol 36:248–252
Leal WS (1998) Chemical ecology of phytophagous scarab beetles. Ann Rev Entomol 43:39–61
Li Z, Liu C, Wang Q, Cui J (1995) Effect of attractants on scarabs. Acta Agric Boreali-Sinica 10:153–156
Lin H, Phelan L (1991) Identification of food volatiles attractive to dusky sap beetle, Carpophilus lugubris (Coleoptera: Nitidulidae). J Chem Ecol 17:1273–1286
Loppez JD Jr, Crocker RL, Shaver TN (2002) Attractant for monitoring and control of adult scarabs. US Patent 6,440,406
Matich AJ, Young H, Allen JM, Wang MY, Fielder M, McNeilage MA, Elspeth A, MacRae EA (2003) Actinidia arguta: volatile compounds in fruit and flowers. Phytochemistry 63:285–301
McGovern TP, Ladd TL (1984) Japanese beetle attractant tested with eugenol substitutes and phenethyl propionate. J Econ Entomol 77:370–373
Meng XZ, Yan XH, Han Y (1999) Chemical communication and semiochemicals in scarab beetles. Chin Bull Life Sci 11:230–232
Park HY, Park SS, Oh HW, Kim JI (1994) General characteristics of the white-spotted flower chafer, Protaetia brevitarsis reared in the laboratory. Korean J Entomol 24:1–5
Phelan L, Lin H (1991) Chemical characterization of fruit and fungal volatiles attractive to dried fruit beetle, Carpophilus hemiptera (Coleoptera: Nitidulidae). J Chem Ecol 17:1253–1272
Reed DK, Lee MH, Kim SH, Klein MG (1991) Attraction of scarab beetle populations (Coleoptera: Scarabaeidae) to Japanese beetle lures in the Republic of Korea. Agric Ecosyst Environ 36:163–174
Reinecke A, Ruther J, Tolasch T, Francke W, Hilker M (2002) Alcoholism in cockchafers: orientation of male Melolontha melolontha toward green leaf alcohols. Naturwissenschaften 89:265–269
Renwick JAA (1989) Chemical ecology of oviposition in phytophagous insects. Experientia 45:223–228
Robertson GW, Griffiths DW, Woodford JAT, Birch ANE (1995) Changes in the chemical composition of volatiles released by the flowers and fruits of the red raspberry (Rubus idaeus) cultivar Glen Prosen. Phytochemistry 38:1175–1179
Rochat D, Meillour JR, Nagnan-Lee P (2000a) Identification of pheromone synergists in American palm weevil, Rhynchophorus palmarum, and attraction of related Dynamis borassi. J Chem Ecol 26(1):155–187
Rochat D, Ramirez-Lucasa P, Malossea C, Aldana R, Kakul T, Morin J-P (2000b) Role of solidphase microextraction in the identification of highly volatile pheromones of the two rhinoceros beetles Scapanes australis and Strategus aloeus (Coleoptera, Scarabaeidae, Dynastinae). J Chromatogr A 885:433–444
Ruther J (2004) Male-biased response of garden chafer, Phyllopertha horticola L., to leaf alcohol and attraction of both sexes to floral plant volatiles. Chemoecology 14:187–192
Ruther J, Hilker M (2003) Attraction of forest cockchafers Melolontha hippocastani to plant volatiles and 1,4-benzoquinone: application aspects. Entomol Exp Appl 107:141–147
Ruther J, Mayer JC (2005) Response of the garden chafer Phyllopertha horticola, to plant volatiles: from screen to application. Entomol Exp Appl 115:51–59
Syed Z, Guerin PM (2004) Tsetse flies are attracted to the invasive plant Lantana camara. J Insect Physiol 50:43–50
Tolasch T, Solter S, Toth M, Ruther J, Francke W (2003) (R)-Acetoin-Female sex pheromone of the summer chafer Amphimallon solstitiale. J Chem Ecol 28:1045–1050
Toth M, Leal WS, Szarukan I, Lesznyak M, Szocs G (1994) 2-(E)-nonen-1-ol: male attractant for chafers Anomala vitis Fabr. and A. dubia Scop. (Coleoptera: Scarabaeidae). J Chem Ecol 20:2481–2487
Toth M, Klein MG, Imrei Z (2003) Field screening for attractants of scarab pests in Hungary (Coleoptera:Scarabaeidae). Acta Phytopathol Entomol Hung 38:323–331
Toth M, Schmera D, Imeri Z (2004) Optimization of a chemical attractant for Epicometis (Tropinota) hirta Poda. Z Naturforsch 59:288–292
Toth M, Vuts J, Difranco F, Tabilio R, Baric B, Razov J, Toshova T, Subchev M, Sredkov I (2009) Detecting and monitoring of Epicometis hirta Poda and Tropinota squalid Scop. with the same trap. Acta Phytopathol Entomol Hung 44:337–344
Vallat A, Gu H, Dorn D (2005) How rainfall, relative humidity and temperature influence volatile emissions from apple trees in situ. Phytochemistry 66:1540–1550
Vuts J, Szarukan I, Subchev M, Toshova T, Toth M (2010a) Improving the floral attractant to lure Epicometis hirta Poda (Coleoptera: Scarabaeidae, Cetoniinae). J Pest Sci 83:15–20
Vuts J, Imrei Z, Toth M (2010b) New co-attractants synergizing attraction of Cetonia aurata aurata and Potosia cuprea to the known floral attractant. J Appl Entomol 134:9–15
Wang Q-h, Zhang J-t (2005) Field screening of attractants for Potosia brevitarsis. J Shanxi Agric Univ (Nat Sci Ed) 28:444–446, 486
Wang Q-h, Zhang J-t (2008) Preliminary study of mating behavior of biology for Potosia brevitarsis. J Shanxi Agric Univ (Nat Sci Ed) 29:150–152
Wei H-J, Zhang Z-L, Wang Y-C (1989) Underground insect pest of China. Shanghai Scientific Expression. 245–270
Wu H-h, Yang M-l, Guoy Y-p, Ma E-b (2004) Comparative study of Malathion toxicity and general esterases in larvae and adults from a field population of Oxya chinensis (Thunberg) (Orthoptera: Acridoidea). Agric Sci China 3:812–821
Zhang S-m, Wang G (1955) Preliminary study on propensity of Potosia brevitaris Lewis. J Entomol 5:199–209
Acknowledgments
This work was supported by the Research Institute of Foliage Protection, Chinese agriculture academy of sciences. We thank Jilin Agricultural University’s experimental Station’s staff for their assistance in conducting this study. We also gratefully acknowledge R. I. Song, assistant professor, Jilin Agricultural University, for his assistance in conducting this study. Michael G. Klein, Adjunct Professor, The Ohio State University, Wooster, OH USA assisted in revising this manuscript into a more standard English format. Robin A. J. Taylor, Research Scientist, The Ohio State University, provided the statistical treatment utilized in Table 2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Toth and M. Traugott.
Rights and permissions
About this article
Cite this article
Chen, Rz., Li, y. A novel plant volatile attractant scheme to protect corn in China from the white-spotted flower chafer (Coleoptera: Scarabaeidae: Cetoniinae). J Pest Sci 84, 327–335 (2011). https://doi.org/10.1007/s10340-011-0353-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-011-0353-6