Abstract
A combination between an ionic liquid and melamine-formaldehyde aerogel on the carbon fibers was developed for in-tube solid-phase microextraction of estrogens with high efficiency. The sorbent has a high enrichment capability for several estrogens. Scanning electron microscopy showed that the aerogel on the carbon fibers has a porous three-dimensional network structure. Several important parameters such as sampling volume, sampling rate, the concentration of organic solvent in sample, pH value of sample as well as desorption time were optimized towards estrogen targets. Comparing with melamine-formaldehyde aerogel coating, the coating gave higher extraction efficiency. Comparing with melamine-formaldehyde aerogel coating, the new coating displays higher extraction efficiency. An online analytical method of estrogens was established, by the combination between in-tube solid-phase microextraction and high performance liquid chromatography with diode array detector. Analytical figures of merit include low limits of detection (<0.20 μg L−1), wide linearity (0.15–20 μg L−1), high enrichment factors (1028–1256), good extraction repeatability (RSDs<2.5%) and satisfactory preparation repeatability (RSDs<10.5%). The method was applied to the determination of trace estrogen targets in plastic bottle, tap water and surface water.

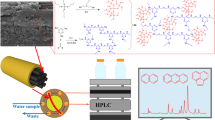
Schematic representation of online combination between in-tube solid-phase microextraction and high performance liquid chromatography, based on an ionic liquid (IL)-modified melamine-formaldehyde (MF) aerogel coating on carbon fibers (CFs) in a polyether-etherketone (PEEK) tube.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aerogels are advanced materials with promising physical properties like large surface area, low density, high thermal stability and porosity. Therefore, it was widely used as catalyst support, biomedicines, sensors and adsorbents [1]. In 1931, the first silica aerogel was created by Kistler and then zirconia, alumina aerogels were also prepared [2]. Up to date, a series of aerogels based on silica [3], carbon [4], metal oxides [5], organic polymers [6, 7] and inorganic-organic hybrids [8] have been developed. In terms of extraction or adsorption, some studies on graphene aerogels [9, 10], silica aerogels [11] and carbon aerogels [12] were conducted. Melamine-formaldehyde (MF) aerogel, as a kind of typical organic aerogels, was firstly developed by Pekala in 1991 [6]. To overcome the disadvantages containing poor-toughness and long-gelation time, MF aerogel was further improved by inorganic hybridization or organic functionalization. A composite aerogel was prepared through doping SiO2 into MF aerogel with sol-gel technology, the gelation time was greatly shortened in this way [13]. The starch as a functional monomer was introduced in MF aerogel, the melamine-starch-formaldehyde aerogel was synthesized and exhibited high surface area, relatively large pore volume, low bulk density and excellent mechanical performance [14]. Ionic liquids (ILs) possess the unique physicochemical properties, such as very low vapor pressure, good thermal stability, facile design by assembling diverse cations and anions, flexible functionalization through introducing specific groups like hydroxyl, carboxyl, amino and sulfonic acid groups [15]. Hence ILs were applied in various fields such as separation, material preparation, electrochemistry, biomass conversion and so on [16, 17]. In our previous work, ILs were used as the adsorbents which exhibited superior performance [18,19,20]. This makes ILs-modified MF aerogel an expected candidate of excellent extraction materials.
Comparing to conventional solid-phase extraction and liquid-liquid extraction, solid-phase microextraction (SPME) is an excellent sample preparation technique with cost-effective, time-efficient and easily automated merits [21,22,23,24,25,26]. In-tube solid-phase microextraction (IT-SPME) can accomplish the convenient automation both extraction and analysis via connecting to high performance liquid chromatography (HPLC). It overcomes common problems of fiber SPME, including the fragility, low sorption capacity, bending issues and short lifetime. A number of adsorbent materials were applied in IT-SPME towards various targets in aqueous solutions. The fused-silica tube coated with oxidized multiwalled carbon nanotube was inserted in a polyetheretherketone (PEEK) tube to detect substituted aniline compounds [27]. An organically modified silica aerogel coating was applied to the analysis of estrogens in sewage sample [28]. The electrochemically modified carbon fibers (CFs) with conducting poly(3,4-ethylenedioxythiophene)selectively extracted sulfonamides [29]. Therefore, one of the most important aspects is to explore the efficient coatings for IT-SPME.
The study described CFs which was in situ deposited with IL and MF aerogel complex. It was placed into an PEEK tube as the extraction material for IT-SPME. The selectivity and stability of extraction tube were investigated. Five estrogens were selected as the targets, the analytical method was established after optimizing the extraction conditions. The method was applied to the analysis of several estrogens in real samples.
Experimental
Materials and reagents
See Electronic Supporting Material.
Apparatus
An P102 HPLC pump from Dalian Elite Analytical Instruments Co., Ltd. (Dalian, China, http://dlwiling164.de.b2b168.com) was used as the sampling pump. An Agilent 1260 HPLC system (Agilent Technologies, USA, https://www.agilent.com) equipped with a 20 μL sample loop, an Zorbax C18 column (250 × 4.6 mm i.d., 5 μm) and a diode array detector (DAD) were used. Extraction material was characterized by a field-emission scanning electron microscopy (SEM, SUPRATM55, Carl Zeiss, AG, Germany, https://www.zeiss.com) with an energy-dispersive X-ray spectrometer (EDS, Oxford INCA X-Act, England, https://www.oxford-instruments.cn). 1H NMR data were obtained using a Bruker Avance DRX600 spectrometer (Bruker BioSpin AG, Fallanden, Switzerland, www.bruker-biospin.com) with DMSO as solvent.
Preparation of extraction tube
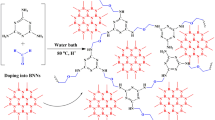
A bundle of carbon fibers (45 cm length) were ultrasonically washed with water and ethanol for 15 min successively. 1-Bromododecane and 1-(3-aminopropyl)imidazole (molar ratio 1:1) were dissolved to 50 mL ethanol, and the mass percentage of 1-bromododecane was 20%. Then the reaction solution was heated under 80 °C for 36 h. After removing ethanol and washing product with ether, 1-dodecyl-3-(3-aminopropyl)imidazolium bromide IL was obtained. 1-Dodecyl-3-(3-aminopropyl)imidazolium bromide (0.3 g) was added into ultrapure water (20 mL), and moved to a water bath (75 °C) till dissolved. Simultaneously, melamine (1.0 g) and sodium carbonate (0.01 g) were also dissolved into ultrapure water (20 mL), and the solution was placed in a water bath (75 °C) for 30 min. Then the bundle of CFs (400 mg) were immersed into the mixture of above two solutions. Formaldehyde (5 mL) was added into the reaction solution drop by drop under stirring. The pH value of the solution was immediately controlled to 1.5 by adding hydrochloric acid. After 30 min, the reaction solution was heated under 90 °C until to get a wet gel. To replace the solvent in the wet gel, it was immersed into ethanol and acetone for 6 h and three times in turn. The IL-modified MF aerogel was in-situ produced on CFs after a freeze-drying treatment. Finally, a bundle of aerogel-coated CFs (98 mg) were packed into a polyetheretherketone tube (30 cm) to obtain the extraction tube.
Online extraction and analysis procedure
As shown in Fig. S1, extraction tube was connected to the HPLC. The extraction and desorption processes are achieved by converting the six-port valve. Under the load state (Fig. S1a), sample solution is transferred in the extraction tube by the pump, and the targets are enriched onto the extraction material. After extraction process, the six-port valve is converted to injection state (Fig. S1b), the mobile phase flows through the extraction tube and the targets are desorbed into the column and detector for separation and detection.
Chromatographic conditions
All chromatographic tests were performed with 1 mL min−1 of mobile phase under 25 °C of column temperature. Estrogens were eluted by acetonitrile-water (50:50, v/v) and detected under 202 nm. Ultraviolet filters were eluted by acetonitrile-water (60:40, v/v) and detected under 290 nm for 2,4-dihydroxybenzophenone and phenyl salicylate and under 245 nm for 2-hydroxy-4-methoxybenzophenone.. Methanol-water (80:20, v/v) was used for the separation of anilines, and the detection wavelength was 254 nm. The runtime of these three types of analytes was 14 min. Phthalates were separated by a gradient methanol-water eluent, in which methanol was maintained as 75% (v/v) during 0–5 min and then gradually increased to 100% (v/v) from 5 to 18 min. Phthalates were detected under 210 nm with 22 min runtime.
Sample preparation
A standard stock solution of estrogens were prepared with a concentration of 10 mg L−1 in methanol solvent and stored at 4 °C untill use. Working solution was prepared daily by diluting the stock solution with ultrapure water to 5 μg L−1. Ultrapure water (1 L) was poured into a new plastic bottle and heat under 100 °C for 2 h to obtain a real sample (water in plastic bottle). Tap water was collected from our lab. Surface water was taken from a local river. Before the detection, all samples were filtrated with 0.45 μm membrane.
Results and discussion
Characterization of the extraction coating
The SEM images in Fig. 1a, b show relatively smooth surface on bare CFs. After the functionalization (Fig. 1c), it is obvious that CFs have been successfully coated by a rough aerogel coating. As can be seen from Fig. 1d, the open-pore-rich structure that is beneficial to increase mass transfer and adsorption sites will improve the extraction efficiency. According to the EDS spectrum of the coating, C, N, O and Br signals are found in Fig. S2, indicating IL has successfully been modified in MF aerogel. 1-Dodecyl-3-(3-aminopropyl)imidazolium bromide IL is confirmed through the 1H NMR spectrum, specific signals including δ 9.36 (s, 1H), 7.65–7.90 (d, 1H), 6.93–7.22 (d, 1H), 4.05–4.35 (t, 4H), 2.78–2.95 (m, 2H), 2.65–2.75 (m, 2H), 1.92–2.25 (m, 2H), 1.55–1.81 (t, 2H), 1.25–1.28 (m, 18H), 0.85–0.87 (t, 3H) can be observed.
Extraction selectivity and stability
Different types of targets including estrogens, ultraviolet filters, anilines and phthalates were tested to study the selectivity of extraction coating. The concentration of estrogens, ultraviolet filters, anilines was 5 μg L−1, while phthalates was 10 μg L−1. The peak areas of estrogens are significantly higher than that of others in Fig. S3. In addition, the enrichment factors (EFs) were calculated to be in the range of 1028–1265 for estrogens, 56–101 for ultraviolet filters, and 9–516 for phthalates. No aniline was extracted. According to these results, the coating exhibited better extraction performance towards estrogens. The extraction mechanism between estrogens and the coating may attribute to π-π, hydrogen-bonding and hydrophobic interactions.
The durability of the extraction tube was also evaluated. After 120 cycles, the loss of peak areas of all analytes on the tube was in the range of 7%–19%. In fact, one tube can be recycled more than 150 times stably. To explore the chemical stability of the extraction tube, 200 mL of ethanol, acid (pH = 4.0), basic (pH = 9.0) and KCl (20.0%, w/v) solutions were sequentially flowed through the tube. After each treatment, the tube was investigated for three times under the same conditions, and there was no significant change between peak areas. The relative standard deviations (RSDs) of all peak areas of each analyte during these tests were lower than 5.6%. Results above indicate that the extraction tube can provide both satisfactory durability and great chemical stability.
Extraction conditions investigation
The extraction performance is greatly affected by the extraction volume, so different extraction volumes (30, 35, 40, 45, 50, 55 and 60 mL) were investigated primarily in this study. The peak areas of all the targets, shown in Fig. 2a, increase as extraction volume increases from 30 to 60 mL, and the adsorption saturation is not approached. These indicates the remarkable extraction capacity of aerogel coating towards estrogens. Considering large extraction volume is generally a negative factor for rapid analysis [30], hence 45 mL was selected to achieve reasonable extraction efficiency.
In order to get a rapid test, a high sampling rate is desirable. As shown in Fig. 2b, the effect of sampling rate is investigated in the range of 0.75–2.00 mL min−1. The response for bisphenol A changes little, while other four targets increase as the sampling rate raises till 1.50 mL min−1. In this case, 1.50 mL min−1 was considered as the optimum rate for all targets.
The distribution coefficient of target between coating and aqueous sample is influenced by the concentration of organic solvent in sample, thereby affecting extraction efficiency. As shown in Fig. 2c, the concentration of methanol is examined in range of 0–5% (v/v). The peak areas of all targets show a decreasing trend with increase of methanol in sample, except for the estrone with a largest peak area at 4% of methanol. More adsorption sites will be released during the peak areas of other species are decreased, the possible reason about the increase of estrone may be a competitive adsorption of estrone with other species. Taking most targets into account, methanol was not added in subsequent experiments.
The pH value has an influence on the presence status of ionic components. As can be seen from Fig. 2d, the peak areas of the five estrogens increase along with the change of pH from 4 to 7, and all peak areas decrease when pH value beyonds 7. According to pKa values of bisphenol A, 17α-ethylestradiol, estrone, diethylstillbestrol and hexestrol corresponding to 9.59, 10.76, 10.34, 10.20, 10.10, the ionization at pH > 8 was increased. This proves that IL-modified MF aerogel coating possesses favorable enrichment ability towards neutral molecules. Therefore, the pH value of sample was controlled to 7 to achieve high extraction efficiency.
Desorption time has an effect on the amount of analytes desorbed from extraction material, it should be as short as possible while carryover effect must be also considered. When the desorption time was regulated from 0.2 to 2.0 min, the influence was investigated. The obvious improvement in peak areas of all estrogens can be seen with an increasing time (0.2–0.6 min) in Fig. 2e. There is no significant change as the desorption time is more than 0.6 min, indicating that 0.6 min is enough for desorbing the estrogens from extraction coating. The residue under different desorption time was also investigated. Similarly, the residual amount was gradually reduced along with the increasing of desorption time, and then remained unchanged more than 0.6 min. And the residue is in the range of 0.2%–0.7%. So, the desorption process was completed in 0.6 min.
Analytical performance and analysis of real samples
The analytical performance of this method was examined using the targets under the optimum conditions. Low-concentration solutions were prepared and tested to achieve limits of detection (LODs) at S/N = 3 (signal-to-noise ratio). As listed in Table 1, LODs of the method are ranged between 0.05 and 0.20 μg L−1. All estrogens possess believable and wide linear ranges like as 0.15–20 μg L−1 for bisphenol A, 0.36–35 μg L−1 for 17α-ethylestradiol, 0.30–20 μg L−1 for estrone, 0.60–25 μg L−1 for diethylstillbestrol, 0.24–20 μg L−1 for hexestrol. Correlation coefficients (r) within 0.9984–0.9993 were obtained. The extraction repeatability and preparation repeatability were evaluated. RSDs of single extraction tube for seven replicate extractions ranged from 0.3%–2.5% (n = 7). The preparation repeatability of three parallel fabricated tubes presented RSDs in the range of 6.9%–10.5%. These results proved the satisfactory extraction repeatability and reproducibility of extraction material. In addition, IL-modified MF aerogel and MF aerogel coating were compared under the same conditions. As shown in Fig. S4, the extraction ability of IL-modified MF aerogel is better than MF aerogel.
The applicability of this method was assessed by detecting five estrogens from real samples including plastic bottle, tap water and surface water in Fig. 3. As listed in Table 2, bisphenol A was detected in plastic bottle with a concentration of 1.2 μg L−1. Others were not detected. No target was detected in tap water and surface water. The relative recoveries spiked at different concentrations (3, 5 and 9 μg L−1) were in the ranges of 80%–129%, 75%–118% and 80%–109% in three real samples, respectively.
The chromatograms of real samples including a water in plastic bottle, b tap water and (3) surface water, spiked at 3 μg L−1, 5 μg L−1 and 9 μg L−1. Chromatographic peaks: (1) bisphenol A, (2) 17α-ethylestradiol, (3) estrone, (4) diethylstillbestrol and (5) hexestrol. Conditions: extraction volume, 45 mL; sampling flow rate, 1.5 mL min−1; methanol content, 0; pH, 7; desorption time, 0.6 min
Comparison with other literature methods for estrogens
The present method was compared with other sample preparation methods for estrogen determination. Several parameters including extraction material, LODs and extraction time were summarized in Table 3. It can be seen that the LODs and extraction time of this method are lower or comparable than other methods [31,32,33,34] except for SCSE-HPLC/DAD [35]. Although the sensitivity of SCSE-HPLC/DAD is better than the method, it requires very long extraction time (150 min) which reduces the work efficiency. The method is online analytical method, but a large sample volume is necessary. In addition, the use of polymeric ionic liquids gives us some inspiration that it can be tried to modify the aerogel for our next work.
Conclusions
In this study, an IL-modified MF aerogel coating was synthesized and used for the IT-SPME of estrogens. Combining the dual advantages of ILs and aerogels, the IL-modified MF aerogel coating shown obvious advantages including good selectivity, considerable stability, great reusability and satisfactory repeatability. Comparing with MF aerogel coating, it exhibited higher extraction efficiency. The established method showed large linear ranges and low LODs. Aerogel materials are rarely reported in terms of SPME, it has great prospect in sample preparation.
References
Moon I, Yoon S, Chun K, Oh J (2015) Highly elastic and conductive N-doped monolithic graphene aerogels for multifunctional applications. Adv Funct Mater 25:6976–6984
Yoldas E (1975) Alumina sol preparation from alkoxides. Am Ceram Soc Bull 54:289–290
Baktash M, Bagheri H (2017) Silica aerogel coated on metallic wire by phase separation of polystyrene for in-tube solid phase microextraction. J Chromatogr A 1500:69–75
Wang X, Lu M, Wang H, Huang P, Ma X, Cao C, Du X (2016) Three-dimensional graphene aerogel-mesoporous carbon composites as novel coatings for solid-phase microextraction for the efficient enrichment of brominated flame retardants. New J Chem 40:6308–6314
Berestok T, Guardia P, Du R, Javier B, Colombo M, Esteade S, Peiro F, Brock S, Cabot A (2018) Metal oxide aerogels with controlled crystallinity and faceting from the epoxide-driven cross-linking of colloidal nanocrystals. ACS Appl Mater Interfaces 10:16041–16048
Alviso C, Pekala R (1992) Melamine-formaldehyde aerogels. Polym Preprints 32:242–243
Al-Muhtaseb S, Ritter J (2003) Preparation and properties of resorcinol-formaldehyde organic and carbon gels. Adv Mater 15:101–114
Zhang M, Cheng J, Xuan X (2017) Pt/graphene aerogel deposited in Cu foam as a 3D binder-free cathode for CO2 reduction into liquid chemicals in a TiO2 photoanode-driven photoelectrochemical cell. Chem Eng J 322:22–32
Wang X, Lu M, Wang H, Pei Y, Rao H, Du X (2015) Three-dimensional graphene aerogels-mesoporous silica frameworks for superior adsorption capability of phenols. Sep Purif Technol 153:7–13
Chen M, Li Z, Geng Y, Zhao H, He S, Li Q, Zhang L (2018) Adsorption behavior of thorium on N,N,N',N'-tqetraoctyldiglycolamide (TODGA) impregnated graphene aerogel. Talanta 181:311–317
Roostaie A, Mohammadiazar S, Bargozin H, Ehteshami S (2018) A modified nanoporous silica aerogel as a new sorbent for needle trap extraction of chlorobenzenes from water samples. Chromatographia 81:649–655
Joul P, Vaher M, Kuhtinskaja M (2018) Evaluation of carbon aerogel-based solid-phase extraction sorbent for the analysis of sulfur mustard degradation products in environmental water samples. Chemosphere 198:460–468
He S, Bi Y, Zhang Y, Cao H, Shi X, Luo X, Zhang L (2015) One-pot synthesis and characterization of acid-catalyzed melamine formaldehyde/SiO2 aerogel via sol-gel technology. J Sol-Gel Sci Technol 74:175–180
Zhang Y, Zhu J, Ren H, Bi Y, Zhang L (2017) Synthesis and properties of melamine-starch hybrid aerogels cross-linked with formaldehyde. J Sol-Gel Sci Technol 83:44–52
Welton T (1999) Room-temperature ionic liquids: solvents for synthesis and catalysis. Chem Rev 99:2071–2084
Huang K, Chen Y, Zhang X, Xia S, Wu Y, Hu X (2014) SO2 absorption in acid salt ionic liquids/sulfolane binary mixtures: experimental study and thermodynamic analysis. Chem Eng J 237:478–486
Harada M, Kawasaki C, Saijo K, Demizu M, Kimura Y (2010) Photochemical synthesis of silver particles using water-in-ionic liquid microemulsions in high-pressure CO2. J Colloid Interface Sci 343:537–545
Feng J, Mao H, Wang X, Tian Y, Luo C, Sun M (2018) Ionic liquid chemically bonded basalt fibers for in-tube solid-phase microextraction. J Sep Sci 41:1149–1155
Feng J, Wang X, Tian Y, Luo C, Sun M (2017) Poly(ionic liquids)-coated stainless-steel wires packed into a polyether ether ketone tube for in-tube solid-phase microextraction. J Sep Sci 40:4773–4779
Sun M, Feng J, Bu Y, Luo C (2016) Ionic liquid coated copper wires and tubes for fiber-in-tube solid-phase microextraction. J Chromatogr A 1458:1–8
Xiao R, Zhang X, Zhang X, Niu J, Lu M, Liu X, Cai Z (2017) Analysis of flavors and fragrances by HPLC with Fe3O4@GO magnetic nanocomposite as the adsorbent. Talanta 166:262–267
Zeng J, Chen J, Li M, Subhan F, Chong F, Wen C, Yu J, Cui B, Chen X (2015) Determination of amphetamines in biological samples using electro enhanced solid-phase microextraction-gas chromatography. J Chromatogr B 1000:169–175
Liu L, Meng W, Zhou Y, Wang X, Xu G, Wang M, Lin J, Zhao R (2019) β-ketoenamine-linked covalent organic framework coating for ultra-high-performance solid-phase microextraction of polybrominated diphenyl ethers from environmental samples. Chem Eng J 356:926–933
Zhang J, Li W, Zhu W, Yang Y, Qin P, Zhou Q, Lu M, Cai Z (2019) Mesoporous graphitic carbon nitride as an efficient sorbent for extraction of sulfonamides prior to HPLC analysis. Microchim Acta 186(279). https://doi.org/10.1007/s00604-019-3394-9
Liu L, Meng W-K, Li L, Xu G-J, Wang X, Chen L-Z, Wang M-L, Lin J-M, Zhao R-S (2019) Facile room-temperature synthesis of a spherical mesoporous covalent organic framework for ultrasensitive solid-phase microextraction of phenols. Chem Eng J 369:920–927
Wen C, Li M, Li W, Li Z, Duan W, Li Y, Zhou J, Li X, Zeng J (2017) Graphene deposited onto aligned zinc oxide nanorods as an efficient coating for headspace solid-phase microextraction of gasoline fractions from oil samples. J Chromatogr A 1530:45–50
Liu X, Jia Y, Zhang H, Liu M (2008) Highly sensitive analysis of substituted aniline compounds in water samples by using oxidized multiwalled carbon nanotubes as an in-tube solid-phase microextraction medium. J Chromatogr A 1212:10–15
Bu Y, Feng J, Tian Y, Wang X, Sun M, Luo C (2017) An organically modified silica aerogel for online in-tube solid-phase microextraction. J Chromatogr A 1517:203–208
Ling X, Zhang W, Chen Z (2016) Electrochemically modified carbon fiber bundles as selective sorbent for online solid-phase microextraction of sulfonamides. Microchim Acta 183:813–820
Saito Y, Kawazoe M, Hayashida M, Jinno K (2000) Direct coupling of microcolumn liquid chromatography with in-tube solid-phase microextraction for the analysis of antidepressant drugs. Analyst 125:807–809
Penalver A, Pocurull E, Borrull F, Marce R (2002) Method based on solid-phase microextraction-high-performance liquid chromatography with UV and electrochemical detection to determine estrogenic compounds in water samples. J Chromatogr A 964:153–160
Wang C, Yang L, Li N, Zhang X, Guo Y, Li C (2017) Development of immunoaffinity solid phase microextraction rods for analysis of three estrogens in environmental water samples. J Chromatogr B 1061:41–48
Zou Y, Li Y, Jin H, Tang H, Zou D, Liu M, Yang Y (2012) Determination of estrogens in human urine by high-performance liquid chromatography/diode array detection with ultrasound-assisted cloud-point extraction. Anal Biochem 421:378–384
Hu C, He M, Chen B, Zhong C, Hu B (2013) Polydimethylsiloxane/metal-organic frameworks coated stir bar sorptive extraction coupled to high performance liquid chromatography-ultraviolet detector for the determination of estrogens in environmental water samples. J Chromatogr A 1310:21–30
Chen L, Mei M, Huang X, Yuan D (2016) Sensitive determination of estrogens in environmental waters treated with polymeric ionic liquid-based stir cake sorptive extraction and liquid chromatographic analysis. Talanta 152:98–104
Acknowledgements
This research work was financially supported by the National Natural Science Foundation of China (NSFC, No. 21777054) and the Shandong Provincial Natural Science Foundation of China (Nos. ZR2017MB043 and ZR2019MB058).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 680 kb)
Rights and permissions
About this article
Cite this article
Feng, J., Wang, X., Han, S. et al. An ionic-liquid-modified melamine-formaldehyde aerogel for in-tube solid-phase microextraction of estrogens followed by high performance liquid chromatography with diode array detection. Microchim Acta 186, 769 (2019). https://doi.org/10.1007/s00604-019-3909-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3909-4