Abstract
To improve the performance of melamine–formaldehyde (MF) aerogel, boron nitride nanosheets (BNNs) were doped in it to get a new aerogel. The aerogel was prepared as a coating, and basalt fibers modified with it were filled in a polyetheretherketone tube for in-tube solid-phase microextraction. Its morphological structure was characterized by scanning electron microscopy. Connected with high performance liquid chromatography, it was investigated with several polycyclic aromatic hydrocarbons (PAHs) as model analytes. An online analytical method was established under the optimized conditions, including extraction volume, extraction flow rate, methanol content in sampling solution and desorption time. The method had wide linear ranges (0.016–15 µg L−1, 0.016–20 µg L−1, 0.030–20 µg L−1), satisfactory correlation coefficients (0.9968–0.9997) and low limits of detection (0.005–0.010 µg L−1). The relative standard deviations (RSDs, n = 3) of the method were in the ranges of 0.5–6.1% (intra-day) and 2.2–8.8% (inter-day). RSDs of preparation repeatability among three tubes ranged from 0.4 to 2.9%. Compared with MF aerogel coating and other materials, BNNs-doped MF aerogel showed better limits of detection, higher enrichment factors (1015–1846) and comparable extraction time, due to large specific surface area and π–π interaction with PAHs. The method was applied to detect PAHs in rainwater and a soil solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid-phase microextraction (SPME) is an effective and environmentally friendly method of sample pretreatment, and it has been extensively used in environmental, medical and biological analysis [1,2,3]. It can be easily coupled with gas chromatography [4,5,6], high performance liquid chromatography (HPLC) [7, 8] and capillary electrophoresis [9] by online or offline modes. Using a tubular extraction device, in-tube solid-phase microextraction (IT-SPME) is valuable for miniaturization, rapidity, and online coupling with analytical instruments. Extraction efficiency depends on the adsorbent coating; various coating materials have been explored for IT-SPME, such as Co/Cr layered double hydroxide nano-sheets [10], poly(glycidyl methacrylate) nanoparticle [11], silica aerogel [12], metal–organic frameworks [13] and so on. Single-walled carbon nanotubes are incorporated into a monolithic polymer material for IT-SPME to extract triazine herbicides in real samples [14]. Gao’s group prepared single layer graphene oxide based IT-SPME inside the capillary wall for the determination of five triazine herbicides in several types of water samples [15].
As a typical organic aerogel, the melamine–formaldehyde (MF) aerogel possesses continuous porosity and the nanostructure composed of interconnected colloidal particles. The nanostructure presents relatively large pore volume and low-bulk densities that exceptionally enlarge the surface area for extraction. It was applied to the catalysis, aerospace, adsorption, sensor and electrochemistry [16, 17]. However, there are some shortcomings in MF aerogel, like weak mechanical strength, poor extraction performance and long preparation time. To solve these issues, a one-pot synthesis method was used for the preparation of MF/SiO2 aerogel, and the gelation time was shortened [18]. A starch-enhanced MF hybrid aerogel was prepared and demonstrated excellent elasticity and mechanical durability [19]. It is worth mentioning that the doping method can improve the performance of MF aerogel.
Recently, nanosilica [20], zinc oxide nanorods [21], carbon nitride nanocomposites [22] and other nanomaterials have received attention owing to their excellent properties, such as high thermal conductivity, better mechanical properties, satisfactory resistance to oxidation, and good chemical stability [23,24,25,26]. Boron nitride nanosheets (BNNs) are also a kind of nanomaterial with high surface area, abundant pore structures, large π–π conjugate system, high extraction capacity and hydrophobic properties, and it has been widely investigated in many fields, such as hydrogen storage, dye removal, water cleaning and so on [27,28,29]. Polycyclic aromatic hydrocarbons (PAHs) are a class of hydrophobic and carcinogenic pollutant. BNNs may have a good extraction effect on PAHs, and a satisfactory adsorption and enrichment result will be obtained.
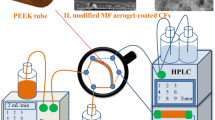
Considering that the incorporation of BNNs will improve the performance of MF aerogel, BNNs were doped in MF aerogel to develop an IT-SPME coating onto basalt fibers (BFs) in this work. They were filled in a polyetheretherketone (PEEK) tube to get an extraction tube. Connecting with HPLC, the extraction tube was evaluated with some PAHs as targets. Extraction and desorption conditions were optimized, and an online analysis method was established to detect PAHs in real samples. It was compared with some extraction tubes based on MF aerogel coating and other materials.
Experimental
Materials and Reagents
Formaldehyde (37%) was of analytical purity from Sinopharm Group Chemical Reagent Co., Ltd. (Shanghai, China). Methanol and acetonitrile were of chromatographic pure grade from Tedia Chemical Reagent Co. (USA). PEEK tube (0.75 mm i.d., 1.5 mm o.d.) was obtained from Haohai Chemical Co., Ltd. (Wuhan, China). BFs were obtained from Zhejiang Jiaxing Co., Ltd. (Zhejiang, China). BNNs were purchased from Beijing Deke Daojin Science and Technology Co., Ltd. (Beijing, China). Melamine was purchased from J&K Technology Co., Ltd. (Beijing, China). Sodium carbonate was provided by Shanghai Liangren Chemical Co., Ltd. (Shanghai, China). Naphthalene (Nap), acenaphthylene (Acy), acenaphthene (Ace), fluorene (Flu), phenanthrene (Phe), anthracene (Ant), fluoranthene (FlA) and pyrene (Pyr) were of analytical reagent grade from Shanghai Pure Reagent Co., Ltd. (Shanghai, China). Hydrochloric acid was obtained from Huantai County Shun Xin Chemical Co., Ltd. (Shandong, China). Ultrapure water (18.25 MΩ cm, 25 °C) was used for the whole experiment.
Apparatus
A field-emission scanning electron microscope (SEM, SUPRATM55, Carl Zeiss AG, Germany) equipped with an energy-dispersive spectrometer (EDS, Oxford INCA X-Act, UK) was used for characterizing extraction materials. Sample solution was moved by a P102 HPLC pump from Dalian Elite Analytical Instruments Co., Ltd. (Dalian, China). All chromatographic tests were performed on an Agilent 1260 HPLC model (Santa Clara, CA, USA) equipped with a Zorbax C18 column (250 × 4.6 mm i.d., 5 µm), a 20 µL sample loop and a diode array detector (DAD). A mobile phase filter with a 0.45 µm membrane was set to a gradient elution for PAHs (A acetonitrile, B ultrapure water; 0 min A = 70%, B = 30%; 10 min A = 70%, B = 30%; gradually increased to 20 min A = 100%; 20 min stop). Multiple detector wavelengths were set to receive sensitive signals, including 220 nm (Nap), 225 nm (Acy and Flu), 230 nm (FlA and Pyr), 250 nm (Phe and Ant) and 260 nm (Ace). The flow rate of the mobile phase was 1 mL min−1 and the column temperature was set at 25 °C.
Preparation of Sample Solutions
The stock solution composed of eight PAHs was prepared with methanol solvent and stored at 4 °C. The concentration of each PAH in the stock solution was 10 mg L−1. Working solution was obtained through diluting stock solution to 5 µg L−1 with ultrapure water. A series of standard solutions were prepared from the stock solution to 0.05, 1.00, 2.00, 4.00, 6.00, 10.00, 15.00, 20.00, 25.00 µg L−1 in the determination of the linear range. Rainwater was collected as the real sample and filtered with a 0.45 µm membrane before analysis. Soil on the edge of the road was collected and 1.00 g of soil was dissolved with 20.0 mL methanol. The solution was ultrasonically extracted for 30 min to accelerate the entry of target components into the methanol. The solution of dissolved soil sample was filtered to obtain the extractant of soil. 200 µL of extractant was diluted to 250.0 mL with ultrapure water to get the soil solution sample.
Preparation of Extraction Tube
Preparation of the Aerogel-Coated Extraction Materials
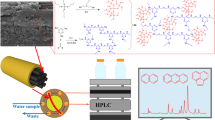
BNNs/MF aerogel was prepared according to the modified preparation method of MF aerogel as describe in a previous report [30]. The preparation schematic is shown in Fig. 1. BNNs were incorporated into MF aerogel during the chemical reaction between melamine and formaldehyde. 1.26 g of melamine and 0.01 g of sodium carbonate were dissolved into 10 mL of ultrapure water in a water bath (70 °C), and then 4 mL of formaldehyde (37%) was added dropwise to the above solution. When the concentration of BNNs exceeded 1.5%, no gel could be formed in the system. In order to allow more BNNs to be doped in the gel, its content was controlled to 1%. Then 0.18 g of BNNs was uniformly dispersed in 4 mL ultrapure water and added to the above mixed solution. A bundle of BFs (60 cm length) was placed in the mixture and pH value was adjusted to about 1.5 by adding HCl (36%). The temperature of the water bath was raised to 80 °C, and the hydrogel was obtained after 9 h. The hydrogel was aged at room temperature for 24 h. Water and the impurities in the hydrogels were washed with ethanol and acetone, successively. BNNs/MF aerogel coated-BFs were obtained after freeze drying. For comparison, MF aerogel-coated BFs were also prepared by the same method, only without BNNs.
Filling of Extraction Tubes with the Aerogel-Coated Basalt Fibers
89 mg of BNNs/MF aerogel-coated BFs were filled in 30 cm length PEEK tube to prepare an extraction tube. For comparison, an equal quantity of MF aerogel-coated BFs were also filled in a PEEK tube of the same length. These extraction tubes were rinsed with ultrapure water and ethanol for 30 min, in turn, before use.
Online Extraction and Analysis Procedure
To achieve an online analysis, the extraction tube was connected to an HPLC instrument via replacing the sampling loop of the six-port valve. As described in our previous report [31], the extraction and desorption processes were carried by turning the six-port valve from “load” to “inject” state. In the “load” position, the sample solution was transported through the extraction tube via P102 HPLC pump, meanwhile, the targets were extracted. HPLC mobile phase was directly used as the desorption solvent. When the valve was changed to “inject”, the analytes were desorbed from the extraction tube by the flow of the mobile phase. Then the analytes were carried to the column and detector by the mobile phase for separation and detection, respectively.
Results and Discussion
Characterization of Extraction Material
MF aerogel-coated BFs and BNNs/MF aerogel-coated BFs were characterized by SEM and EDS. Compared with the MF aerogel coating in Fig. 2a, the formation of the colloidal coating was not changed with the doping of BNNs (Fig. 2b). As shown in Fig. 2c, d, two coatings were evenly coated onto the surface of BFs, and they were homogenous and rough. As can be seen from Fig. 2d, the BNNs/MF aerogel coating possesses a sheet structure that is not found in the MF aerogel, which can provide more adsorption sites and enhance extraction capacity. We conducted an EDS characterization for qualitative and approximate quantitative analysis. In Fig. S1, the EDS spectrum shows the five main signals including B, N, C, O and Si peaks, however the signal of B is not found in Fig. S2. This is evidence of the successful incorporation of BNNs in the MF aerogel coating. The EDS data in Table S1 give rough atomic content, atomic contents of B, N, C, O and Si as 44.98%, 33.74%, 4.11%, 15.64% and 1.52%, respectively. In Table S2, atomic contents of N, C, O and Si are 37.90%, 11.88%, 25.60% and 15.13%, respectively. Si and a part of O are sourced from the BFs, other atomic signals are attributed to the aerogel.
Optimization of Extraction and Desorption Factors
Eight PAHs were used as the model analytes to evaluate the extraction tube. In order to obtain high extraction and desorption efficiency, several main factors were investigated and optimized, including extraction volume, extraction flow rate, methanol content in sample and desorption time.
Extraction Volume
The extraction efficiency of IT-SPME is greatly influenced by the extraction volume. It is often increasing with the enlargement of sampling volume. Nevertheless, the higher the extraction volume, the more extraction time that will usually be wasted. Extraction volume was evaluated in the range from 30 to 80 mL. As shown in Fig. 3a, peak areas of all analytes increase quickly with the increase of the extraction volume from 30 to 70 mL, especially for Ant. When extraction volume further increased more than 70 mL, the increase of peak areas was unremarkable. Taking into account both high extraction efficiency and short extraction time, 70 mL was selected as the optimal extraction volume.
Extraction Flow Rate
A vital parameter affecting extraction efficiency and extraction time is the flow rate of the sample. To get high extraction efficiency, flow rate was investigated from 1.25 to 2.50 mL min−1 under the extraction volume of 70 mL. As shown in Fig. 3b, peak areas of Phe, Ant, FlA and Pyr were slowly increased by increasing the flow rate from 1.25 to 2.00 mL min−1, and the maximum values were reached at 2.00 mL min− 1 . However peak areas of other PAHs, except Nap, have little change in the range from 2.00 to 2.50 mL min−1, which means that 2.00 mL min−1 is better extraction flow rate for targets. In addition, excessive flow rate reduces mass transfer efficiency and raises the pressure in the extraction tube. To achieve quick analysis and get satisfactory extraction efficiency, 2.00 mL min−1 was chosen as the suitable extraction rate for further tests.
Methanol Content
PAHs have poor solubility in aqueous solution due to their strong hydrophobicity, but adding suitable amounts of organic solvent will change their solubility. In this paper, methanol as a common organic solvent was added to the sample solution and its content was regulated from 0 to 5.0% (v/v). As shown in Fig. 3c, the extraction efficiency is improved with the increase of methanol from 0.5 to 1.0% (v/v), the trend remains stable when its content is more than 1.0% (v/v). To reduce damage to the environment and humans by organic solvents, 1.0% (v/v) of methanol content was added to the sample for the whole experiment.
Desorption Time
In order to obtain accurate analytical results, the desorption of all analytes was as possible after the extraction process. Desorption of analytes was performed by 1 mL min− 1 of acetonitrile–water (70:30, v/v), therefore desorption time directly affected the analysis result. If it was too short, targets were not be completely desorbed from the extraction tube; otherwise the analysis time was delayed. To determine the optimal time, different desorption times in the range of 0.2–2.0 min were tested. As can be seen from Fig. 3d, the peak areas of all analytes increased obviously with desorption times from 0.2 to 0.6 min, and they had no significant change when the time was more than 0.6 min. When the desorption process was completed at different desorption times, the tube was rinsed without extracting the sample. As shown in Fig. S3, the residual amount was investigated after elution at corresponding desorption times, and the residual areas no longer decreased after more than 0.6 min. It means that 0.6 min is enough to fully desorb all analytes from extraction tube.
Evaluation and Analysis of Real Samples
An analysis method was established under the optimum conditions. A series of standard solutions with the concentrations of 0.05–25 µg L−1 (calibration level, n = 9) were prepared to determine the linear ranges, correlation coefficients (r), limits of detection (LODs) and limits of quantification (LOQs). As listed in Table 1, all analytes show relatively wide linear ranges from 0.016 to 15 µg L−1 for Nap and Phe, 0.016 to 20 µg L−1 for Acy, Ace and Flu, 0.030 to 20 µg L−1 for Ant, FlA and Pyr. The correlation coefficients range from 0.9968 to 0.9997. LODs (S/N = 3) and LOQs (S/N = 10) are in the ranges of 0.005–0.010 µg L−1 and 0.016–0.030 µg L−1, respectively. The RSDs (n = 3) of intra-day and inter-day by a parallel test on the same extraction tube are 0.5–6.1% and 2.2–8.8%, respectively. Preparation repeatability was also evaluated among three extraction tubes, and their RSDs (n = 3) range from 0.4 to 2.9%. The low RSD values bear evidence to the fact that the developed IT-SPME-HPLC is reliable and reproducible.
In order to evaluate the applicability of the analytical method, it was applied to detect PAHs in real samples, including rainwater and a soil solution. Their chromatograms are shown in Fig. 4. As listed in Table 2, only Nap and Ant can be detected in rainwater, but they are unable to be quantified because their concentrations are lower than the LOQ. In the soil solution, Nap, Acy, Flu, Phe and Ant are found but not quantified, and other targets are not detected. Their recoveries, ranging from 81.3 to 120.0%, were obtained by spiking the standard PAHs solution in two real samples at the concentration levels of 2 and 6 µg L−1, respectively. The partial lower recoveries are due to the complex matrix of the real sample.
Chromatograms of real samples including (a) rainwater and (b) soil solution. Chromatographic peaks, (1) Nap, (2) Acy, (3) Ace, (4) Flu, (5) Phe, (6) Ant, (7) FlA and (8) Pyr. Conditions, analytes concentration, 5 µg L−1; extraction volume, 70 mL; extraction flow rate, 2.00 mL min− 1; methanol content, 1.0% (v/v); desorption time, 0.6 min. Other conditions are the same as in Fig. 3, detection wavelength, 230 nm
Comparison with Melamine–Formaldehyde Aerogel and Other Extraction Materials
The extraction efficiency of BNNs/MF aerogel-coated BFs was compared with that of MF aerogel-coated BFs in Fig. 5. No peak was found with the direct injection of the 5 µg L−1 sample, and peak signals of all analytes are presented on MF aerogel-coated BFs, but these are more obvious on BNNs/MF aerogel-coated BFs, even more than with direct injection of the 5 mg L−1 sample. Enrichment factors (EFs) can express the extraction efficiency of extraction materials. EF values of extraction tubes can be calculated through the ratio between the corresponding concentration after to that before extraction (EFs = CSPME/CO). The EFs on two aerogels were investigated, and the corresponding peak areas and calculated CSPME are listed in Table 3, where EFs on BNNs/MF aerogel-coated BFs are in the range of 1015–1846. However, the EFs on MF aerogel-coated BFs are in the range from 186 to 1433. According to the ratio of their EFs in the range about 1.1–5.7, indicating BNNs/MF aerogel-coated BFs have better extraction capability than MF aerogel-coated BFs.
Chromatograms on MF aerogel coated-BFs, BNNs/MF aerogel coated-BFs and direct injection at 5 µg L−1 and 5 mg L−1. Chromatographic peaks, (1) Nap, (2) Acy, (3) Ace, (4) Flu, (5) Phe, (6) Ant, (7) FlA and (8) Pyr. Conditions, extraction volume, 30 mL; extraction flow rate, 2.00 mL min− 1; methanol content, 1.0 (v/v); desorption time, 0.6 min. Detection wavelength, 230 nm
LODs, EFs and extraction time of this method for PAH analytes in Table 4 were compared with some methods based on other extraction materials, including C12-Ag wire [32], polyester fibers [33], zeolitic imidazolate framework-8 polydopamine PEEK tube [34] CP-Sil 19CB capillary [35] and TRB-5 capillary column [36]. BNNs/MF aerogel coating provides higher EFs, it also gives comparable sensitivity to the TRB-5 capillary column, and better sensitivity than other extraction materials. Extraction time of this work is comparable for other methods. Results confirm good extraction performance of the BNNs/MF aerogel-coated BFs for PAHs.
Conclusion
In this work, BFs coated with BNNs/MF aerogel were developed for IT-SPME. It was filled into PEEK tube and then coupled with HPLC, and the extraction tube was evaluated using eight PAH analytes. Several main extraction conditions were optimized thoroughly. A sensitive, convenient and online analytical method was established with wide linear ranges, low LODs, LOQs and relative recoveries. From these parameters, BNNs/MF aerogel-coated BFs demonstrated better extraction ability and enrichment effects for eight PAHs than did MF aerogel-coated BFs. Compared with other SPME material-based extraction tubes, it is superior in some aspects including LODs, enrichment factors and extraction time. The established method was successfully applied to detect PAHs in real samples. It indicated BNNs/MF aerogel-based extraction tube has good prospects for development in the field of sample preparation.
References
Cheng H, Song Y, Bian Y, Wang F, Ji R, He W, Gu C, Ouyang G, Jiang X (2018) A nanoporous carbon material coated onto steel wires for solid-phase microextraction of chlorobenzenes prior to their quantitation by gas chromatography. Microchim Acta 185:56–64
Souza-Silva EA, Jiang R, Rodríguez-Lafuente A, Gionfriddo E, Pawliszyn J (2015) A critical review of the state of the art of solid-phase microextraction of complex matrices I. Environmental analysis. Trac-Trend. Anal Chem 71:224–235
Liu L, Meng W-K, Zhou Y-S, Wang X, Xu G-J, Wang M-L, Lin J-M, Zhao R-S (2019) β-ketoenamine-linked covalent organic framework coating for ultra-high-performance solid-phase microextraction of polybrominated diphenyl ethers from environmental samples. Chem Eng J 356:926–933
Zhou S, Wang H, Jin P, Wang Z, Wang X, Du X (2017) Electrophoretic deposition strategy for the fabrication of highly stable functionalized silica nanoparticle coatings onto nickel-titanium alloy wires for selective solid-phase microextraction. J Sep Sci 40:4796–4804
Meng W-K, Liu L, Wang X, Zhao R-S, Wang M-L, Lin J-M (2018) Polyphenylene core-conjugated microporous polymer coating for highly sensitive solid-phase microextraction of polar phenol compounds in water samples. Anal Chim Acta 1015:27–34
Zeng J, Zhao C, Chen J, Subhan F, Luo L, Yu J, Cui B, Wang W, Chen X, Yan Z (2014) Ordered mesoporous carbon/Nafion as a versatile and selective solid-phase microextraction coating. J Chromatogr A 1365:29–34
Pang J, Mei M, Yuan D, Huang X (2018) Development of on-line monolith-based in-tube solid phase microextraction for the sensitive determination of triazoles in environmental waters. Talanta 184:411–417
Wang H, Du J, Zhen Q, Zhang R, Wang X, Du X (2019) Selective solid-phase microextraction of ultraviolet filters in environmental water with oriented ZnO nanosheets coated nickel-titanium alloy fibers followed by high performance liquid chromatography with UV detection. Talanta 191:193–201
Li T, Shi Z-G, Zheng M-M, Feng Y-Q (2008) Multiresidue determination of sulfonamides in chicken meat by polymer monolith microextraction and capillary zone electrophoresis with field-amplified sample stacking. J Chromatogr A 1205:163–170
Asiabi H, Yamini Y, Shamsayei M (2018) Using cobalt/chromium layered double hydroxide nano-sheets as a novel packed in-tube solid phase microextraction sorbent for facile extraction of acidic pesticides from water samples. New J Chem 42:9935–9944
Ying L-L, Ma Y-C, Xu B, Wang X-H, Dong L-Y, Wang D-M, Liu K, Xu L (2017) Poly(glycidyl methacrylate) nanoparticle-coated capillary with oriented antibody immobilization for immunoaffinity in-tube solid phase microextraction: preparation and characterization. J Chromatogr A 1509:1–8
Baktash MY, Bagheri H (2017) Silica aerogel coated on metallic wire by phase separation of polystyrene for in-tube solid phase microextraction. J Chromatogr A 1500:69–75
Zhang B, Xu G, Li L, Wang X, Li N, Zhao R-S, Lin J (2018) Facile fabrication of MIL-96 as coating fiber for solid-phase microextraction of trihalomethanes and halonitromethanes in water samples. Chem Eng J 350:240–247
Wang X, Li X, Li Z, Zhang Y, Bai Y, Liu H (2014) Online coupling of in-tube solid-phase microextraction with direct analysis in real time mass spectrometry for rapid determination of triazine herbicides in water using carbon-nanotubes-incorporated polymer monolith. Anal Chem 86:4739–4747
Tan F, Zhao C, Li L, Liu M, He X, Gao J (2015) Graphene oxide based in-tube solid-phase microextraction combined with liquid chromatography tandem mass spectrometry for the determination of triazine herbicides in water. J Sep Sci 38:2312–2319
Meador M, Wright S, Sandberg A, Nguyen B, Van Keuls F, Mueller C, Rodriguez-Rolis R, Miranda F (2012) Low dielectric polyimide aerogels as substrates for lightweight patch antennas. ACS Appl Mater Inter 4:6346–6353
Guilminot E, Fischer F, Chatenet M, Rigacci A, Berthon-Fabry S, Achard P, Chainet E (2007) Use of cellulose-based carbon aerogels as catalyst support for PEM fuel cell electrodes: electrochemical characterization. J Power Sources 166:104–111
He S, Bi Y, Zhang Y, Cao H, Shi X, Luo X, Zhang L (2015) One-pot synthesis and characterization of acid-catalyzed melamine formaldehyde/SiO2 aerogel via sol-gel technology. J Sol-gel Sci Techn 74:175–180
Zhang Y, Zhu J, Ren H, Bi Y, Shi X, Wang B, Zhang L (2017) A novel starch-enhanced melamine-formaldehyde aerogel with low volume shrinkage and high toughness. J Porous Mat 24:1303–1307
Long Z, Xu W, Lu Y, Qiu H (2016) Nanosilica-based molecularly imprinted polymer nanoshell for specific recognition and determination of rhodamine B in red wine and beverages. J Chromatogr B 1029–1030:230–238
Wen C, Li M, Li W, Li Z, Duan W, Li Y, Zeng J (2017) Graphene deposited onto aligned zinc oxide nanorods as an efficient coating for headspace solid-phase microextraction of gasoline fractions from oil samples. J Chromatogr A 1530:45–50
Chen J, Chen Q, Chen J, Qiu H (2016) Magnetic carbon nitride nanocomposites as enhanced peroxidase mimetics for use in colorimetric bioassays, and their application to the determination of H2O2 and glucose. Microchim Acta 183:3191–3199
Li L, Chen Y, Cheng B-M, Lin M-Y, Chou S-L, Peng Y-C (2012) Photoluminescence of boron nitride nanosheets exfoliated by ball milling. Appl Phys Let 100:404–404
Falin A, Cai Q, Santos E, Scullion D, Qian D, Zhang R, Yang Z, Huang S, Watananbe K, Taniguchi T, Barnett M, Chen Y, Ruoff R, Li L (2017) Mechanical properties of atomically thin boron nitride and the role of interlayer interaction. Nat Commun 8:15815–15815
Li L, Cervenka J, Watanabe K, Taniguchi T, Chen Y (2014) Strong oxidation resistance of atomically thin boron nitride nanosheets. ACS Nano 8:1457–1462
Zhu Y, Bando Y, Yin L, Golberg D (2016) Field nanoemitters: ultrathin BN nanosheets protruding from Si3N4 nanowires. Nano Lett 6:2982–2986
Lian G, Zhang X, Zhang S, Liu D, Cui D, Wang Q (2012) Controlled fabrication of ultrathin-shell BN hollow spheres with excellent performance in hydrogen storage and wastewater treatment. Energy Environ Sci 5:7072–7080
Li J, Huang Y, Liu Z, Zhang J, Liu X, Luo H, Ma Y, Xu X, Lu Y, Lin J, Zou J, Tang C (2015) Chemical activation of boron nitride fibers for improved cationic dye removal performance. J Mater Chem A 3:8185–8193
Lei W, Portehault D, Liu D, Qin S, Chen Y (2013) Porous boron nitride nanosheets for effective water cleaning. Nat Commun 4:1777–1777
Zhang Y, Zhu J, Ren H, Bi Y, Zhang L (2017) Synthesis and properties of melamine-starch hybrid aerogels cross-linked with formaldehyde. J Sol-Gel Sci Techn 83:44–52
Feng J, Wang X, Tian Y, Luo C, Sun M (2017) Poly(ionic liquids)-coated stainless-steel wires packed into a polyether ether ketone tube for in-tube solid-phase microextraction. J Sep Sci 40:4773–4779
Li J, Ma L, Tang M, Xu L (2013) C12-Ag wire as solid-phase microextraction fiber for determination of benzophenone ultraviolet filters in river water. J Chromatogr A 1298:1–8
Bu Y, Feng J, Sun M, Zhou C, Luo C (2016) Facile and efficient poly(ethylene terephthalate) fibers-in-tube for online solid-phase microextraction towards polycyclic aromatic hydrocarbons. Anal Bioanal Chem 408:4871–4882
Zhang J, Zhang W, Bao T, Chen Z (2015) Polydopamine-based immobilization of zeolitic imidazolate framework-8 for in-tube solid-phase microextraction. J Chromatogr A 1388:9–16
Ishizaki A, Saito K, Hanioka N, Narimatsu S, Kataoka H (2010) Determination of polycyclic aromatic hydrocarbons in food samples by automated on-line in-tube solid-phase microextraction coupled with high-performance liquid chromatography-fluorescence detection. J Chromatogr A 1217:5555–5563
Fernandez-Amado M, Prieto-Blanco M, Lopez-Mahia P, Muniategui-Lorenzo S, Prada-Rodriguez D (2016) A novel and cost-effective method for the determination of fifteen polycyclic aromatic hydrocarbons in low volume rainwater samples. Talanta 155:175–184
Acknowledgements
This research work was financially supported by the National Natural Science Foundation of China (NSFC, No. 21777054) and the Shandong Provincial Natural Science Foundation of China (No. ZR2017MB043).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection Recent Trends in Solid-Phase Extraction for Environmental, Food and Biological Sample Preparation with guest editors Anna Laura Capriotti, Giorgia La Barbera, and Susy Piovesana.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, C., Feng, J., Wang, X. et al. Melamine–Formaldehyde Aerogel Doped with Boron Nitride Nanosheets as the Coating of In-Tube Solid-Phase Microextraction. Chromatographia 82, 757–766 (2019). https://doi.org/10.1007/s10337-019-03707-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-019-03707-y