Abstract
Understanding patterns in avian migration phenology and the proximate mechanisms for such patterns is important for assessing behavioural responses of individuals or populations to climate change. Among songbirds, protandry in spring is a common pattern; phenology in fall is less well described. Using tracking data collected from geolocators deployed at a breeding site, and capture data from banding stations, we assessed fall and spring migration phenology of an Arctic-breeding passerine, the Snow Bunting (Plectrophenax nivalis), by sex and age. We measured migration timing, speed, and distance, as well as duration of migration stopovers to test proximate mechanisms for observed sex and age differences in spring and fall migration phenology. During fall migration, hatch-year birds preceded adults, and adult males tended to precede adult females; however, there remained extensive variation by year. Males and females tracked directly arrived at winter sites at approximately the same time. During early spring migration, Snow Buntings exhibited moderate protandry, where after-second-year males preceded all other age-sex classes by ~6 days, on average. Surprisingly, protandry was not apparent at late spring migration or at breeding arrival. Instead, arrival dates by sex and age appeared highly variable between years. The winter site arrival date was predicted by fall migration departure date, total number of stopover days, migration speed, and migration distance. The breeding site arrival date was similarly predicted by spring migration departure date, total stopover days, and migration speed. Our results provide key baseline data for monitoring ongoing changes in migration phenology of this important Arctic-breeding songbird, as climate change effects become more pronounced across temperate and Arctic regions.
Zusammenfassung
Zugphänologie in Frühjahr und Herbst bei einem in arktischen Regionen brütenden Singvogel
Um Verhaltensreaktionen von Individuen oder Populationen auf den Klimawandel beurteilen zu können, ist es wichtig, die Muster der Vogelzugphänologie sowie die ihnen unmittelbar zugrunde liegenden Mechanismen zu verstehen. Bei Singvögeln ist Protandrie im Frühling ein häufig zu beobachtendes Muster; die Phänologie im Herbst ist weniger gut beschrieben. Anhand von Peildaten aus Geolokatoren, die den Vögeln an einem Brutplatz angelegt wurden, sowie Fangdaten von Beringungsstationen untersuchten wir die Herbst- und Frühjahrs-Zugphänologie eines in arktischen Regionen brütenden Singvogels, der Schneeammer (Plectrophenax nivalis), nach Alter und Geschlecht. Wir bestimmten den zeitlichen Ablauf des Zuggeschehens, Geschwindigkeit und Entfernung ebenso wie die Dauer von Zugunterbrechungen, um die den beobachteten Geschlechts- und Altersunterschieden in der Frühjahrs- und Herbst-Zugphänologie unmittelbar zugrunde liegenden Mechanismen zu untersuchen. Auf dem Herbstzug flogen diesjährige Vögel früher als die Adulten weg; adulte Männchen zogen tendenziell vor den adulten Weibchen; allerdings gab es hier eine breitgestreute Variation von Jahr zu Jahr. Die durch Besenderung direkt verfolgten Männchen und Weibchen kamen etwa zeitgleich in den Überwinterungsgebieten an. Während des zeitigen Frühjahrszuges zeigten die Schneeammern gemäßigte Protandrie, wobei Männchen über dem zweiten Lebensjahr allen anderen Alters- und Geschlechtsklassen im Schnitt um etwa sechs Tage voraus waren. Überraschenderweise war weder auf dem späten Frühjahrszug noch bei der Ankunft im Brutgebiet Protandrie zu beobachten. Stattdessen erschienen die Ankunftsdaten nach Geschlecht und Alter von Jahr zu Jahr höchst variabel. Das Ankunftsdatum im Überwinterungsgebiet konnte mithilfe des Abzugsdatums beim Herbstzug, der Gesamtzahl von Rasttagen und der Zugstrecke vorhergesagt werden. Das Ankunftsdatum im Brutgebiet ließ sich auf ähnliche Weise anhand des Abzugsdatums beim Frühjahrszug, der Summe der Rasttage und der Zuggeschwindigkeit vorhersagen. Unsere Ergebnisse liefern wichtige Grunddaten zum Monitoring stattfindender Veränderungen der Zugphänologie dieses wichtigen arktischen Brutvogels, während sich die Auswirkungen des Klimawandels in gemäßigten und arktischen Regionen stärker bemerkbar machen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent global warming has resulted in changes in migration phenology for many bird species (Mills 2005a; Thorup et al. 2007; Tøttrup and Thorup 2008; Travers et al. 2015). Migration phenology has important consequences for fitness of migratory animals, as organisms must synchronize their arrival and subsequent breeding activities to the conditions at distant breeding sites to optimize reproductive success (Gienapp et al. 2014). Because of climate-induced changes in resource phenology, some migratory birds are now arriving and nesting out-of-sync with key breeding-site resources, with negative consequences for overall population persistence (Both et al. 2006). In some species, differences in spring migration phenology exist between sexes and age classes (Maggini and Bairlein 2012; McKinnon et al. 2014) and may differentially affect the response by each group to changing environmental conditions over time (Harnos et al. 2015). Fall migration phenology, though less well studied, may also influence subsequent winter survival (Stutchbury et al. 2011), and may be changing in response to global warming (Mills 2005a; Tøttrup et al. 2006). Thus, predicting individual fitness or population-level responses to climate change requires full-life-cycle information on migration phenology in spring and fall, for both males and females and different age classes.
Spring migration phenology in migratory songbirds is driven by a balance between sexual selection on individuals to arrive first at breeding sites to claim territories and mates and natural selection against arriving too early (Coppack and Pulido 2009). This typically results in migratory protandry, where males arrive earlier to breeding sites than females (Morbey and Ydenberg 2001). Ultimate hypotheses for protandry at breeding arrival have been explored using both empirical (Coppack et al. 2006; Saino et al. 2010; Tøttrup and Thorup 2008) and theoretical data (Kokko et al. 2006). In migratory birds, the majority of results to date support the ‘mate opportunity’ hypothesis, where selection acts directly on males and females to arrive at breeding sites at an optimal time to maximize reproductive success through mate selection (Canal et al. 2012; Coppack et al. 2006; Kokko et al. 2006; Tøttrup and Thorup 2008). Natural selection on early-arriving males may be reduced, if mortality associated with cold weather extremes in spring is lower due to a warming climate (Gienapp et al. 2014). Strong sexual selection is also associated with more significant increases in phenology for populations overall, with a trend for advances in timing of the earliest birds (i.e., males) (Spottiswoode et al. 2006). This emphasizes the need to examine intra-specific patterns in migration phenology, as population-level analyses may obscure changes that occur only in some groups.
Phenology in fall is much less studied than in spring, despite the importance of many fall processes and events in influencing fitness (Gallinat et al. 2015). In songbirds, fall migratory protogyny (females preceding males) may be a more common pattern (Mills 2005b). Predictions about fall phenology can be derived from the same hypotheses that predict migratory protandry in spring. For example, if males have an advantage from defending future breeding territories (mate opportunity hypothesis) males may be selected to remain at breeding sites as long as possible (Bai and Schmidt 2012). In contrast, breeding systems where females invest proportionally more in provisioning young later in the breeding season, and where environmental conditions at breeding sites deteriorate rapidly, may promote migratory protandry in fall. This pattern is found in ducks and some shorebirds (Newton 2008), and also in Aquatic Warblers (Acrocephalus paludicola) (Wojczulanis-Jakubas et al. 2013).
The mechanisms that account for differences in phenology by age and sex include: (1) differential migration speed, (2) differential initiation of migration, and (3) differential migration distance/destination (e.g., latitudinal segregation of sexes) (Coppack and Pulido 2009). In some birds, males may have more efficient wing morphology (e.g., Swainson’s Thrushes, Catharus ustulatus) (Bowlin and Wikelski 2008), which could lead to more efficient refuelling and shorter stopovers, and, thus, an overall advance in arrival dates. Both corticosterone and testosterone can increase migratory preparedness in birds by stimulating hyperphagia and fat deposition (Holberton 1999; Tonra et al. 2011), which likely contributes to the finding that males in some species refuel at stopovers significantly faster in spring relative to females (Common Yellowthroat, Geothlypis trichas; Yellow-rumped Warbler, Setophaga coronata) (Seewagen et al. 2013). If males arrive earlier in spring because of more efficient flight, we might also predict that they arrive at wintering sites faster in autumn, barring other selective pressures. Differential refuelling rates were not apparent between adults and hatch-year birds (Common Yellowthroat, Yellow-rumped Warbler, Swainson’s Thrush, and White-throated sparrow, Zonotrichia albicollis) during autumn (Seewagen et al. 2013), suggesting that in at least some species, migration of adults and hatch-year birds should be similar in autumn.
Intra-specific differences in arrival at breeding or wintering sites may also be related to differences in initiation of migration. Recent controlled laboratory studies have found that male Northern Wheatears (Oenanthe oenanthe) have an earlier endogenous clock than females such that under constant photoperiod and access to food, males exhibit zugunruhe (migratory restlessness) earlier in spring (Maggini and Bairlein 2012). Males and or dominant adults of both sexes may monopolize high-quality winter habitats or food resources, allowing them to prepare for spring migration faster and depart earlier than females or young birds wintering in the same sites (Marra and Holmes 2001; Studds and Marra 2011). During fall migration, differential timing of breeding across age classes resulted in first-year breeding Dunlin (Calidris alpina) arriving before older birds when reproductive success was high, and after older birds when reproductive success was low (Meissner 2015). Thus, endogenous programs, differential winter habitat occupancy, timing of breeding, and breeding success can all result in intra-specific differences in migration initiation that carry-over to affect arrival phenology.
Finally, latitudinal segregation by sex, where males winter closer to breeding sites than females, has been documented in many species across broad taxonomic groups (Cristol et al. 1999). Such range-wide differences in winter site occupancy between sex classes can result in protandry in spring and fall, even if birds of both sexes depart on migration synchronously. However, there are few species in which winter segregation between the sexes is enough to account for large observed lags between male and female arrival at breeding sites. As such, multiple interacting mechanisms likely contribute to intra-specific migration phenology in both spring and fall. For example, males may winter closer to breeding sites, depart earlier on spring migration, and fly/refuel faster, all of which contribute to sex differences in arrival phenology.
Using geolocators and data from banding stations, we assessed migration phenology by sex and age in Snow Buntings (Plectrophenax nivalis). Snow Buntings are small long-distance Arctic-breeding migrants, and males have been recorded at breeding sites as much as 6 weeks in advance of females (Montgomerie and Lyon 2011). Sexual dimorphism and high capture rates at banding stations make Snow Buntings an excellent passerine model for examining sex and age-patterns in migratory phenology. We first quantified the general phenology patterns by sex and age during both spring and fall migration. We then tested proximate mechanisms accounting for variability in winter and breeding site arrival date. Our previous work indicated that males and females tracked directly did not have significant spring migration distances (Macdonald et al. 2015); therefore, in this study we ruled out latitudinal segregation of the sexes as a mechanism contributing to differences in phenology. If males benefit from remaining at breeding sites as long as possible to defend territories (mate-opportunity hypothesis), we predicted that Snow Buntings would show an overall pattern of migratory protandry in spring and protogyny in fall. In order to arrive at breeding sites early and in advance of females, we predicted faster speeds and fewer stopovers for males relative to females. We also predicted that the migration initiation date would contribute to variation in arrival dates. In fall we predicted that males would show later migration timing but continue to migrate faster and stopover for fewer days relative to females. Finally, we tested for effects of age on migration patterns, with the prediction that first-year birds would be later than adults on fall and spring migration (Newton 2008). This could be owing to less experience (Mitchell et al. 2015; Sergio et al. 2014), less efficient wing morphology (Alatalo et al. 1984), or selection for differing migration strategies (Hill 1989).
Methods
Animal care statement
All methods followed the Canadian Council for Animal Care recommendations, as reviewed by Environment Canada. Bird banding and handling permission was obtained from the Bird Banding Office of Canada (East Bay Island, Nunavut: permit 10808; Thunder Cape Bird Observatory, Ontario and Rivère-St.-Jean, Quebec, various permit holders). Geolocator protocols were reviewed and approved by the University of Windsor’s Animal Utilization Committee (protocol AUPP # 9-14).
Light-level geolocators
We deployed light-level geolocators (British Antarctic Survey, 2010: model MK12S, 2011 and 2012: MK20AS) at a long-term study site at East Bay Island (EBI), in Nunavut, Canada (‘EBI’, 64.01N, 81.47W), on locally breeding male and female Snow Buntings in 2010 (n = 30), 2011 (n = 28), and 2012 (n = 25). Geolocators weighed 0.8 g (total weight 1.1 g, or ~3 % of mean adult body weight) and were attached by a leg-loop harness (Rappole and Tipton 1991) of 2.5 mm wide Teflon ribbon (Stutchbury et al. 2009). Geolocators were deployed post-breeding in 2010 and 2011; they were deployed earlier in 2012 but only on known local breeders (recaptures from previous years or other signs of breeding such as a brood patch). In previous studies we report on tests for negative effects of geolocators on return rates by sex and age (Macdonald et al. 2015). We found no detectable negative effects of 1.1 g geolocator leg-loop harness backpacks on Snow Buntings (no difference in return rates by age, sex, or size). We deployed 83 geolocators on adult Snow Buntings and retrieved 21 (2011, n = 6; 2012, n = 7; 2013, n = 8). One geolocator failed before recording any migration information, and two others recorded fall migration but failed prior to spring migration. Thus, the total sample size for fall migration was 20 and for spring migration was 18.
Light data from geolocators retrieved from Snow Buntings were downloaded, decompressed, and visually inspected to score the quality of the light transitions indicating sunrise and sunset. We used a sun elevation angle of −4.05 to transform light levels into latitudes and longitudes using the software program Locator (British Antarctic Survey). This sun elevation was an average calculated from light data recorded when each bird was still known to be at the breeding site (EBI). Snow Buntings are an open-country species, thus habitat shading contributed very little to errors in light data (Lisovski et al. 2012). Snow Buntings nest and roost in cavities at breeding sites, thus the breeding arrival date was evident by a drastic shift in light levels corresponding with cavity use. We estimated the departure date from breeding sites by examining longitudes for consistent shifts by more than 2°. We relied primarily on longitude, because it is more accurate than latitude, and can provide information even during the autumnal and vernal equinoxes (~15 days before and after the equinox dates), when latitude estimates cannot be calculated (as day length is similar at all latitudes) (Fudickar et al. 2012; McKinnon et al. 2013). Consecutive noon locations that were more than 2° different in longitude were considered migrations, and locations less than 2° different were considered stopovers. Stopover locations were estimated by taking an average of midnight and noon latitudes (when available) and longitudes.
Migration route and distance were estimated by connecting consecutive stopovers with straight lines. Where latitude estimates were unavailable (during equinoxes), we assumed that stopovers were located on the straight line between the previous known stopover and the next known stopover. Arrival at winter sites was defined as the first of 7 or more days in the same location, south of the boreal forest, within the Snow Bunting winter range. Similarly, spring migration initiation was defined as movement northeast towards the breeding site (i.e., >2° shift in longitude) that continued northwards of the boreal forest and outside the winter range. To estimate ‘flight speed’, we divided migration distance by the number of flight nights less the number of stopover nights. This is not a measure of actual air or groundspeed, but instead an estimate of overall travel speed during flight phases of migration.
Phenology data from banding sites
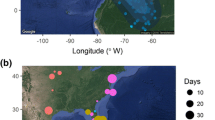
To examine patterns of migration phenology by sex and age in fall, we used banding data collected at Thunder Cape Bird Observatory, Ontario (“TCBO”, http://www.tbfn.net, 48°15′N 88°55′W, n = 553 birds, n = 3 years: 1999, 2002, 2003), located at the northern edge of the winter range. Banding of buntings at TCBO occurs simultaneously with their fall migration-monitoring program (which uses captures with mistnets, ground-traps, and observations) and traps are deployed daily (weather permitting) from July 1 until the end of the October. We included only years for which at least 150 buntings were captured, to avoid sex- or age-biases associated with small sample sizes (note that total numbers of buntings observed annually at this site are usually >400 individuals; thus high-capture years are not anomalous in terms of total buntings in the area). For spring phenology, we used banding data from a banding station at Rivière-St.-Jean, Quebec (“RSJ”, 50°16′N 64°47′W; n = 2110 birds, n = 1 year), also located at the northern edge of the wintering range. Birds at this site were also trapped by using baited ground traps, deployed daily for the duration of the overwintering and spring migration period. Birds captured at TCBO and RSJ likely belong to the western-Greenland breeding population, based on range-wide connectivity analyses (Macdonald et al. 2012).
We also used data from our long-term breeding study site (EBI; 2009: n = 145, 2011: n = 180, 2012: n = 103) since we regularly captured flocks of migrants passing through the site early in the season (i.e., transient, non-local breeders). These birds likely breed further north in the Canadian Arctic (Macdonald et al. 2012). The two spring banding sites were considered representative of early (28 Mar–28 Apr, 2012) and late (25 May–15 Jun, 2008, 2009, 2011) spring migration. We truncated our late spring banding data at EBI by 15 June as local breeding birds are known to commence laying at that time.
Birds at all banding sites were captured in baited ground-based walk-in traps, generally following the trap design and protocols of the Canadian Snow Bunting Banding Network (Love et al. 2015). Other species captured (data not shown here) at banding sites in low numbers include Lapland Longspurs (Calcarius lapponicus) and Horned Larks (Eremophila alpestris).
Statistical analysis
To quantify fall migration phenology by sex and age using banding data, we compared ordinal capture dates (Jan 1 = 1) of HY (‘hatch-year’, i.e., first winter) and AHY (‘after-hatch-year’, i.e., at least second winter) within each sex by using general linear models. We nested age classes by sex and by year (3 years total, 1999, 2002, 2003) to obtain model estimates for ordinal capture date of each sex-age class in each year of banding. We also quantified fall phenology in arrival at winter sites by using our geolocator-tracked sample from EBI, with a linear model including sex nested within the year as the predictor.
To quantify migration phenology at early spring (RSJ) and late spring (EBI) banding sites, we compared ordinal capture dates for SY (‘second-year’, i.e., first time spring migrants) and ASY (‘after-second-year’, i.e., migrating north for at least the second time) birds within each sex by using general linear models. For early spring migration (RSJ), we only had 1 year (2012); for late spring migration (EBI), we nested age and sex by year to obtain model estimates of ordinal capture date for each sex-age class in each year (4 years total). We also used our geolocator sample to examine phenology in arrival date of breeding birds at EBI by using a linear model with sex nested within the year as a predictor. We report overall model fit, t statistics, and P values for each individual model parameter.
We tested proximate hypotheses for winter site arrival date by using a linear model with fall departure date, total number of fall stopover days, fall migration speed (total duration in days less stopover days, divided by distance) and fall migration distance as predictors. We also included a term for sex and year of migration. We used the function ‘step’ in R to drop model terms and obtain the simplest model (backwards stepwise regression, using AIC as a measure of model fit). We used a similar approach with breeding site arrival date, including spring migration start date, total number of spring stopover days, spring migration speed, spring migration distance, sex, and year. All analyses were conducted using the statistical program R (R Development Core Team 2014).
Results
Overall, age and sex nested within the year explained 26 % of the variation in capture-date at our fall migration banding station (r 2 = 0.26, F = 18.35, df = 11,541, P < 0.001). There was a small trend towards protandry in fall within AHY birds, where AHY males tended to be captured earlier than AHY females, although the estimate for males was only significantly earlier than AHY females in 1 year (1999) (Table 1; Fig. 1). HY birds of both sexes were captured before AHY females in all years (Fig. 2), and before AHY males in 1999 (Table 1). There were no significant differences between sexes within the HY age class, except in 1999, where HY females were significantly later than HY males (Fig. 2). Annual differences were apparent in that AHY females were captured significantly later in 2002 and 2003 than in 1999 (Table 1; Fig. 2). Within the geolocator-tracking sample (n = 20), males did not arrive significantly earlier at their winter sites relative to females overall (linear model with sex nested within year: r 2 = −0.07, F = 0.73, df = 5.14, P = 0.61; Table 2).
Mean capture dates of males and females during fall migration (October) at Thunder Cape Bird Observatory over 3 years (total n = 553). a AHY females were captured significantly later than AHY males in 1999, and significantly earlier than AHY females in 2002 and 2003. b Capture dates of HY birds were not significantly different by sex; HY females were captured significantly earlier than adult females in 1999
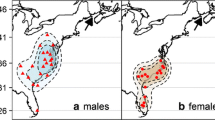
In spring, patterns of protandry were clearer within banding station captures during early migration. Banding data from RSJ indicated that age-sex patterns were significant predictors of the variation in capture date in early spring 2012 (r 2 = 0.13, F = 57.12, df = 3,1084, P < 0.001), where ASY males were captured approximately 7 days earlier on average than other age sex-classes (Table 1; Fig. 3). SY birds of both sexes were captured slightly earlier than ASY females (1–2 days), but still nearly 6 days behind the average ASY males (Fig. 3). In contrast, banding data from late spring migration at EBI showed no overall pattern of protandry within either ASY or SY captures (Table 1; Figs. 4, 5). Captures of ASY birds significantly preceded SY birds only in 1 year at EBI (Fig. 5).
Early spring migration captures of Snow Buntings (n = 1088) in 2012 at Rivière St. Jean (RJS): a Density of captures by sex shows males were captured before females overall. b Within-age class (ASY after-second year, SY second year), only ASY males were significantly different, arriving on average 6 days earlier than ASY females and SY birds of both sexes
Average date of capture of non-local breeding Snow Buntings by sex during late spring migration at East Bay Island (EBI) over 3 years. a ASY females were significantly later than ASY males in year 2011 and trended towards so in 2012. b SY birds tended to arrive later than ASY birds overall, but this was only significant in 2009
Snow Buntings tracked directly by using geolocators travelled, on average 2660 ± 59 km during fall migration, and took 34.6 ± 1.2 days in total (Table 2). Birds departed on fall migration the last week of September (range 17 Sep–8 Oct), and arrived at winter sites by the end of October (range 19 Oct–6 Nov). In fall, birds stopped 2–5 times (average 4.5 ± 0.4 stopovers) for a total of 27.6 ± 1.26 days. This resulted in an overall fall migration rate of 78.7 ± 3.1 km/days; on travelling days only (excluding stopovers) this resulted in a speed of 427.3 ± 36.7 km/days. Snow Buntings spent on average 9.5 ± 0.3 % of their annual cycle on fall migration. In spring, Snow Buntings travelled slightly shorter distances, on average: 2147 ± 69 km. Departure dates were highly variable (Table 2, overall range 27 Apr–18 May, average 6 May), as were arrival dates (overall range 20 May–11 Jun, average 28 May). Their overall migration duration was shorter, at 22.2 ± 2 days and they stopped fewer times (range 2–5, but average 2.3 ± 0.2) and for fewer days (average 16.5 ± 1.9 days). Overall spring migration rate was 118.2 ± 14.8 km/day, and flight speed only was 446.2 ± 31 km/day. This resulted in Snow Buntings spending 6.1 ± 0.5 % of their annual cycle on spring migration.
Geolocator-tracking data revealed a similar pattern at EBI for arriving breeding birds with no consistent pattern of migratory protandry. When nested within the year, males were significantly earlier to arrive than females in 2011 (2011 males = 16.5 day earlier than females, t = −3.70, df = 12, P = 0.003). However, the sample size within-year was small (Table 2); 2012 and 2013 showed less dramatic or reverse (and non-significant) differences between the sexes (2012: males = 6 days earlier than females, t = −1.73, df = 12, P = 0.12; 2013: males 2 days later than females, t = 0.64, df = 12, P = 0.53).
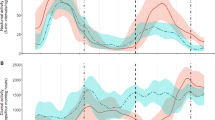
Using geolocator data to examine proximate mechanisms accounting for variation in arrival at winter sites revealed that fall migration start date, fall migration speed, fall migration distance, and total number of stopover days all contributed to the winter site arrival date (overall model r 2 = 0.96, F = 117.5, df = 4,13, P < 0.001) (Fig. 6). Sex and year of migration were not significant predictors of winter site arrival date and were not retained in the best model after stepwise regression. Fall migration start date positively influenced winter arrival date, in that birds initiating fall migration later arrived later (estimate 1.1 ± 0.06 days, t = 17.99, df = 13, P < 0.001; Fig. 6a). Fall migration speed had a small effect on arrival date in that birds migrating at slower speeds arrived slightly later (estimate −0.16 ± 0.001 days, df = 13, t = −7.81, P < 0.001; Fig. 6b). More fall migration stopover days also resulted in later winter site arrival (estimate 1.00 ± 0.06 days, t = 16.89, df = 13, P < 0.001; Fig. 6c). Fall migration distance had a very small but significant effect in that travelling further resulted in later winter site arrival (distance 0.003 ± 0.001 days, t = 3.17, df = 13, P = 0.007; Fig. 6d).
Proximate mechanisms accounting for variation in winter arrival date for Snow Buntings tracked using geolocators from East Bay Island. Fall departure date (a), total number of stopover days (b), fall migration speed (overall distance/duration) (c), and fall migration distance (d), were all significant predictors of fall arrival date
For breeding site arrival, a similar pattern to fall was found, in that spring migration initiation date, spring migration speed, and total number of spring stopover days were all significant predictors of arrival date (r 2 = 0.88, F = 44.5, df = 3,14, <0.001; Fig. 7). Similar to winter arrival date, sex and year were not retained in the best model after stepwise regression. Birds initiating spring migration later arrived later at breeding site (estimate 1.0 ± 0.12, t = 8.5, df = 14, P < 0.001; Fig. 7a). Slower spring migration speeds were related to later arrival to the breeding site (speed, −0.02 ± 0.004, t = −5.54, df = 14, P < 0.001; Fig. 7b). Finally, birds stopping for more days during spring migration arrived later (estimate 0.96 ± 0.08, t = 11.23, df = 14, P < 0.001; Fig. 7c). Interestingly, spring migration distance was not a significant predictor of breeding arrival date (estimate 0.0005 ± 0.003, t = 0.15, df = 14, P = 0.88).
Discussion
We examined migration phenology by combining data from banding station captures with light-level geolocators to directly track Snow Buntings and found that overall intra-specific migration patterns (i.e., migratory protandry in spring) were much less pronounced than predicted and previously suggested for this species. However, the extensive variation we detected between years and individuals (Table 2) indicates that a larger sample size from direct-tracking would be useful for further explorations of patterns in phenology. During early spring migration, older (ASY) males were captured earlier than the other sex-age classes (Fig. 3), but during late spring migration, and among birds arriving to breed at East Bay Island (EBI), protandry was not apparent and arrival date of males and females varied extensively between years (Figs. 4, 5). We predicted that migratory protogyny would be more apparent during fall migration; however, our banding station data indicate a weak pattern of protandry on fall migration (Figs. 1, 2) and direct tracking data from geolocators showed no significant difference in winter site arrival date by sex (Table 2). We found that migration departure date, number of stopover days, and migration speed were all significant predictors of timing of arrival at both winter and breeding sites. Fall migration distance showed a small but significant effect on winter site arrival date; an effect not detected for spring migration. Our data suggest that these factors are important predictors of variation in migration phenology.
Differential migration patterns in fall are often expected to be somewhat weaker than in spring (Mills 2005b), owing to a less time-selected migration strategy in fall, as early winter site arrival provides fewer fitness benefits than early breeding site arrival (Alerstam 2006). In this context, our observation of no or little differential fall migration by sex is perhaps not surprising. Our fall departure dates from direct tracking (average late Sep) were consistent with previous records from North American Snow Buntings (Montgomerie and Lyon 2011). We found no support for the prediction that selection on males to defend valuable breeding resources resulted in males remaining onsite later into the fall than females. Instead, males showed some protandry at a fall migration banding station, arriving after hatch-year birds of both sexes but significantly earlier than adult females in 1 year (Fig. 2). Although not significant, a similar overall trend was evident in our geolocator-tracked sample, where males, on average, arrived at winter sites 3 days in advance of females (Table 2).
We found that the arrival date in winter was related to the fall migration departure date, speed, distance, and total number of stopovers. There is some evidence that female Snow Buntings may invest more in provisioning chicks (Falconer et al. 2008), which could explain why females migrate after males in fall. Parents provisioning more, or for later into the season, may be delayed in post-breeding moult (Stutchbury et al. 2011) and on fall migration (Meissner 2015). Males in our geolocator sample tended to depart slightly earlier, migrate shorter distances, travel faster, and stop for fewer days relative to females (Table 2). It is possible that with a larger sample size the subtle differences we observed would become more clear; regardless, the substantial variation we detected between years suggests that fall migration phenology is highly variable in Snow Buntings (Table 2). Studies at breeding sites quantifying body condition late in the breeding season and fall departure dates could elucidate whether parental efforts carry-over to affect fall migration in Snow Buntings.
One of the most surprising results of our study was the lack of consistent or extensive spring protandry in late spring migration captures and in arrival timing of breeding birds tracked directly. Sex was not a significant predictor of arrival date, likely due to variability in the magnitude of this trend over the three tracking years: males preceded females by 16 days in 2011, while in 2012 the difference was only 6 days, and in 2013, two females tracked arrived within a few days of the three males tracked, even preceding one male. Even where protandry was evident, for example, at RJS during early spring migration, the degree of protandry (~6 days) was much less pronounced than anticipated. In the context of other species, our results are not that surprising: the degree of protandry of mean breeding arrival or spring passage dates is often less than 7 days, and varies extensively between years (Bauboeck et al. 2012; Hedlund et al. 2015). Previous studies of Snow Buntings at breeding sites in Greenland and North America also indicate large annual variation in arrival timing (Montgomerie and Lyon 2011). Here we explore several hypotheses accounting for the differences between phenological patterns we measured and those reported previously for Snow Buntings (Montgomerie and Lyon 2011).
First, evidence of extreme protandry in Snow Buntings is based on first observed birds (i.e., first-arrival date or FAD) and not mean arrival dates (observations cited within Montgomerie and Lyon 2011). First arrival dates are poorly correlated with mean arrival dates, especially for long-distance migrants (Goodenough et al. 2015). We measured passage of males and females within set windows on migration, e.g., early or late spring, and exact arrival of known locally breeding birds by using geolocators. It is possible that ‘outlier’ males do arrive at EBI much earlier than the rest of the birds in our study populations.
Another possibility is that in Snow Buntings, the degree of protandry has changed over time. Recent studies have shown that changes in arrival phenology are largely due to broad phenotypic plasticity in this trait (Tarka et al. 2015). Some studies have found that protandry has increased (Harnos et al. 2015; Moller 2004), presumably as a result of decreased costs for early arriving birds (Spottiswoode et al. 2006). Other studies have found no change in protandry over time (Bauboeck et al. 2012; Rainio et al. 2007) or even that breeding females are advancing arrival dates in a population where males are not (Hedlund et al. 2015). In Snow Buntings, protandry may have decreased over time, if females are surviving better (Kokko et al. 2006) or are in better condition prior to spring migration. Our previous work suggested that small-bodied female Snow Buntings are largely constrained in winter distribution by weather (Macdonald et al. 2015). Temperate winters are warming (IPCC 2014), which may be reducing temperature constraints on females. In our geolocator sample, departure dates, migration speed, and number of stopovers predicted breeding arrival dates. Males tended to depart on migration 5 days earlier than females, and stopped for fewer days, but females actually travelled faster (Table 2). More information is required on overwinter survival and pre-migration body conditions of male and female Snow Buntings to test mechanisms for the phenological patterns.
Phenology in Snow Buntings may vary depending on the specific breeding population measured and or depending on carry-over effects from breeding success in previous years (Meissner 2015). Protandry was evident (Fig. 3) during early spring migration measured at Rivière St. Jean (RSJ), where migrants likely represent populations breeding in Greenland (Macdonald et al. 2012). A long-term study at breeding sites in Greenland that spanned 1969–1982 documented clear migratory protandry (Meltofte 1983), following the pattern we observed during early spring migration. In contrast, late spring migrants and breeding birds captured at our more southern breeding site at EBI showed much less evidence of protandry in spring. These two breeding ranges are thought to be separated by a migratory divide (Macdonald et al. 2012), and it is possible that selection on migration phenology is different at this scale. Differences in phenology across this migratory divide were unexpected, and direct tracking data from birds in the Greenland-breeding population (i.e., breeding site for birds in our banding database) would be useful to determine if proximate mechanisms accounting for arrival phenology also differ.
The relatively small and variable differences by sex in arrival timing in our geolocator-tracked sample resulted in no clear intra-specific phenology patterns. Banding data in general supported these results, although there was a trend towards earlier hatch-year and adult male passage in fall, and adult male arrival in spring. Overall our data indicate that arrival phenology at both winter and breeding sites is related to departure timing, number of stopover days, and migration speed. Understanding controls and constraints on each of these factors would allow further predictions about the potential for Snow Buntings to respond to warming temperatures across their range.
References
Alatalo RV, Gustafsson L, Lundbkrg A (1984) Why do young passerine birds have shorter wings than older birds? Ibis 126:410–415. doi:10.1111/j.1474-919X.1984.tb00264.x
Alerstam T (2006) Strategies for the transition to breeding in time-selected bird migration. Ardea 94:347–357
Bai ML, Schmidt D (2012) Differential migration by age and sex in central European Ospreys Pandion haliaetus. J Ornithol 153:75–84
Bauboeck L, Miller-Rushing AJ, Primack RB, Evans TLL, Wasserman FE (2012) Climate change does not affect protandry in seven passerines in North America. Wilson J Ornithol 124:208–216
Both C, Bouwhuis S, Lessells CM, Visser ME (2006) Climate change and population declines in a long-distance migratory bird. Nature 441:81–83. doi:10.1038/nature04539
Bowlin MS and Wikelski M (2008) Pointed wings, low wingloading and calm air reduce migratory flight costs in songbirds. PLOS One 3:e2154. doi:10.1371/journal.pone.0002154
Canal D, Jovani R, Potti J (2012) Multiple mating opportunities boost protandry in a pied flycatcher population. Behav Ecol Sociobiol 66:67–76. doi:10.1007/s00265-011-1253-8
Coppack T, Pulido F (2009) Proximate control and adaptive potential of protandrous migration in birds. Integr Comp Biol 49:493–506. doi:10.1093/icb/icp029
Coppack T, Tøttrup AP, Spottiswoode C (2006) Degree of protandry reflects level of extrapair paternity in migratory songbirds. J Ornithol 147:260–265. doi:10.1007/S10336-006-0067-3
Cristol DA, Baker MB, Carbone C (1999) Differential migration revisited: latitudinal segregation by age and sex class. Curr Ornithol 15:33–67
Falconer CM, Mallory ML, Nol E (2008) Breeding biology and provisioning of nestling snow buntings in the Canadian High Arctic. Polar Biol 31:483–489
Fudickar AM, Wikelski M, Partecke J (2012) Tracking migratory songbirds: accuracy of light-level loggers (geolocators) in forest habitats. Methods Ecol Evol 3:47–52. doi:10.1111/J.2041-210x.2011.00136.X
Gallinat AS, Primack RB, Wagner DL (2015) Autumn, the neglected season in climate change research. Trends Ecol Evol 30:169–176. doi:10.1016/j.tree.2015.01.004
Gienapp P, Reed TE, Visser ME (2014) Why climate change will invariably alter selection pressures on phenology. Proc Biol Sci 281:20141611. doi:10.1098/rspb.2014.1611
Goodenough AE, Fairhurst SM, Morrison JB, Cade M, Morgan PJ, Wood MJ (2015) Quantifying the robustness of first arrival dates as a measure of avian migratory phenology. Ibis 157:384–390. doi:10.1111/ibi.12227
Harnos A, Nora A, Kovacs S, Lang Z, Csoergo T (2015) Increasing protandry in the spring migration of the Pied Flycatcher (Ficedula hypoleuca) in Central Europe. J Ornithol 156:543–546. doi:10.1007/s10336-014-1148-3
Hedlund JSU, Jakobsson S, Kullberg C, Fransson T (2015) Long-term phenological shifts and intra-specific differences in migratory change in the willow warbler Phylloscopus trochilus. J Avian Biol 46:97–106. doi:10.1111/jav.00484
Hill GE (1989) Late spring arrival and dull nuptial plumage—aggression avoidance by yearling males. Anim Behav 37:665–673. doi:10.1016/0003-3472(89)90045-6
Holberton RL (1999) Changes in patterns of corticosterone secretion concurrent with migratory fattening in a neotropical migratory bird. Gen Comp Endocrinol 116:49–58. doi:10.1006/gcen.1999.7336
IPCC (2014) Climate Change 2014: Synthesis Report. In: Core Writing Team, Pachauri RK, Meyer LA (eds) Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva, Switzerland, p 151
Kokko H, Gunnarsson TG, Morrell LJ, Gill JA (2006) Why do female migratory birds arrive later than males? J Anim Ecol 75:1293–1303. doi:10.1111/j.1365-2656.2006.01151.x
Lisovski S, Hewson CM, Klaassen RHG, Korner-Nievergelt F, Kristensen MW, Hahn S (2012) Geolocation by light: accuracy and precision affected by environmental factors. Methods Ecol Evol 3:603–612. doi:10.1111/j.2041-210X.2012.00185.x
Love OP, Macdonald C, McKinnon EA (2015) Canadian snow bunting banding network protocol 2012. figshare. doi:10.6084/m9.figshare.1588581.v1
Macdonald CA, Fraser KC, Gilchrist HG, Kyser TK, Fox JW, Love OP (2012) Strong migratory connectivity in a declining Arctic passerine. Animal Migration 1:23–30. doi:10.2478/ami-2012-0003
Macdonald CA, McKinnon EA, Gilchrist HG, Love OP (2015) Cold tolerance, and not earlier arrival on breeding grounds, explains why males winter further north in an Arctic-breeding songbird. J Avian Biol 46:001–009. doi:10.1111/jav.00689
Maggini I, Bairlein F (2012) Innate sex differences in the timing of spring migration in a songbird. PLoS One 7:e31271. doi:10.1371/journal.pone.0031271
Marra PP, Holmes RT (2001) Consequences of dominance-mediated habitat segregation in American Redstarts during the nonbreeding season. Auk 118:92–104
McKinnon EA et al (2013) Estimating geolocator accuracy for a migratory songbird using live ground-truthing in tropical forest. Animal Migr 1:31–38. doi:10.2478/ami-2013-0001
McKinnon EA, Fraser KC, Stanley CQ, Stutchbury BJM (2014) Tracking from the Tropics reveals behaviour of juvenile songbirds on their first spring migration. PLoS One 9:e105605
Meissner W (2015) Immature dunlins Calidris alpina migrate towards wintering grounds later than adults in years of low breeding success. J Ornithol 156:47–53. doi:10.1007/s10336-014-1132-y
Meltofte H (1983) Arrival and pre-nesting period of the Snow Bunting Plectrophenax nivalis in east Greenland. Polar Res 1:185–198
Mills AM (2005a) Changes in the timing of spring and autumn migration in North American migrant passerines during a period of global warming. Ibis 147:259–269
Mills AM (2005b) Protogyny in autumn migration: Do male birds “play chicken”? Auk 122:71–81
Mitchell GW, Woodworth BK, Taylor PD, Norris DR (2015) Automated telemetry reveals age specific differences in flight duration and speed are driven by wind conditions in a migratory songbird. Mov Ecol 3:19. doi:10.1186/s40462-015-0046-5
Moller AP (2004) Protandry, sexual selection and climate change. Glob Change Biol 10:2028–2035. doi:10.1111/j.1365-2486.2004.00874.x
Montgomerie R, Lyon B (2011) Snow Bunting (Plectrophenax nivalis). Cornell Lab of Ornithology, Ithaca. doi:10.2173/bna.198
Morbey YE, Ydenberg RC (2001) Protandrous arrival timing to breeding areas: a review. Ecol Lett 4:663–673
Newton I (2008) The migration ecology of birds. Academic Press, London
R Development Core Team (2014) R: a language and environment for statistical computing, version 3.1.0 edn. R Foundation for Statistical Computing, Vienna
Rainio K, Tottrup AP, Lehikoinen E, Coppack T (2007) Effects of climate change on the degree of protandry in migratory songbirds. Climate Rese 35:107–114. doi:10.3354/cr00717
Rappole JH, Tipton AR (1991) New harness design for attachment of radio transmitters to small passerines. Condor 62:335–337
Saino N, Rubolini D, Serra L, Caprioli M, Morganti M, Ambrosini R, Spina F (2010) Sex-related variation in migration phenology in relation to sexual dimorphism: a test of competing hypotheses for the evolution of protandry. J Evol Biol 23:2054–2065. doi:10.1111/j.1420-9101.2010.02068.x
Seewagen CL, Guglielmo CG, Morbey YE (2013) Stopover refueling rate underlies protandry and seasonal variation in migration timing of songbirds. Behav Ecol 24:634–642. doi:10.1093/Beheco/Ars225
Sergio F et al (2014) Individual improvements and selective mortality shape lifelong migratory performance. Nature 515:410–413. doi:10.1038/nature13696
Spottiswoode CN, Tøttrup AP, Coppack T (2006) Sexual selection predicts advancement of avian spring migration in response to climate change. Proc Roy Soc B Biol Sci 273:3023–3029. doi:10.1098/Rspb.2006.3688
Studds CE, Marra PP (2011) Rainfall-induced changes in food availability modify the spring departure programme of a migratory bird. Proc Roy Soc B Biol Sci 278:3437–3443. doi:10.1098/rspb.2011.0332
Stutchbury BJM et al (2009) Tracking long-distance songbird migration by using geolocators. Science 323:896. doi:10.1126/science.1166664
Stutchbury BJM, Gow EA, Done T, MacPherson M, Fox JW, Afanasyev V (2011) Effects of post-breeding moult and energetic condition on timing of songbird migration into the tropics. Proc Roy Soc B Biol Sci 278:131–137. doi:10.1098/Rspb.2010.1220
Tarka M, Hansson B, Hasselquist D (2015) Selection and evolutionary potential of spring arrival phenology in males and females of a migratory songbird. J Evol Biol 28:1024–1038. doi:10.1111/jeb.12638
Thorup K, Tøttrup AP, Rahbek C (2007) Patterns of phenological changes in migratory birds. Oecologia 151:697–703. doi:10.1007/S00442-006-0608-8
Tonra CM, Marra PP, Holberton RL (2011) Early elevation of testosterone advances migratory preparation in a songbird. J Exp Biol 214:2761–2767. doi:10.1242/Jeb.054734
Tøttrup AP, Thorup K (2008) Sex-differentiated migration patterns, protandry and phenology in North European songbird populations. J Ornithol 149:161–167. doi:10.1007/S10336-007-0254-X
Tøttrup AP, Thorup K, Rahbek C (2006) Changes in timing of autumn migration in North European songbird populations. Ardea 94:527–536
Travers SE, Marquardt B, Zerr NJ, Finch JB, Boche MJ, Wilk R, Burdick SC (2015) Climate change and shifting arrival date of migratory birds over a century in the Northern Great Plains. Wilson J Ornithol 127:43–51
Wojczulanis-Jakubas K, Jakubas D, Foucher J, Dziarska-Palac J, Dugue H (2013) Differential autumn migration of the aquatic warbler Acrocephalus paludicola. Naturwissenschaften 100:1095–1098. doi:10.1007/s00114-013-1108-4
Acknowledgments
We thank the following sources of funding for this work: Natural Sciences and Engineering Research Council (NSERC) of Canada (Discovery and Research Tools and Instruments grants to OPL, Canada Graduate Scholarship to CAM), Canada Research Chairs (CRC) program (OPL), Aboriginal Affairs and Northern Development Canada’s Northern Scientific Training Program (NSTP), the Polar Continental Shelf Program (PSCP), Bird Studies Canada’s James L. Baillie Memorial Fund (to the Canadian Snow Bunting Network), Environment Canada, and the University of Windsor. We also thank staff at the Canadian Bird Banding Office, members of the Canadian Snow Bunting Banding Network, and field crews at East Bay Island. Two anonymous reviewers provided helpful comments on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. G. Guglielmo.
Rights and permissions
About this article
Cite this article
McKinnon, E.A., Macdonald, C.M., Gilchrist, H.G. et al. Spring and fall migration phenology of an Arctic-breeding passerine. J Ornithol 157, 681–693 (2016). https://doi.org/10.1007/s10336-016-1333-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-016-1333-7