Abstract
We studied the temporal–spatial distribution of Common Terns Sterna hirundo along the East Atlantic Flyway. In 2009 and 2010 experienced adults from a colony on the German North Sea coast were tagged with geolocators recording light intensity and saltwater contact. Main objectives were the inter-individual temporal–spatial variation of migration routes and wintering areas, wintering site fidelity, and time spent at sea across the annual cycle. Geolocators had no effects on various traits of breeders, but their reproductive output suffered from egg breakage. This can be avoided by artificially incubating the eggs. Twelve routes of nine individuals were tracked. Transponder readings at the breeding site showed that birds left the colony 4 weeks before starting autumn migration. In spring and autumn, Common Terns stopped over around the Canary Islands. Main wintering distribution was the upwelling seas alongside the West African coast and similar between years, but different among individuals. Three females wintered further north and more offshore than six males. Pair mates wintered at different locations. Spring migration was longer (56 ± 8 days) than autumn migration (37 ± 17 days). During both migration and wintering the terns spent more time on salt water than during breeding and post-breeding. In most individuals saltwater contact was higher during the day than at night, reduced at sunrise and sunset likely due to foraging, and peaked about noon possibly related to resting or thermoregulation. Detailed ecological and behavioral studies of common terns during wintering are needed to clarify the results based on geolocators.
Zusammenfassung
Flussseeschwalben entlang des Ostatlantischen Zugweges: Raumzeitliche Verteilung außerhalb der Brutperiode Wir untersuchten die raumzeitliche Verteilung von Flussseeschwalben Sterna hirundo entlang des Ostatlantischen Zugweges. 2009 und 2010 wurden erfahrene Brutvögel einer Kolonie an der deutschen Nordseeküste mit Geolokatoren versehen, die Lichtintensität und Salzwasserkontakt aufzeichneten. Ziele der Untersuchungen waren die interindividuelle raumzeitliche Variation der Zugrouten und Überwinterungsgebiete, die Winterortstreue und die Dauer des Seewasserkontakts im Jahreszyklus. Die Geolokatoren beeinträchtigten die Flussseeschwalben nicht, der Reproduktionserfolg jedoch war durch Bruch der Eier verringert, was durch Austausch und Ausbrüten der Eier in einem Inkubator vermieden werden kann. 12 Routen von 9 Individuen wurden verfolgt. Die Registrierungen der zusätzlich mit Transpondern gekennzeichneten Vögel zeigten, dass sie den Koloniestandort vier Wochen vor Beginn der Herbstwanderung verließen. Im Frühjahr und Herbst legten einige Flussseeschwalben bei den Kanarischen Inseln einen Zwischenhalt ein. Die Hauptverbreitung im Winter erstreckte sich entlang der Westafrikanischen Küste und war ähnlich zwischen den Jahren, aber verschieden zwischen Individuen. Die drei Weibchen überwinterten weiter nördlich als die sechs Männchen und die Paarpartner an verschiedenen Orten. Der Frühjahrszug dauerte länger (56 ± 8 d) als der Herbstzug (37 ± 17 d). Während der Wanderung und Überwinterung verbrachten die Flussseeschwalben mehr Zeit auf dem Salzwasser als im Brutgebiet. Die meisten Individuen hatten tagsüber längere Salzwasserkontakte als nachts, die bei Sonnenaufgang und -untergang stark reduziert waren, vermutlich aufgrund der Nahrungssuche. Während der Mittagszeit waren Salzwasserkontakte besonders intensiv, möglicherweise bedingt durch Rasten oder Thermoregulation. Detaillierte Studien zu Ökologie und Verhalten im Winter sollten folgen, um die auf den Geolokatoren basierten Ergebnisse zu klären.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seabirds spend most of their non-breeding period far offshore at the oceans, e.g. Shaffer et al. (2006), Guilford et al. (2009), and Egevang et al. (2010). This makes it difficult studying their behavior during these times. By analyzing the stable isotope composition of feathers grown outside the breeding area, we gain information about the birds’ diet composition and how this might affect other life-history stages, e.g. Sorenson et al. (2009). Ring recoveries might give us some indication about the birds’ whereabouts during the non-breeding period, but these recoveries seem to be highly aged-biased in seabirds (Wendeln and Becker 1999; Bairlein et al. 2014). Although both methods can be used to study the ecology and the behavior of seabirds away from their breeding areas to a certain extent, different types of loggers offer the opportunity to estimate seabirds’ behavior during migration and winter on a more precise scale. After recapture such loggers provide data about, e.g. GPS coordinates (Weimerskirch et al. 2002), light-level geolocations (Weimerskirch and Wilson 2000), three-dimensional acceleration (Sommerfeld et al. 2013), heart rate (Ropert-Coudert et al. 2006), water depth (Garthe et al. 2000), temperature (Wilson et al. 1992a), saltwater contact (Wilson et al. 1995), and others (Wilson et al. 2002). So far these studies have been limited to rather large seabirds, because neither the size nor the weight of the specific loggers have allowed deploying these devices to small seabirds, i.e., with body mass <100 g. Only little, therefore, was known about the whereabouts and their behavior during the migration and wintering period of such seabirds. The miniaturization of light-level geolocators now allows tracking also these smaller seabirds such as terns (e.g. Egevang et al. 2010; Nisbet et al. 2011a, b; Fijn et al. 2013; van der Winden et al. 2014).
Here we add to better knowledge about the ecology of seabirds during the non-breeding period by estimating the temporal–spatial distribution of European Common Terns (Sterna hirundo) along the East Atlantic Flyway. To do so we tagged adult Common Terns with data loggers at a breeding colony site in northwestern Germany (e.g. Becker et al. 2008) to record light levels and wet–dry conditions. The main objectives of this study were to estimate the inter-individual temporal–spatial variation of both their migration and wintering period, to explore potential sex-specific and within-pair differences of the wintering area, and to quantify the birds’ behavior across the annual cycle in relation to the individual time spent on sea water.
Methods
Study site
Common Terns considered in this study bred at a monospecific colony of about 400 breeding pairs located at “Banter See” at Wilhelmshaven on the German North Sea coast (53°36′N, 08°06′E, Becker et al. 2001, 2008; Becker 2010). This colony is the focus of an integrated, long-term population study, and about half of the breeders are aged, sexed, and marked with transponders (e.g. Szostek and Becker 2012). The colony site consists of six rectangular concrete islands (10.7 × 4.6 m), surrounded by a wall of 60 cm height. The walls are equipped with 44 elevated platforms for terns to land and rest on. Each platform contains an antenna reading transponder codes every 5 s, and half of them contain an electronic balance (accuracy ±1 g). This allows reliable automatic and remote detection of the birds’ presence at the colony site, arrival, and body mass (Limmer and Becker 2007), with a reencounter probability of almost 1 (Szostek and Becker 2012). Colony site fidelity is very high (adult local return rate ca. 90 %; Ezard et al. 2006; Szostek and Becker 2012). The first and last transponder reading of an individual in a season indicated that the bird had arrived and left the breeding colony, respectively (Becker et al. 2008). For simplicity birds are called by individual names. Reproductive performance and output was determined for each clutch including those of geolocator-marked parents using standard protocols (e.g. Becker and Wink 2003; Zhang et al. 2015). For chicks, maximum mass, mass at fledging (±1 g), and age at fledging (±1 day) were recorded (Becker and Wink 2003).
Capture and deployment of light-level geolocators
Experienced breeders (9–14 years old, in 2009 and 2010 both pair members; Table S1) were identified by the transponder with a nest antenna and caught on the nest with an electronically released drop trap (or spring trap in exceptional cases) during incubation, on average 12 days after laying the first egg (Table S1). Before catching the birds, their eggs were replaced by dummy eggs to avoid egg breakage. The captured adults were weighed (±1 g, digital balance), measured (head and bill length ±0.1 mm; wing length 0.5 mm), and tagged with light-level geolocators (Fig. S9). Total handling time was 3–6 min. Most individuals returned to the clutch a few minutes after release and started incubation soon [on average after 13±11 (2–38) min, n = 11]. No clutch was deserted owing to catching the breeders. In 2011 when light-level geolocators had to be only recovered, the eggs were removed immediately from the clutch after laying of the identified individuals, put in an incubator and were replaced by dummy eggs. Eggs remained in the incubator until light-level geolocators were retrieved from the adults to avoid any egg breakage. After that original eggs were exchanged again. Captures were performed earlier during incubation than in the previous years. Most individuals were captured in three successive years (Table S1).
Light-level geolocators
We used miniature light-level geolocators, Mk 10, from the British Antarctic Survey (BAS). They were fixed with layers of self-amalgamating tape to a plastic ring with cable tie (Fig. S9; 10 mm height, 5 mm internal diameter, 1.0 mm thickness). In 2010, three geolocators were attached to an aluminum ring for a Black-headed Gull (Croicocephalus ridibundus, 10 mm height). Mass of the ring and fixing materials was <1.7 g (about 1.3 % of Common Tern body mass). At recapture, the geolocator from the previous year was removed and replaced by a new one (Table S1). During the pre-calibration period light-level geolocators experienced the unhindered natural change in light conditions at the colony site for 7–19 days. After removal a post-calibration was conducted with each light-level geolocator for 5–18 days (in 2011 at the colony, in 2009 and 2010 at the Institute of Avian Research, 53°33′N, 08°06′E). Twelve of the 24 geolocators had failed (see Table S1); reasons for data loss were infiltrated water, non-realistic shift in longitude due to internal clock shifts (Fig. S8), or insufficient lifetime of batteries.
Light-level geolocators used in the present study archive maximum light intensity every 10 min. Sunrise and sunset times allow inferring length of day and night and the timing of midday and midnight, and finally estimate latitude and longitude twice a day (Wilson et al. 1992b; Hill 1994). As a matter of principle, latitude cannot be estimated on about 10 days around the equinoxes (Wilson et al. 1992b; Hill 1994; Lisovski et al. 2012). The general uncertainty of the estimated locations is generally on the order of magnitude of about 150 km (Phillips et al. 2004; Fudickar et al. 2012; Lisovski et al. 2012).
Light-level geolocation data were analyzed using the statistical software R 3.1.2 (R Core Team 2014) and the freely available SGAT package (https://github.com/SWotherspoon/SGAT). This packages combines tools of the R package GeoLight (Lisovski and Hahn 2012), which uses the threshold approach (Hill 1994; Ekstrom 2004), and the R package tripEstimation (Sumner et al. 2009), which uses the curve-fitting approach (Ekstrom 2004; Nielsen and Sibert 2007) to estimate the animals' locations. Here a threshold-based approach was used to estimate the birds' locations via an Estelle model. A probability distribution of these locations is derived from the Markov chain Monte Carlo method with a metropolis sampler. In comparison to other methods of estimating birds’ locations from light-level geolocation data, here a priori knowledge can be used to estimate locations by considering (1) a species-specific movement model, which is described by a bird’s ground speed, (2) a species-specific land mask model, and (3) that the errors in the twilight times, which follow a log normal distribution. Following these assumptions, probability distributions of the locations are estimated. The movement model defines the density distribution of travel speed, which is described here by a gamma distribution. As air speed of common terns is about 11 m/s (Bruderer and Boldt 2001; Pennycuick et al. 2013) and as terns in general exploit favorable wind conditions (Egevang et al. 2010), we arbitrarily set mean ground speed to 15 m/s. To determine the density distribution of ground speeds, all locations of a bird were initially estimated with the threshold-sensitivity twilight function threshold.path and used to estimate the ground speed for the initial track. This was on average 14.66 ± 1.05 m/s (mean ± SD; n = 11) and similar to the arbitrarily chosen ground speed. In a second step, we excluded extremely high speeds which are associated with erroneously estimated locations. The mean and SD of these remaining speed values were used to estimate both the shape parameter (1.51) and rate parameter (0.13) of the corresponding gamma distribution (Becker et al. 1988). This gamma distribution fitted well the density distribution of the ground speed during the tracking period (Fig. S1). The land mask model allows setting different probabilities for the bird being on land or on water. We set the probability of a Common Tern to be near or over water two times higher than being over land because Common Terns are typical seabirds (Harrison 1997; Nisbet et al. 2011a; Neves et al. 2015) and because the vast majority of ring recoveries from mid-European breeding populations comes from the West African coast and not from inland sites, indicating the wintering grounds to be on or even off the West African coast (Wernham et al. 2002; Bairlein et al. 2014). When sunrise and sunset events are not affected by artificial light, light cannot be detected before sunrise or after sunset by the light sensor. Hence, twilight errors are not normally distributed, but described by a lognormal distribution, as twilight error of recorded light cannot be negative (Fig. S2).
We considered these assumptions in our analyses of estimating birds’ locations (for details and R-code see https://github.com/SWotherspoon/SGAT). The resulting estimates in respect of longitude and latitude and their corresponding 95 % confidence intervals are given for each individual in the electronic supplemental material (Fig. S3).
We defined departures and arrivals from stationary sites, i.e., breeding area, stopover sites, and wintering grounds, as obvious changes in longitude and/or latitude (Fig. S3). In the latter, changes were only considered outside 10 days before and 10 days after the equinoxes. Because of corrupt data and heavy outliers (Fig. S3) the “changeLight” function of the “GeoLight” R packages (Lisovski and Hahn 2012) to estimate the migration schedule did not work properly. The values describing the individual migratory schedules should be treated cautiously. The estimated start of spring migration, e.g. in Cornelia and Joachim (Table 1; Fig. S3) could also be attributed to the start of movements in the wintering area. Some light-level geolocators broke before detachment, and in some the internal geolocator clock drifted (Figs. S3, S8). The area that was visited during winter time was individually estimated based on light-level geolocation estimates (Fig. S3; Table S2). However, we did not consider location estimates derived before 1 November and after 28 February to minimize the influence of the equinoxes on the latitudinal estimates (Table S2). The centroid of the wintering ground for each individual was estimated as the mean ± SD of the estimated locations which are all shown in the corresponding figures. Stopover sites could only be determined for three individuals (Table S2). Kernel densities (45, 75, and 95 %; Epanechnikov kernel) were calculated for wintering grounds of different sets (sex, year) of individuals using the kernelUD function of the R-packages adehabitatHR (Calenge 2006). The ad hoc method was used for the smoothing parameter. The grid was set to 500. The same settings were applied when estimating kernel densities for stopover sites. The distance between the breeding area and the average wintering ground was calculated as the great circle distance between these locations.
Time spent on salt water
The Mk 10 BAS geolocators also recorded saltwater immersion every 3 s and stored number of positive records ranging from 0 (continuously dry) to 200 (continuously wet) at the end of each 10-min period (“wet–dry” information). Immersion data were available for eight individual tracks (two females, six males, Table S3). We estimated the average proportion of time spent on saltwater per hour (0–24 h, Greenwich Mean Time, GMT) and per day (in hours or % of 24 h, and for wintering at the latitude of Dakar, Senegal, we differentiated between daylight (7:30–18:45) and night hours (18:45–7:30).
Defining stages of the annual cycle
Based on the individual light-level geolocation data combined with data from transponders at the colony site (Table 1; Fig. S3) we defined for each individual six different annual stages:
Breeding stage: the bird was at the colony.
Post-breeding stage: the bird had left the colony, but remained in the vicinity of the German Bight and did not start its autumn migration.
Autumn migration: the bird was on the move, but had not reached its wintering area.
Wintering: the time after arrival at the wintering area and before spring migration.
Spring migration: the bird started its spring migration and had not reached the colony.
Pre-breeding: spring migration was finished, but the colony site not reached (sufficient data only in one individual, Table S3).
Defining these stages based on light-level geolocation data was a rough estimate, and small differences between these stages with respect to saltwater contact should be interpreted cautiously.
Statistics
Data were analyzed using the statistical software R 3.1.2 (R Core Team 2014). To assess whether individual birds being tracked for two consecutive winters showed significantly higher winter area fidelity than the population on average, we performed a randomization test, randomly selecting 10,000 pairs of mean wintering locations from our data set. We did not allow that a pair of mean locations consisted of the same locations. If the within-individual difference of the two tracked mean wintering locations were shorter than the 250 shortest distances between randomly selected pairs of mean wintering locations, birds were assessed more faithful than expected by chance.
We tested for seasonal differences in at-sea activity between stages (without the pre-breeding period, owing to insufficient data) using GLMRM (generalized linear model for repeated measurements, SPSS 22). The Mann–Whitney U test was applied when comparing non-parametric differences between two groups. The Wilcoxon signed-rank test was used as a non-parametric test for paired samples. If not otherwise stated values are reported as mean ± 1 SD.
Results
Retrieval of geolocators
Twenty-five out of the 29 tagged birds, i.e., 86 %, returned to the breeding colony the year after deployment. All individuals carrying a light-level geolocator bred in their returning year (Table S1). No bird showed any signs of leg injuries when light-level geolocators were removed. One female had lost her light-level geolocator (Table S1). Twelve of the 24 light-level geolocators contained analyzable data by nine adults (three females and six males, including three pairs).
Potential effects of geolocators
Carrying light-level geolocators did not significantly affect both arrival and laying date, mass at arrival, mass at catching, clutch size, body mass growth of chicks, and ability to fledge chicks (see chapter “Additional information about potential effects of geolocators on common terns” in Electronic Supplementary Material). However, we recorded a strong and significant deterioration of hatching success from 86 to 43 % reducing reproductive output of pairs marked with geolocators severely (Tables S5, S6). The reduced hatchability was caused by eggshell breakage owing to fine fissures increasing with time advancing of incubation by the marked individuals (Figs. S9, S10). In 2011, i.e., the last year of this study, reproductive success of geolocator-birds was successfully increased by exchanging pairs’ original eggs with dummy eggs, and incubating the original eggs in an incubator until geolocators were retrieved. These measures had increased hatching success to 89 % (for details see Electronic Supplementary Material, Table S6).
General temporal–spatial distribution of Common Terns during the non-breeding period
As Common Terns mainly migrated during both equinoxes (Fig. S3), we dispensed with a detailed temporal–spatial analysis of individual movements between the colony and the wintering areas.
Birds left the colony on average on 4 August ±20 days (range 12 July–2 September) and abandoned the German Bight on 2 September ±19 days (22 July–21 September; Table 1). In general, the data suggested that common terns moved along the East Atlantic Flyway and that they predominantly used offshore migration routes (Fig. S3). The sea around the Canaries was identified as a stopover area (Fig. 1; Table S2): two individuals stopped there during autumn migration. One remained in this area approximately for 7 days (Moses in 2010) and the other slightly less than a month (Cornelia in 2009; Table 1, Table S2). Also, during spring migration one individual (Kasimir) stopped there (Table S2). Within 13 days after resuming migration from this stopover area the bird (Kasimir) reached the colony (Table 1, Table S2).
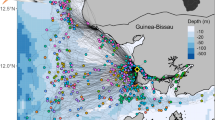
Wintering and stopover locations at Canary Islands of 12 routes of nine Common Terns tracked with light-level geolocators between 2009 and 2011. Breeding site large black dot. Large black triangles (females) and black circles (males) mean winter locations ±SD. Dotted lines 95 kernel densities; dashed lines 75 kernel densities; solid lines 45 kernel densities. Kernel densities at wintering sites were highlighted in three different shades of grey. Birds migrated to their winter locations by flying mainly over water. Small black dots indicate African ring recoveries during December and January of adult common terns from northwest German breeding sites (Helgoland ringing center, n = 30; age at ringing older than 1 year or period between ringing and recovery date >3 years; cf. Bairlein et al. 2014). Map is Mercator projection
Common Terns arrived at the wintering areas on 13 October ±25 days (29 July–1 November, Table 1). Mean wintering period lasted 136 ± 34 days (n = 7, calculated by the individual differences, cf. Table 1). Their preferred wintering areas were the upwelling seas alongside the West African coast of Morocco, Western Sahara, Mauritania, Senegal, The Gambia, Guinea Bissau, Guinea, and Sierra Leone (Fig. 1). Mean great circle distance between the colony and the individual mean wintering locations was 4,782 ± 467 km (range 3,881–5,368 km, n = 12). In autumn, this distance was covered in 41 ± 17 days (n = 12, calculated by the individual differences, cf. Table 1). The mean distances covered per day during southward migration was 158 ± 132 km (n = 12). The four females spent the winter further north (females 20 ± 2.5°N, range 18–24°N, males 13 ± 3.8°N, range 9–19°N; Mann–Whitney U test: U = 30, p = 0.016; Fig. 1) and seemingly more offshore than the eight males (males 107 ± 57 km, range 30–217 km; females 293 ± 255 km, range 86–624 km; Mann–Whitney U test: U = 44, p = 0.174).
The winter distributions were not obviously different between the 2 years (Fig. S5). There was no indication for significant wintering site fidelity, however, as the within-individual distance of the tracked mean wintering locations were not shorter than expected by chance in comparison to the between-individual distance of the mean wintering locations (Figs. S4–S6).
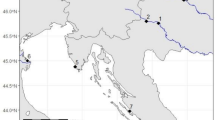
In the three pairs for which light-level geolocation data were available for both partners (Table 1), the general wintering areas and the estimated mean wintering locations did not overlap between the sexes (Fig. 2). There was some spatial overlap of the general wintering area of Cornelia and Kasimir (Fig. 2), but they seemed to be temporally separated (Fig. S3). Distance of pair members’ mean wintering locations was 897 ± 320 km (530–1,120 km, n = 3) and with longer than the median great circle distance (647 km) of the 10,000 randomly chosen mean wintering location pairs (Fig. S4). These sex-specific differences in the mean location of the general wintering areas within breeding pairs supported the general picture of females wintering further off-shore and unrelated to their mates.
Wintering areas of pair mates tracked during the same winter (Ayla, Heiner 2009/2010; Cornelia, Kasimir 2010/2011) or with male one winter later (Marianna 2009/2010, Wieland 2010/2011). Grey dots female; black dots male locations. Symbols and kernel densities (females highlighted in grey) as described in Fig. 1
Spring migration started on average on 22 February ±8 days (15 February–8 March, Table 1). Common terns arrived at the breeding grounds on 20 April ±7 days (11–28 April) so that total time of migration was about 56 ± 8 days (mean ± SD, n = 7) in spring. The mean distance covered per day during northward migration was 88 ± 20 km (n = 7). For these seven birds spring migration lasted significantly longer than autumn migration (autumn: 37 ± 17 days; Wilcoxon signed-rank test: V = 0, p = 0.036, n = 7). Common Terns spent about 117 ± 8 days (n = 11) at the breeding colony or in the vicinity of the colony during the reproductive season. Based on transponder data only the tracked common terns stayed 96 ± 23 days (n = 16) at the colony site.
The within-individual variation of the migration schedule between 2 years varied in general by a few days (Table 1). In 2009 Joachim and Cornelia and in 2010 only Cornelia left the colony and the breeding area on the same day, i.e., autumn migration started on the day individuals were last recorded at the colony by their transponder. Cornelia arrived at the wintering area in the beginning of October in 2009, but to the end of July in 2010. This between-year difference in the estimated arrival time at the wintering area was not explained by the between-year variation in the start of autumn migration (about 1 week).
The return of the young of Ayla, Heiner, and Ernst (Table 1) as prospectors to the colony 2 years later showed that post-fledging parental care of these parents was successful. The temporal patterns of Ayla’s, Heiner’s, and Ernst’s autumn migration, however, were not distinctively different from the adults failing to produce fledglings (Table 1).
Arrival and departure dates at the colony site: a comparison of transponder data and light-level geolocation estimates
After leaving the breeding colony (transponder data) it took on average 31 days before Common Terns started their autumn migration (Table 1; Fig S3). Only two birds had left both the colony site and the breeding area on the same day (Joachim and Cornelia, Table 1; Fig S3). In spring, however, arrival date at the breeding colony detected with the transponder recording system was similar to the estimated arrival date by light-level geolocation data (Table 1).
Saltwater contact during the annual cycle
The proportion of time spent on salt water varied among individuals and stages (Fig. 3, Fig. S7; Table S3). The differences between the stages of the annual cycle were highly significant (F = 10.228, p < 0.001, n = 6; 3 stages, F = 11.711, p = 0.002, n = 8; Fig. 3). During breeding and post-breeding, common terns spent only a small proportion of time on saltwater (1.1–3.5 %). During autumn migration, wintering, and spring migration, however, individuals spent significantly more time on salt water (8.6–13.9 %; Fig. 3, Fig. S7; Table S3 with statistics among single periods). Inter-individual differences were consistent between stages: during all periods, e.g. Ayla or Joachim spent more time at sea than, e.g. Heiner and Moses (between subject effects, F = 37.325, p = 0.002, n = 8; Fig S7; Table S3). There was a tendency that individuals wintering more offshore had more water contact than birds wintering closer to the coast (correlation between proportion of time at sea water with distance from the coast, Pearson, r = 0.624, p = 0.098, n = 8). Furthermore, the daily proportion of time spent at seawater during winter was significantly and positively correlated with the latitude of mean wintering locations of the common terns studied (Pearson, r = 0.743, p = 0.035, n = 8).
Seasonal variation in the temporal proportion of saltwater contact across different stages of the annual cycle. Means of daily percentage of time eight common terns had contact with salt water recorded by using saltwater immersion data from geolocators (B breeding, PB post-breeding, AM autumn migration, W wintering, SM spring migration)
The time spent with saltwater contact varied over the course of the day with respect to the stages of the annual cycle (Fig. 4). During both autumn and spring migration and during winter, Common Terns spent about 10–15 % of the time on salt water during the night. At times around sunrise and sunset proportion of water contact was minimal, but highest between these events (Fig. 4), peaking between 11 and 15 GMT. There was no clear daytime pattern for the other stages of the annual cycle (Fig. 4). With respect to day and night differences in winter, five out of seven individuals had more saltwater contact at daylight than during the night, for the other two individuals it was vice versa (Cornelia and Joachim, who also had most saltwater contact in total, cf. Table S3).
Daily saltwater contact pattern. Mean hourly percentage of time spent on salt water ± standard error of seven common terns recorded using geolocation-immersion loggers during different stages of the annual cycle. Means of values were first calculated for individual birds, then averaged for all birds (without Ayla owing to clock shift, Fig. S8). Vertical lines refer to mean sunrise and sunset hour during wintering. Codes for stages as in Fig. 3
Discussion
Our results show that common terns from the breeding colony in Germany winter in the fish-rich upwelling off the West African coast (Grecian et al. 2016; Fig. 1). Females’ wintering areas were situated further to the north by 7° than that of males. The proportion of time birds had direct contact with salt water varied between the different stages of the annual cycle: While at the breeding area saltwater contact was low, it was high during the migration and wintering periods (Fig. 3). This difference across the annual cycle might be explained by the daily variation of saltwater contact (Fig. 4).
Potential effects of geolocators
Despite the phenomenon of egg breakage (Fig. S10 and below) we found no adverse effects of birds being tagged at the tarsus with a light-level geolocator neither on return rate, body condition, nor arrival date after spring migration or laying date. Return rate to the colony was in the range known for this and other colonies of the common tern (Ezard et al. 2006; Szostek and Becker 2012; Nisbet and Cam 2002; Breton et al. 2014; Palestis and Hines 2015). Return rate of tagged birds was also similar to the rates as reported from other light-level geolocation studies of Sterna terns in general (Nisbet et al. 2011a; Fijn et al. 2013). Returned Common Terns equipped with geolocators were in good physical condition like Arctic Terns (Sterna paradisaea, Egevang et al. 2010; Fijn et al. 2013) and showed no reduction of body mass at arrival or when recaptured. This is in contrast to the findings of Nisbet et al. (2011a) in Common Terns and Mostello et al. (2014) in Roseate Terns Sterna dougallii. Neither arrival date of the birds repeatedly measured before, during, or after deployment of the geolocators nor laying date was affected (for further details see Electronic Supplemental Material). Thus, the various parameters recorded in the individuals tagged with light-level geolocators make us confident that the geolocators did not negatively affect the temporal–spatial distribution of the Common Terns during their non-breeding period.
After return all experimental birds produced normal clutch sizes (in contrast to Arctic Terns, Egevang et al. 2010), but suffered from increased egg breakage (cf. Nisbet et al. 2011a). This was caused by the geolocator and dependent on the number of days the eggs were incubated by a parent carrying a geolocator. Thus, effects of geolocators on the individual fitness can be serious (cf. Scandolara et al. 2014 for barn swallows Hirundo rustica). This effect, however, can be minimized by exchanging natural eggs with dummy eggs soon after laying and by artificially incubating the natural eggs until deployment of the geolocator, or even until hatching.
General temporal–spatial distribution of common terns during the non-breeding period
In agreement with recoveries of adult Common Terns ringed during the breeding period in Germany, this study confirms that individuals from our study site mainly winter in coastal West Africa (Fig. 1). However, ring recoveries suggested that the wintering area of adults from eastern, but also from western Germany is further extended to the south of western Africa than pictured by the birds from Banter See colony (Fig. 1, cf. Neubauer 1982; Bairlein et al. 2014). Common Terns made use of the upwelling zone supplied by the cold Canary current off the northwest African coast (Brenninkmeijer et al. 2002), where primary productivity is higher than in other areas (McGregor et al. 2007; Arístegui et al. 2009). Accordingly, the coastline of about 2,200 km along Mauritania, Senegal, Gambia, Guinea Bissau, Guinea, Sierra Leone to Liberia is a very attractive and important wintering area for many seabird species (Grecian et al. 2016). To reach and leave this area, Common Terns might make use of stopover sites at the seas around the Canary Islands (Fig. 1), similarly to Black Terns Chlidonias niger (van der Winden et al. 2014). Like other tern species passing West African waters, Common Terns mainly use offshore migration routes (Figs. 3, 4, Figs. S3, S7), cf. Arctic Terns (Fijn et al. 2013) and Black Terns (van der Winden et al. 2014).
Wintering site fidelity is described for some seabird species (Phillips et al. 2005; Guilford et al. 2011; Dias et al. 2013). On average the three birds tracked for two seasons did not revisit the exact same wintering area (see “Results”), suggesting a low wintering site fidelity at a narrow spatial scale. However, this may result from a low sample size and indeed site fidelity varied substantially among individuals (Figs. S5, S6). The habitat which common terns seek for wintering is not fixed to a certain location, because biotic and abiotic environmental conditions are on the move with the actual currents. Hence, we do not predict a similar level of high winter site fidelity as found in terrestrial bird species, e.g. Salewski et al. (2000).
The general data indicate that Common Tern females wintered further north than males (Fig. 1), which was supported by within-pair data (Fig. 2). Causes are unknown, but could be related to different nutritional requirements between male and female Common Terns: Nisbet et al. (2002) showed that pair members of Common Terns breeding at Bird Island, MA, USA, had different diets in winter. Females were supposed to feed on a higher trophic level than males. A stable-isotope analysis of feathers from individuals whose gender and wintering site are known could enlighten these interesting findings. Based on our light-level geolocation data, we argue that pair mates do not meet during their wintering period and that in consequence they likely migrated separately from their mate to the colony. Similar results have been found for other seabird species, e.g. the Cory’s Shearwater Calonectris borealis (Müller et al. 2015).
Time schedule of the annual cycle
The general timing of the stages within a year was similar between Common Terns on their East and West Atlantic Flyways (Table 1, cf. Nisbet et al. 2011a). In contrast to the more general pattern that avian spring migration is faster than autumn migration (Nilsson et al. 2013), Common Terns reached their seasonally appropriate migratory goal in on average 41 days in autumn, but 55 days in spring. This may be a consequence of prevailing winds, rotating clockwise in the North Atlantic and offering tailwind during autumn migration, but headwind during spring migration (Liechti 2006). For the few birds tracked along the West Atlantic Flyway, however, spring migration was faster than autumn migration (Nisbet et al. 2011a) again in agreement with prevailing wind directions. However, these results should be treated cautiously given the location error in light-level geolocation estimates and the low sample sizes.
Most adult Common Terns lingered for 4 weeks around the breeding area, as inferred by the time passed between the last detection at the colony site by the transponder system and the first sign of migration from geolocation. A similar pattern was described by Nisbet et al. (2011a) showing that adult Common Terns stayed about 100–200 km to the east or the west of the breeding colony before starting autumn migration. The reason for this behavior remains speculative. Possibly, adults care for their offspring, which they may guard and feed up to several weeks after fledging (Burger 1980; Becker and Ludwigs 2004; Nisbet et al. 2011b: at least until end of September; for other tern species see Ashmole and Tovar 1968). Parents may familiarize their offspring with the extended surroundings of the colony site or to reach more productive feeding grounds (cf. Fijn et al. 2013). Adults may also accumulate energy, in terms of fat and muscle mass, as a preparation for the upcoming migrations. Our light-level geolocator data indicated that the delay until the final departure of adults for migration was independent of sex (Table 1). This is in contrast to the findings of Nisbet et al. (2011a, b) showing that females started earlier than males presumably because the post-fledgling guarding is mostly provided by the fathers (Nisbet et al. 2011b).
Saltwater contact during the annual cycle
Common terns spent small proportions of time resting on saltwater during the breeding period (Figs. 3, 4). This saltwater contact was likely explained by bathing as Common Terns do not swim in the breeding area (PHB personal observations; Nisbet 2002; Nisbet et al. 2011a). During the non-breeding season, however, the birds spent more time on salt water, confirming observations of Common Terns from the West Atlantic Flyway (Nisbet et al. 2011a; Neves et al. 2015). The inter-individual differences in saltwater contact during both migration periods and wintering along the West African coast might be due to individual selection of habitats. In contrast to other individuals who spent most time resting at sea water during the day, Cornelia and Joachim showed high saltwater contact during the night, which they obviously had spent offshore (Fig. S8). Perhaps inter-individual variation in wintering habitat selection may be influenced by an extended parental care; hence, wintering on the coast might be beneficial if parents still care for their offspring (e.g. potentially in Heiner, Ayla and Ernst), so that juveniles in poor body condition can easily find sites for resting on beaches or sandbars (e.g. Bugoni et al. 2005; Blokpoel et al. 1982, 1984). Whether Common Terns care for their offspring at wintering sites is still unclear, but juvenile Royal Terns Thalasseus maximus were fed by adults during wintering in Peru in December and January, when they were about 7 months old (Ashmole and Tovar 1968).
Changes in the daily routines of Common Terns as suggested by the saltwater contact data could likely be explained to a certain extent by their daily foraging pattern. Radio-tracked Common Terns spending the non-breeding season in southern Brazil usually started foraging from roosting sites on the beach or sandbars in the morning or late afternoon (Bugoni et al. 2005). The low proportion of saltwater contact during sunrise and sunset (Fig. 4) is, therefore, likely to be related to the foraging behavior of Common Terns, considering that during the short plunge dives no saltwater contact was recorded, cf. in the breeding period (Figs. 3, 4). Another explanation of the high proportion of saltwater contact during the non-breeding period in common terns on the West (Nisbet et al. 2011a) and East Atlantic Flyways could be thermoregulatory necessities: during noon at areas close to the equator (Fig. 4) they may cool down their body temperature, which might be heated up considerably by the high solar irradiation. This is corroborated by the significant positive correlation of Common Terns’ saltwater contact per day with higher latitude of the wintering locations coming along with decreasing sea water temperatures. Moreover, water contact was highest during spring migration (Figs. 3, 4) when also sunshine duration is highest in Senegal and Mauretania, concomitant with lowest sea surface temperatures due to upwelling (19–20 °C, February and March; e.g. Hayward and Oguntoyinbo 1987; http://www.iten-online.ch/klima/afrika) that the temperature gradient between birds’ legs and sea water should warrant body heat release. Another explanation of longer resting times at sea during noon (Fig. 4) may be related to winds, since wind speed is typically higher at midday than at sunrise and sunset, possibly handicapping the terns’ flight. Gannets Sula bassana, too, wintering off West Africa spend more time on the sea water during daylight than conspecifics wintering at the Bay of Biscaya or the North Sea (Garthe et al. 2012). There is a need of detailed behavioral observations of terns and other seabirds in their wintering areas to clarify these speculations on persisting parental care and thermoregulation by offshore swimming.
General migration patterns of Common Tern populations studied by geolocation
Our study adds to the three investigations published to date of Common Tern migration based on light-level geolocators (Nisbet et al. 2011a, b; Neves et al. 2015; Moore et al., personal communication). Overall, these studies clearly show a strong east–west separation in their migration routes and wintering areas among breeding populations and connectivity at broad spatial scales (Fig. 5). Some studies on pelagic seabirds have also found a certain degree of migratory connectivity (e.g. Cory’s Shearwater Calonectris diomedea, González-Solís et al. 2007, Bulwer’s petrel Bulweria bulwerii, Ramos et al. 2015), but Common Terns are more coastal seabirds and their longitudinal change in migratory routes parallel those found in terrestrial birds of the Palearctic–Tropical and Nearctic–Neotropical migratory systems (e.g. Trierweiler et al. 2014; Hallworth et al. 2015). Such knowledge is important to understand migration strategies and for conservation concerns. Based on information about the migratory connectivity we can recognize and elucidate impacts of population-level threats during the non-breeding period, which may affect demographic rates or traits of migration timing (e.g. in Common Terns: Szostek and Becker 2015; Szostek et al. 2015). The differences in the wintering areas and migratory flyways of Common Terns breeding, in geographical terms, in relative close vicinity to each other are striking for seabirds. Common Terns breeding in northwest Germany and on the Azores are separated to a larger scale in winter when visiting the West African coast or the eastern South American coast, respectively, than in summer. A similar pattern exists for the breeding populations in North America: Common Terns from the northeast Atlantic coast (Bird Island) spent their winter along the eastern South American coast and mix with birds from the Azores breeding population, whereas Common Terns from the Great Lakes winter along the eastern Pacific coast in South America (Fig. 5). Ring recoveries suggest similar divergence of wintering sites for further common tern populations (Neubauer 1982; Bairlein et al. 2014; Cohen et al. 2014). The origin and causes of the population-specific migration patterns and wintering areas in Common Terns may be driven by geographical structures and barriers such as mountains, coastline courses, wind patterns, currents, water bodies, or oceans.
Breeding grounds (indicated by different symbols), migration routes (diverse lines), and wintering areas (differently shaded areas) of Common Terns tracked with light-level geolocators. Migration routes are rough estimates. Data are from four populations of Common Terns breeding in north Germany (this study), on the Azores (Neves et al. 2015), at MA, USA (Nisbet et al. 2011a, b), and Great Lakes, Canada (Moore et al., personal communication)
References
Arístegui J, Gasol JM, Duarte CM, Herndl GJ (2009) Microbial oceanography of the dark ocean’s pelagic realm. Limnol Oceanogr 54:1501–1529
Ashmole NP, Tovar HS (1968) Prolonged parental care in royal terns and other birds. Auk 85:90–100
Bairlein F, Dierschke J, Dierschke V, Salewski V, Geiter O, Hüppop K, Köppen U, Fielder W (2014) Atlas des Vogelzugs. AULA, Wiebelsheim
Becker PH (2010) Populationsökologie der Flussseeschwalbe: Das Individuum im Blickpunkt. In: Bairlein F, Becker PH (eds) 100 Jahre Institut für Vogelforschung “Vogelwarte Helgoland”. Aula, Wiebelsheim, pp 137–155
Becker PH, Ludwigs J-D (2004) Sterna hirundo common tern. In: Parkin D (ed) BWP update, vol 6, nos 1/2. Oxford University Press, NY, pp 93–139
Becker PH, Wink M (2003) Influences of sex, sex composition of brood and hatching order on mass growth in common terns (Sterna hirundo). Behav Ecol Sociobiol 54:136–146
Becker RA, Chambers JM, Wilks AR (1988) The new S language. Wadsworth and Brooks/Cole, Belmont
Becker PH, Wendeln H, González-Solís J (2001) Population dynamics, recruitment, individual quality and reproductive strategies in common terns marked with transponders. Ardea 89(special issue):239–250
Becker PH, Dittmann T, Ludwigs J-D, Limmer B, Ludwig SC, Bauch C, Braasch A, Wendeln H (2008) Timing of initial arrival at the breeding site predicts age at first reproduction in a long-lived migratory bird. PNAS 105:12349–12352
Blokpoel H, Morris R, Trull P (1982) Winter observations of common terns in Trinidad, Guyana, and Suriname. Colon Waterbirds 5:144–147
Blokpoel H, Morris R, Tessier G (1984) Field investigations of the biology of common terns wintering in Trinidad. J Field Ornithol 55:424–434
Brenninkmeijer A, Stienen EWM, Klaassen M, Kersten M (2002) Feeding ecology of wintering terns in Guinea-Bissau. Ibis 144:602–613
Breton AR, Nisbet ICT, Mostello CS, Hatch JJ (2014) Age-dependent breeding dispersal and adult survival within a metapopulation of common terns Sterna hirundo. Ibis 156:534–547
Bruderer B, Boldt A (2001) Flight characteristics of birds: 1. Radar measurements of speeds. Ibis 143:178–204
Bugoni L, Cormons TD, Boyne AW, Hays H (2005) Feeding grounds, daily foraging activities, and movements of common terns in Southern Brazil, determined by radio-telemetry. Waterbirds 28:468–477
Burger J (1980) The transition to independence and post fledging parental care in seabirds. In: Burger J, Olla BL, Winn HE (eds) Marine birds. Behavior of marine animals, vol 4. Plenum Press, New York, pp 367–447
Calenge C (2006) The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519
Cohen EB, Hostetler JA, Royle JA, Marra PP (2014) Estimating migratory connectivity of birds when re-encounter probabilities are heterogeneous. Ecol Evol 4:1659–1670
Dias MP, Granadeiro JP, Catry P (2013) Individual variability in the migratory path and stopovers of a long-distance pelagic migrant. Anim Behav 86:359–364
Egevang C, Stenhouse IJ, Phillips RA, Petersen A, Fox JW, Silk JRD (2010) Tracking of Arctic terns Sterna paradisaea reveals longest animal migration. Proc Natl Acad Sci 107:2078–2081
Ekstrom PA (2004) An advance in geolocation by light. Mem Natl Inst Polar 58:210–226
Ezard T, Becker PH, Coulson T (2006) The contributions of age and sex to variation in common tern population growth rate. J Anim Ecol 75:1379–1386
Fijn RC, Hiemstra D, Phillips RA, van der Winden J (2013) Arctic terns Sterna paradisaea from the Netherlands migrate record distances across three oceans to Wilkes Land, East Antarctica. Ardea 101:3–12
Fudickar AM, Wikelski M, Partecke J (2012) Tracking migratory songbirds: accuracy of light-level loggers (geolocators) in forest habitats. Methods Ecol Evol 3:47–52
Garthe S, Benvenuti S, Montevecchi WA (2000) Pursuit plunging by northern gannets (Sula bassana) “feeding on capelin (Mallotus villosus)”. Proc R Soc B 267:1717–1722
Garthe S, Ludynia K, Hueppop O, Kubetzki U, Meraz JF, Furness RW (2012) Energy budgets reveal equal benefits of varied migration strategies in northern gannets. Mar Biol 159:1907–1915
González-Solís J, Croxall JP, Oro D, Ruiz X (2007) Trans-equatorial migration and mixing in the wintering areas of a pelagic seabird. Front Ecol Environ 5:297–301
Grecian WJ, Witt MJ, Attrill MJ (2016) Multi-species top predator tracking reveals link between marine biodiversity and ocean productivity. Biol Lett (in revision)
Guilford T, Meade J, Willis J, Phillips RA, Boyle D, Roberts S, Collet M, Freeman R, Perrins CM (2009) Migration and stopover in a small pelagic seabird, the Manx shearwater Puffinus puffinus: insights from machine learning. Proc R Soc B 276:1215–1223
Guilford T, Freeman R, Boyle D, Dean B, Kirk H, Phillips R, Perrins C (2011) A dispersive migration in the Atlantic puffin and its implications for migratory navigation. PLoS One 6:e21336
Hallworth MT, Sillett TS, Van Wilgenburg SL, Hobson KA, Marra PP (2015) Migratory connectivity of a Neotropical migratory songbird revealed by archival light-level geolocators. Ecol Appl 25:336–347
Harrison P (1997) Seabirds of the world. Princeton University Press, Princeton
Hayward DF, Oguntoyinbo J (1987) The climatology of West Africa. Hutchinson, London
Hill RD (1994) Theory of geolocation by light levels. In: Burney J, Boeuf BJ, Laws RM (eds) Elephant seals: population ecology, behavior, and physiology. University of California Press, Berkeley, pp 227–236
Liechti F (2006) Birds: Blowin’ by the wind? J Ornithol 147:202–211
Limmer B, Becker PH (2007) The relative role of age and experience in determining variation in body mass during the early breeding career of the common tern (Sterna hirundo). Behav Ecol Sociobiol 61:1885–1896
Lisovski S, Hahn S (2012) GeoLight—processing and analysing light-based geolocator data in R. Methods Ecol Evol 3:1055–1059
Lisovski S, Hewson CM, Klaassen RHG, Korner-Nievergelt F, Kristensen MW, Hahn S (2012) Geolocation by light: accuracy and precision affected by environmental factors. Methods Ecol Evol 3:603–612
McGregor HV, Dima M, Fischer HW, Mulitza S (2007) Rapid 20th-century increase in coastal upwelling off northwest Africa. Science 315:637–639
Mostello CS, Nisbet ICT, Oswald SA, Fox JW (2014) Non-breeding season movements of six North American roseate terns Sterna dougallii tracked with geolocators. Seabird 27:1–21
Müller MS, Massa B, Phillips RA, Dell’Omo G (2015) Seabirds mated for life migrate separately to the same places: behavioural coordination or shared proximate causes? Anim Behav 102:267–276
Neubauer W (1982) Der Zug mitteleuropaeischer Flusseeschwalben (Sterna hirundo) nach Ringfunden. Ber Vogelwarte Hiddensee 2:59–82
Neves VC, Nava CP, Cormons M, Bremer E, Castresana G, Lima P, Azevedojun SM, Phillips RA, Magalhães MC, Santos RS (2015) Migration routes and non-breeding areas of common terns (Sterna hirundo) from the Azores. Emu 115:158–167
Nielsen A, Sibert JR (2007) State-space model for light-based tracking of marine animals. Can J Fish Aquat Sci 64:1055–1068
Nilsson C, Klaassen RH, Alerstam T (2013) Differences in speed and duration of bird migration between spring and autumn. Am Nat 181:837–845
Nisbet ICT (2002) Common tern. Birds N Am 618:1–39
Nisbet ICT, Cam E (2002) Test for age-specificity in survival of the common tern. J Appl Stat 29:65–83
Nisbet ICT, Montoya JP, Burger J, Hatch JJ (2002) Use of stable isotopes to investigate individual differences in diets and mercury exposures among common terns Sterna hirundo in breeding and wintering grounds. Mar Ecol Prog Ser 242:267–274
Nisbet ICT, Mostello CS, Veit RR, Fox JW, Afanasyev V (2011a) Migrations and winter quarters of five common terns tracked using geolocators. Waterbirds 34:32–39
Nisbet ICT, Szczys P, Mostello CS, Fox JW (2011b) Female common terns Sterna hirundo start autumn migration earlier than males. Seabird 24:103–106
Palestis BG, Hines JE (2015) Adult survival and breeding dispersal of common terns (Sterna hirundo) in a declining population. Waterbirds 38:221–228
Pennycuick CJ, Åkesson S, Hedenström A (2013) Air speeds of migrating birds observed by ornithodolite and compared with predictions from flight theory. J R Soc Interface 10:20130419
Phillips RA, Silk JRD, Croxall JP, Afanasyev V, Briggs DR (2004) Accuracy of geolocation estimates for flying seabirds. Mar Ecol Prog Ser 266:265–272
Phillips RA, Silk JRD, Croxall JP, Afanasyev V, Briggs DR (2005) Summer distribution and migration of nonbreeding albatrosses: individual consistencies and implications for conservation. Ecology 86:2386–2396
R Core Team (2014) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Ramos R, Sanz V, Militão T et al (2015) Leapfrog migration and habitat preferences of a small oceanic seabird, Bulwer’s petrel (Bulweria bulwerii). J Biogeogr 42:1651–1664
Ropert-Coudert Y, Wilson RP, Grémillet D, Kato A, Lewis S, Ryan PG (2006) Electrocardiogram recordings in free-ranging gannets reveal minimum difference in heart rate during flapping versus gliding flight. Mar Ecol Prog Ser 328:275–284
Salewski V, Bairlein F, Leisler B (2000) Recurrence of some palaearctic migrant passerine species in West Africa. Ringing Migr 20:29–30
Scandolara C, Rubolini D, Ambrosini R, Caprioli M, Hahn S, Liechti F, Romano A, Romano M, Sicurella B, Saino N (2014) Impact of miniaturized geolocators on barn swallow Hirundo rustica fitness traits. J Avian Biol 45:417–423
Shaffer SA, Tremblay Y, Weimerskirch H, Scott D, Thompson DR, Sagar PM (2006) Migratory shearwaters integrate oceanic resources across the Pacific Ocean in an endless summer. Proc Nat Acad Sci 103:12799–12802
Sommerfeld J, Kato A, Ropert-Coudert Y, Garthe S, Hindell MA (2013) The individual counts: within sex differences in foraging strategies are as important as sex-specific differences in masked boobies Sula dactylatra. J Avian Biol 44:531–540
Sorenson MC, Hipfner JM, Kyser TK, Norris DR (2009) Carry-over effects in a Pacific seabird: stable isotope evidence that non-breeding diet quality influences reproductive success. J Anim Ecol 78:460–467
Sumner MD, Wotherspoon SJ, Hindell MA (2009) Bayesian estimation of animal movement from archival and satellite tags. PLoS One 4:e7324
Szostek L, Becker PH (2012) Terns in trouble: demographic consequences of low breeding success and recruitment on a common tern population in the German Wadden Sea. J Ornithol 153:313–326
Szostek KL, Becker PH (2015) Marine primary productivity in the wintering area influences survival and recruitment in a migratory seabird. Oecologia 178:643–657
Szostek KL, Bouwhuis S, Becker PH (2015) Are arrival date and body mass after spring migration influenced by large-scale environmental factors in a migratory seabird? Front Ecol Evol 3:42
Trierweiler C, Klaassen RGH, Drent RH, Exo K-M, Komdeur J, Bairlein F, Koks BJ (2014) Population specific migration routes and migratory connectivity in a long-distance migratory raptor. Proc R Soc B Lond 281:1471–2954
van der Winden J, Fijn RC, van Horssen PW, Gerritsen-Davidse D, Piersma T (2014) Idiosyncratic migrations of black terns (Chlidonias niger): diversity in routes and stopovers. Waterbirds 37:162–174
Weimerskirch H, Wilson RP (2000) Oceanic respite for wandering albatrosses. Nature 406:955–956
Weimerskirch H, Bonadonna F, Bailleul F, Mabille G, Dell’Omo G, Lipp H-P (2002) GPS tracking of foraging albatrosses. Science 295:1259
Wendeln H, Becker PH (1999) Significance of ring removal in Africa for a common tern Sterna hirundo colony. Ringing Migr 19:210–212
Wernham CV, Toms MP, Marchant JH, Clark JA, Siriwardena GM, Baillie SR (2002) The migration atlas: movements of the birds of Britain and Ireland. T & AD Poyser, London
Wilson RP, Cooper J, Plötz J (1992a) Can we determine when marine endotherms feed? A case study with seabirds. J Exp Biol 167:267–275
Wilson RP, Ducamp JJ, Rees G, Culik BM, Niekamp K (1992b) Estimation of location: global coverage using light intensity. In: Priede IG, Swift SM (eds) Wildlife telemetry: remote monitoring and tracking of animals. Ellis Horwood Ltd, Chichester, pp 131–134
Wilson RP, Weimerskirch H, Lys P (1995) A device for measuring seabird activity at sea. J Avian Biol 26:172–175
Wilson RP, Grémillet D, Syder J, Kierspel MAM, Garthe S, Weimerskirch H, Schäfer-Neth C, Scolaro JA, Bost C-A, Plötz J, Nel D (2002) Remote-sensing systems and seabirds: their use, abuse and potential for measuring marine environmental variables. Mar Ecol Prog Ser 228:241–261
Zhang H, Vedder O, Becker PH, Bouwhuis S (2015) Age-dependent trait variation: the relative contribution of within-individual change, selective appearance and disappearance in a long-lived seabird. J Anim Ecol 84:797–807
Acknowledgments
We thank Christina Bauch, Alexander Braasch, Julia Spieker, Lesley Szostek, Katharina Weißenfels, Silas Wolf, and Christian Wolter for their help with field work. Simeon Lisovski helped with analyzing the light-level geolocation data. Kathrin Hüppop helped preparing the figures. We thank Dave Moore giving access to unpublished data of migration of common terns breeding at the Great Lakes and Olaf Geiter for providing ring recovery data of common terns. The manuscript was improved by helpful comments of Franz Bairlein and two anonymous reviewers. The studies were performed under license of the Nds. Landesamt für Verbraucherschutz und Lebensmittelsicherheit Oldenburg and of the Stadt Wilhelmshaven. H.S. is financed by the Deutsche Forschungsgemeinschaft (SCHM 2647/1-1) which also supported the project (BE 916/8 and 9).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by N. Chernetsov.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Becker, P.H., Schmaljohann, H., Riechert, J. et al. Common Terns on the East Atlantic Flyway: temporal–spatial distribution during the non-breeding period. J Ornithol 157, 927–940 (2016). https://doi.org/10.1007/s10336-016-1346-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-016-1346-2