Abstract
Using direct observations and camera traps at eight sites across Indonesian Borneo we show how red langurs (Presbytis rubicunda) are more terrestrial than previously believed, regularly coming to the ground. This unusual behavior has been found at six of the eight sites surveyed. We find that red langurs come to the ground more frequently in disturbed forests, specifically ones which have been impacted by logging, fire, and hunting, though more data are needed to confirm this as a direct correlation. We also found a trend towards decreased ground use with increased elevation of the habitat. When on the ground, red langurs are predominantly engaged in feeding (50% direct observations, 61% camera traps) and traveling (29% direct observations, 13% camera traps). Red langurs are found on the ground throughout the day, at similar times to activity periods of the apex predator, the Sunda clouded leopard (Neofelis diardi). We suggest that ground use by red langurs could be an adaptation to disturbed forest to exploit additional food sources and to facilitate travel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat conversion, degradation, and loss continues at a rapid rate in many tropical forests and has mixed effects on forest animal diversity (van Niewstadt et al. 2001; Wells et al. 2004; Meijaard et al. 2005; Wilcove et al. 2013). The effects of logging also change over time and is the subject of increasing research. Species composition in logged forests approaches that of unlogged forests just a few decades after logging has ceased (Brodie et al. 2014; Danielsen and Heegaard 1994; Slik et al. 2002). Selectively logged forests are becoming an increasingly dominant component of many tropical landscapes. Yet, the conservation value of selectively logged tropical forests is less well studied (Samejima et al. 2012; Burivalova et al. 2014; Ehlers Smith 2014; Bernard et al. 2016) than those of more dramatic land cover changes, such as deforestation driven by fire, agriculture or tree-plantation developments (Wilson and Wilson 1975; Estrada and CoatesEstrada 1996; Oka et al. 2000; Slik et al. 2002; O’Brien et al. 2003; Meittinen et al. 2012). What is less studied is the impact on animal behavior, and if novel behaviors, e.g., coming to the ground, are a result of disturbance or actually a previously understudied part of a normal behavioral repertoire (Miller 2002; Zuberbuhler and Jenny 2002; Campbell et al. 2005; Mourthé et al. 2007; Loken et al. 2013, 2015; Ancrenaz et al. 2014; Ashbury et al. 2015)
Terrestrial behavior in arboreal primates is recorded from many sites and has been attributed to socio-ecological factors such as food acquisition and aggression avoidance, and/or the anthropogenic effects of habituation to observer presence and habitat disturbance (Campbell et al. 2005; Mourthé et al. 2007). Increased terrestriality in arboreal primates is thought to increase the risk of predation (Miller 2002; Ancrenaz et al. 2014) and disease (Chapman et al. 2005), which has implications for the conservation of many threatened primate species. Increased use of the ground may be an indicator of lower quality habitat (Wells et al. 2004) or could indicate a broader behavioral repertoire and ability to make use of different space (Ancrenaz et al. 2014).
The Asian colobines of genus Presbytis are small-bodied (~ 6 kg), gracile monkeys restricted to the tropical rainforests of Southeast Asia (Oates et al. 1994) and are almost entirely arboreal (Davies and Oates 1994). Indeed, Presbytis monkeys are poorly adapted to terrestriality, with a low inter-membral index (Fleagle 1999) and relatively very long hindlimbs and phalanges, meaning they move quadrupedally on the ground and indicating a strong adaptation to arboreality (Strasser 1992). Relative to their close relatives the Trachypithecus monkeys, which have shorter hindlimbs, Presbytis monkeys engage in less quadrupedal walking, favoring arboreal leaping (Fleagle 1977, 1978; Strasser 1992). However, when investigating positional behavior in Delacour’s langurs Workman and Schmitt (2012) found no real morphological adaptation to living on limestone karsts but more flexibility of behavior. Eighty % of locomotion was on rocks, but there was barely any leaping (4%). Workman and Schmitt (2012) suggest that the generalized locomotor capabilities of cercopithecids allow them this behavioral flexibility despite morphology (Workman and Schmitt 2012).
The red langur, Presbytis rubicunda, is endemic to Borneo and the adjacent island of Karimata (Medway 1970) and occupies the majority of the habitat types across the island (Davies 1984; Supriatna et al. 1986; Marshall 2010). From two ecological studies at different sites and habitats, P. rubicunda is not known to descend to the forest floor (Supriatna et al. 1986) except for rare occasions to engage in geophagy (Davies and Baillie 1988; Davies et al. 1988; Rawson and Tuong Bach 2011). However this species has increasingly been reported on cameras traps placed on the ground, though these studies present encounter rates only and do not discuss behavior (Giman et al. 2007; Samejima et al. 2012; Loken et al. 2013; Cheyne et al. 2015a, b, 2016).
In Sabangau peat-swamp forest, Central Kalimantan, we recorded P. rubicunda engaging in terrestrial behavior through researcher observation and remote observation using camera trapping over an 18-month period. In seven other sites where these primates are not habituated, we used camera traps which were placed for 30–160 days.
We looked at these data to address the following questions:
-
1.
Were red langurs predominantly using the ground to travel, as might be expected in heavily disturbed forests?
-
2.
If travel was not the primary activity, what behaviors were red langurs engaged in on the ground?
-
3.
Which age/sex class was using the ground more? If access to food was a driving influence we may expect adults to use the ground more, and younger animals to avoid the ground due to increased predation risks.
-
4.
Was frequency of ground use affected by logging, burnt forest, habitat type and presence of hunting?
-
5.
Is terrestrial behavior affected by season?
Here, we detail the circumstances under which terrestrial behavior was observed and discuss the ecological and conservation implications.
Study species
P. rubicunda is physiologically adapted for folivory (Bauchop and Martucci 1968), although a large amount of its diet comprises seeds of unripe fruits (Supriatna et al. 1986; Davies 1991; Marshall et al. 2009; Ehlers Smith et al. 2013a). The social unit is the single-male mixed-sex group, with extra males forming all-male bands or ranging alone (Davies 1984; Supriatna et al. 1986; Ehlers Smith et al. 2013b). Currently listed as Least Concern on the IUCN Red List, this species inhabits the same forests as the four species of Bornean gibbons (all listed as Endangered on the IUCN Red List (Geissmann and Nijman 2008a, b, c; Nijman et al. 2008) and the Bornean orangutan [listed as critically endangered (Ancrenaz et al. 2016)]. Red langurs are being upgraded to Red List Vulnerable across their range based on a recent review predicting an estimated population reduction of over 50% when considering the past 30 years and habitat change due to draining of peat swamps, logging and burning into the next 15 years (totaling 45 years which is approximately three generations (Cheyne et al. 2017). Age of first reproduction for red langurs is not confirmed but is estimated to be between 7 and 20 years based on other langur species (Harley 1990). Between 2000 and 2010 the species experienced a mean 10% habitat occupancy reduction across all subspecies; P. r. chrysea experienced a ~ 21% habitat occupancy reduction (Ehlers Smith 2014). Where the species persists, there is hunting and collection for the wildlife trade and for human consumption. A large part of the species range is in peat swamp—an extremely threatened ecosystem.
Study grids
We present data from our long-term site in Sabangau and from seven other sites surveyed for durations ranging from 30 to 180 days. In two of the short-term sites (Murung Raya and Mungku Baru), the camera-trap survey areas were considerably smaller due to access and time limitations (Table 1). The study sites differ in habitat type, altitude, size and human pressures which also impacted the size of the area surveyed by the cameras (Cheyne et al. 2016). The number of functional trap nights is presented (i.e., one camera operating for one calendar night is one trap night), 44 cameras operating for one calendar night is 44 trap nights. Functional trap nights account for the number of trap nights with deductions made for when individual cameras were not functioning (Andrew Royle et al. 2009; Cheyne et al. 2016).
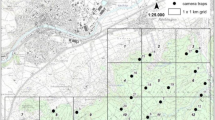
Unlogged areas were sampled in all study grids. Four grids had forest, which was logged > 20 years ago. Due to the size of area surveyed, all grids crossed several habitat types and all were affected by some level of disturbance. Burnt refers to areas where vegetation has been cleared by fire in the last ≤ 15 years and is recovering (Fig. 1).
Map of the study region in Indonesian Borneo showing for each study grid the proportion of camera stations in logged (light grey), burnt (mid-grey), undisturbed (dark grey) and plantation (black) forest. The elevation model is from Google Earth. White shading indicates high elevation; dark grey shading indicates low elevation. See Supplementary Information for a map detailing exact study grid location
Methods
Direct observations (Sabangau only)
We collected data on four habituated groups between March 2010 and January 2017 as part of a long-term ecological study of P. rubicunda. We followed groups from the morning sleeping tree until the evening sleeping tree and collected instantaneous behavioral data on a focal adult female every 5 min, including activity and height of the focal animal. We collected feeding data on a focal adult female at the instant the behavior began until it stopped, including food item species; part eaten, and height or position of the food item (for full details of observation methods see (Ehlers Smith and Ehlers Smith 2013; Ehlers-Smith et al. 2013a, b).
Camera trapping
Following Cheyne et al. (2016) Cuddeback Capture IR® (Cuddeback Digital, Non-Typical) camera traps were placed along established trails and, where possible, watering areas, located so as to maximize the success rate of photographic ‘detections’ (Wilting et al. 2006; Gordon and Stewart 2007; Cheyne et al. 2013a, b). Two cameras were placed opposite each other, 7–10 m apart to create a paired station at each location with the aim of photographing each flank of the animal simultaneously (Brodie and Giordano 2012). In Murung Raya and Mungku Baru only one camera was placed at each location (Cheyne et al. 2015a, b). The passive infrared sensors were set at about 50 cm height off the ground and on a tree to record the majority of biodiversity present in the study areas (Cheyne et al. 2013a, 2017; Adul et al. 2015). The Capture IR cameras use an infrared flash to minimize disturbance to wildlife and reduce trap avoidance (O’Connell et al. 2011). There are some logging roads in some of the study areas, all cameras were placed along established trails at cross-roads and near fallen logs or man-made boardwalks, which may facilitate felid movements during the flooded wet season (Gordon and Stewart 2007; Cheyne and Macdonald 2011; Cheyne et al. 2013a).
The grids were surveyed consecutively except for Sabangau which was surveyed concurrently with the Kutai and Lesan grids. Cameras were ideally set in a grid system with ± 0.5 to 1 km between camera stations. This layout was not possible in Bawan due to the disturbed nature of the forest and issues of water accessibility when setting the cameras thus the cameras were placed along established trails. A general linear model with general contrasts and survey effort as a covariate was performed to assess the impact of logging age (number of years since logging occurred), presence of hunting and fire on the presence of red langurs on the ground. We ranked models based on the Akaike Information Criterion (AIC) values: models with the lowest AIC values were identified as the best output models.
Results
Frequency of encounters on the ground
We recorded 82 counts (0.32% of all focal observations) of ground use by the focal individual at the instantaneous data point, characterized by four distinct behaviors [total data points 25,502 (taken at 5-min intervals following (Ehlers Smith et al. 2013a)]: feeding 50, traveling 29, resting 20 and social behavior 1. We further catalogued 31 of the 82 occasions of the focal individual feeding from the ground independent of the instantaneous point over 100 min by breakdown of time and food types consumed on the forest floor by the focal individual: water from the ground 17 (25 min), water from nepenthes 5 (10 min), fungi 10 (50 min), pith 6 (13 min), leaves 2 (4 min) and unknown 41 (103 min). Images and videos of red langurs on the ground were obtained from in six of the eight sites (a total of 78 independent images/videos). Red langurs were confirmed present in the two remaining sites (Rodman 1977; Bersacola et al. 2014) but the langurs were not seen on the ground (Table 2).
Images of all age sex classes and of multiple individuals at the same time were obtained (Fig. 2).
Red langur behavior on the ground
Red langurs predominantly used the ground for traveling and feeding across study sites (Fig. 3). Feeding was classified when the langurs were holding food items or clearly seen to have food in their mouth. Traveling was classified as any movement and resting where the animals are not in motion. Social activity was classified as any interaction between two or more individuals and direct gaze at the cameras was classed as looking at the camera.
Red langurs came to the ground throughout the active period with peaks between 1000 and 1100 h, 1200–1300 h and 1400–1500 h though there is variation between sites (Fig. 4). These times of greatest terrestrial behavior are not significantly affected by the behavior being performed on the ground (Friedman ANOVA: χ2 = 0.74, n = 6, df = 4, p = 0.69).
Of the eight sites, only five had cameras present consecutively across wet and dry seasons (Kutai, Lesan, Mungku Baru, Sabangau and Sungai Wain). Only three of those sites had images/sightings of red langurs on the ground: Lesan 2 (one wet season, one dry season); Mungku Baru 21 (12 wet season, nine dry season) and Sabangau 40 (24 wet season, 16 dry season).
The number of sightings each month was generally higher in the dry season though the average number of sightings and images per month taken as an average was not significantly different (10.63 wet season and 9.25 dry season, χ2 = 22.01, p > 0.05; Fig. 5).
Habitat and disturbance
There is a possible negative relationship between habitat/altitude and number of images obtained (Pearson correlation p = 0.09). While we recognize that habitat and mammalian guild structure, e.g., forest types/structure, food availability and animal diversity are likely important determinants affecting their terrestrial behaviors (Cheyne et al. 2016), red langurs are recorded on the ground more frequently at low altitudes (Fig. 6). Though this trend does not suggest causality. It might be that the study sites at lower altitudes were different from the others in other aspects (presence of predators, vegetation structure etc). The possible influence of altitude was added as a proxy for the variation in forest structure but also there are some suggestions that predation pressure could be higher in higher altitudes, due to an increased presence of clouded leopards (Macdonald et al. 2018).
Of the 78 images/sightings on the camera traps 4% (three images) were in forests unaffected by fires and 96% (75 images) were obtained in areas where the forests had experienced at least one fire event. A possible reason for the langurs making use of the ground could be impacts of logging. Using logging data obtained for these areas (Cheyne et al. 2016) we assessed the number of images of red langurs on the ground as a function of total primate images and compared this to this logging history of the area at a broad scale. The GLM predicted that the age of logging model most consistently explains presence of red langurs on the ground (F = 5.1, p = 0.036), followed by fire F = 6.4, p = 0.04). The presence of hunting model did not explain any of the variation F = 4.3, p = 0.08 (Fig. 7).
Discussion
Red langur behavior on the ground
Asian colobines are infrequently recorded on the ground and are poorly adapted to terrestrialism. Colobines do use the ground for feeding e.g., geophagy, drinking water, and eating fungi but in the absence of long-term data regarding this behavior it is important to investigate other drivers for terrestriality (McKey et al. 1981; Davies and Oates 1994; Rawson and Tuong Bach 2011). Arboreal primates may descend to the ground in more disturbed habitats and in the presence of researchers (to whom they are habituated) in order to flee (Ehardt et al. 2005). The degree of terrestrial behavior may be related to habituation and has implications for conservation owing to vulnerability from predation/disease/parasite transmission (Hart 2007; Matsuda et al. 2008; Morino 2011; Hilser et al. 2011). Predation rate is difficult to evaluate, and may not be a limiting factor, but the few data which are available on gibbons (primates of a similar size) suggest that there is a predation risk (Uhde and Sommer 2002; Hart 2007; Morino 2011; Clarke et al. 2012; Burnham et al. 2012; Wilcox et al. 2017). Hunting pressure was measured by presence of hunters on the camera traps and is a rough proxy for pressure of human hunting. Most hunters were observed in Bawan forest, where red langurs were observed on the ground. As monkeys are infrequently targeted by human hunters they may come to the ground more frequently (Marshall et al. 2006; Meijaard et al. 2012). Species that travel along the ground and live in more open forests are more prone to predation (by other animals and humans; Rudran 1973; Stanford 1989). Predation of arboreal primates by other animals is hardly ever reported in SE Asia (Davies 1984; Burnham et al. 2012) and much lower than African forests (Aldrich-Blake 1970; Busse 1977). Pythons were thought to have a minimal influence on predators (Whitten 1980). Longer-term studies and increased focus on predation does show that snakes and raptors are important predators of primates in the wild (Shine et al. 1998; Wiens and Zitzmann 1999; Fam and Nijman 2011; Matsuda et al. 2011; Tsuji et al. 2014, 2016) and in rescue centers (SMC pers. obs.). Descending to the forest floor could be a defense strategy against aerial predators e.g., (Seyfarth and Cheney 1980). Forest monkeys travel and forage on the ground far more in the absence of leopards (Struhsaker 2000). The monkeys on the islands do so much more frequently in the absence of leopards. In areas where people hunt primates colobine populations may be reduced (Bennett and Sebastian 1988).
Age/sex class ground use
The use of the ground does not appear to be restricted to one age class or sex though adults are recorded on the ground more than younger animals. Adults may use the forest floor more often than that in juveniles simply because juveniles are more vulnerable than adults in terms of their body size, though this has not been borne out from data on orangutans in the same forests (Ancrenaz et al. 2014). Additionally, clouded leopards in the study sites are predominantly active between 1100 and 1600 h (Adul et al. 2015; Cheyne et al. 2016), similar times to when the red langurs are most active on the ground indicating that predation risk from clouded leopards may not be an explanatory factor.
Habitat and disturbance
Higher quality foods (leaves containing higher protein but lower fiber) may be more available in disturbed forests (Ehlers-Smith et al. 2013; Matsuda et al. 2013), and there are also potentially more shade-tolerant plants on the ground in disturbed forests (Estrada and CoatesEstrada 1996; Munoz et al. 2006; Anderson et al. 2007). Thus, red langurs may have more opportunities to come down to the ground in these habitats.
These findings are preliminary and require further investigation to find correlations, unless the use of the ground is unrelated to habitat disturbance. Only one site (Sungai Wain) has no regular encroachment for hunting and this site did not yield any images of red langurs on the ground. With these data, it is not possible to determine any relationship between hunting presence and frequency of ground use by red langurs. There was no obvious influence of season on the frequency with which red langurs came to the ground either from direct observational data or from the camera traps. However, the impacts of hunting, logging and fire have been observed for other species (Brodie et al. 2014; Cheyne et al. 2016), highlighting the need to investigate this more fully for red langurs.
The more recently disturbed areas (recent logging) had more frequent occurrences of red langurs on the ground, perhaps due to increased loss of large trees and/or canopy connectivity as has been seen for gibbons (Cheyne et al. 2013b). Thus, this behavior could be a potential indicator of habitat quality. Selective logging is likely to influence the ecology of P. rubicunda as the practice removes the largest trees, upon which feeding (Ehlers Smith et al. 2013a), distribution (Ehlers Smith and Ehlers Smith 2013), and sleeping sites (Ehlers Smith 2014) are dependent. Given the increased incidences of terrestrial behaviors in logged forests and the frequency with which feeding behaviors were associated with terrestriality, it is likely that terrestrial foraging forms an important part of the behavioral ecology of P. rubicunda in logged forests. Consequently, there is an increased risk associated with parasite transmission through increased time on the ground (Chapman et al. 2005) and predation for populations in logged forests; despite the success of logging concessions in maintaining overall forest cover in comparison to protected areas (Ehlers Smith 2014), logged forests could be sub-optimal habitat for P. rubicunda populations therein. However more data and longer studies are needed so we can only conclude that this species does come to the ground more often in logged forest. Why red langurs do this remains unclear, as are any impacts on long term population viability.
Change history
09 August 2018
In the original publication of this article, the Table 2 was published incorrectly. The revised Table 2 is given on the following page.
References
Adul R, Capilla B, Limin SH, Cheyne SM (2015) Felids of Sebangau: camera trapping to estimate activity patterns and population abundance in Central Kalimantan, Indonesia. Biodiversitas 16:151–155
Aldrich-Blake FP (1970) Problems of social structure in forest monkeys. In: Crook JH (ed) Social behaviour in birds and mammals. Academic Press, London, pp 79–101
Ancrenaz M, Sollmann R, Ambu L et al (2014) Coming down from the trees: is terrestrial activity in Bornean orangutans natural or disturbance driven? Sci Rep 4:1–5
Ancrenaz M, Gumal M, Marshall A et al (2016) Pongo pygmaeus. In: IUCN red list threat. Species. http://dx.doi.org/10.2305/IUCN.UK.2016-1.RLTS.T17975A17966347.en. Accessed 4 Apr 2018
Anderson J, Cowlishaw G, Rowcliffe JM (2007) Effects of forest fragmentation on the abundance of Colobus angolensis palliatus in Kenya’s coastal forests. Int J Primatol 28:637–655. https://doi.org/10.1007/s10764-007-9143-7
Andrew Royle J, Nichols DJ, Ullas Karanth K, Gopalaswamy AM (2009) A hierarchical model for estimating density in camera-trap studies. J Appl Ecol 46:118–127
Ashbury AM, Posa MRC, Dunkel LP et al (2015) Why do orangutans leave the trees? Terrestrial behavior among wild Bornean orangutans (Pongo pygmaeus wurmbii) at Tuanan, Central Kalimantan. Am J Primatol. https://doi.org/10.1002/ajp.22460
Bauchop T, Martucci R (1968) Ruminant-like digestion of the langur monkey. Science (80) 161:698–699
Bennett EL, Sebastian A (1988) Social organization and ecology of proboscis monkeys (Nasalis larvatus) in mixed coastal forest in Sarawak. Int J Primatol 9:233–256
Bernard H, Rayner B, Matsuda I et al (2016) Species richness and distribution of primates in disturbed and converted forest landscapes in Northern Borneo. Trop Conserv Sci 9:1–11. https://doi.org/10.1177/1940082916680104
Bersacola E, Smith DAE, Sastramidjaja WJ et al (2014) Population density of Presbytis rubicunda in a small primary Dipterocarp forest in East Kalimantan, Indonesian Borneo. Asian Primates J 4:16–26
Brodie J, Giordano A (2012) Density of the vulnerable Sunda clouded leopard Neofelis diardi in a protected area in Sabah, Malaysian Borneo. Oryx 46:427–430
Brodie JF, Giordano A, Zipkin E et al (2014) Correlation and persistence of hunting and logging impacts on tropical rainforest mammals. Conserv Biol 29:110–121. https://doi.org/10.1111/cobi.12389
Burivalova Z, Şekercioğlu C, Pin Koh L (2014) Thresholds of logging intensity to maintain tropical forest biodiversity. Curr Biol 24:1893–1898
Burnham D, Bearder SK, Cheyne SM et al (2012) Predation by mammalian carnivores on nocturnal primates: is the lack of evidence support for the effectiveness of nocturnality as an anti-predator strategy? Folia Primatol 83:236–251
Busse CE (1977) Chimpanzee predation as a possible factor in the evolution of red colobus social organization. Evolution (N Y) 31:907–911
Campbell CJ, Aureli F, Chapman CA et al (2005) Terrestrial behavior of Ateles spp. Int J Primatol 26(5):1039–1051
Chapman C, Gillespie TR, Goldberg TL (2005) Primates and the ecology of their infectious diseases: how will anthropogenic change affect host–parasite interactions? Evol Anthropol 14:134–144
Cheyne SM (2010) Behavioural ecology and socio-biology of gibbons (Hylobates albibarbis) in a degraded peat-swamp forest. In: Supriatna J, Gursky SL (eds) Indonesian Primates. Springer, New York, pp 121–156
Cheyne SM, Macdonald DW (2011) Wild felid diversity and activity patterns in Sabangau peat-swamp forest, Indonesian Borneo. Oryx 45:119–124
Cheyne SM, Stark D, Limin SH, Macdonald DW (2013a) First estimates of population ecology and threats to Sunda clouded leopards (Neofelis diardi) in a peat-swamp forest, Indonesia. Endanger Species Res 22:1–9
Cheyne SM, Thompson CJH, Chivers DJ (2013b) Travel adaptations of gibbons Hylobates albibarbis (Primates: Hylobatidae) in a degraded secondary forest, Indonesia. J Threat Taxa 5:3963–3968
Cheyne SM, Ehlers Smith D, Nijman V, Traeholt C (2015a) Presbytis rubicunda. In: IUCN red list threat. Species
Cheyne SM, Höing A, Houlihan PR et al (2015b) Report on the large mammals of the Uut Murung Region, Central Kalimantan, Indonesia. J Indones Nat Hist 3:38–45
Cheyne SM, Sastramidjaja WJ, Muhalir RY et al (2016) Mammalian communities as indicators of disturbance across Indonesian Borneo. Glob Ecol Conserv 7:157–173
Cheyne SM, Adul Van Veen FJF et al (2017) First record of the bay cat in mosaic heath/peat-swamp forest, Kalimantan, Indonesia. Cat News 65:48
Clarke E, Reichard UH, Zuberbühler K (2012) The anti-predator behaviour of wild white-handed gibbons (Hylobates lar). Behav Ecol Sociobiol 66:85–96
Danielsen F, Heegaard M (1994) The impact of logging and forest conversion of lowland forest birds and other wildlife in Seberida, Riau Province, Sumatra. In: Sandbukt OW (ed) Proceedings of NORINDRA seminar. Indonesian Institute of Sciences. Jakarta, pp 59–60
Davies AG (1984) An ecological study of the red leaf monkey (Presbytis rubicunda) in the dipterocarp forests of Sabah, northern Borneo. University of Cambridge, Cambridge
Davies AG (1991) Seed-eating by red leaf monkeys (Presbytis rubicunda) in dipterocarp forest of northern Borneo. Int J Primatol 12:119–144
Davies AG, Baillie I (1988) Soil-eating by red leaf monkeys (Presbytis rubicunda) in Sabah, northern Borneo. Biotropica 20:252–258
Davies GA, Oates JF (1994) Colobine monkeys: their ecology, behaviour and evolution. Cambridge University Press, Cambridge
Davies AG, Bennett EL, Waterman PG (1988) Food selection by two south-east Asian colobine monkeys (Presbytis rubicunda and P. melalophos) in relation to plant chemistry. Biol J Linn Soc 34:34–56
Ehardt CL, Jones TP, Butynski TM (2005) Protective status, ecology and strategies for improving conservation of Cercocebus sanjei in the Udzungwa Mountains, Tanzania. Int J Primatol 26:557–583. https://doi.org/10.1007/s10764-005-4366-y
Ehlers Smith DA (2014) The effects of land-use policies on the conservation of Borneo’s endemic Presbytis monkeys. Biodivers Conserv. https://doi.org/10.1007/s10531-014-0639-0
Ehlers Smith DA, Ehlers Smith YC, Cheyne SM (2013a) Home-range use and activity patterns of the red langur (Presbytis rubicunda) in Sabangau tropical peat-swamp forest, Central Kalimantan, Indonesian Borneo. Int J Primatol. https://doi.org/10.1007/s10764-013-9715-7
Ehlers Smith DA, Ehlers Smith YC, Cheyne SM (2013b) Home range use and activity patterns of red langurs in Sabangau tropical peat-swamp forest, Central Kalimantan, Indonesia. Int J Primatol 34:957–972. https://doi.org/10.1007/s10764-013-9715-7
Ehlers-Smith DA, Ehlers Smith YC (2013) Population density of red langurs in Sabangau tropical peat-swamp forest, Central Kalimantan, Indonesia. Am J Primatol 75:837–847
Ehlers-Smith DA, Husson SJ, Ehlers Smith YC, Harrison ME (2013) Feeding ecology of red langurs in Sabangau tropical peat-swamp forest, Indonesian Borneo: extreme granivory in a non-masting forest. Am J Primatol 75:848–859
Estrada A, CoatesEstrada R (1996) Tropical rain forest fragmentation and wild populations of primates at Los Tuxtlas, Mexico. Int J Primatol 17:759–783
Fam S, Nijman V (2011) Spizaetus hawk-eagles as predators of arboreal colobines. Primates 52:105–110
Fleagle J (1977) Locomotor behavior and muscular anatomy of sympatric Malaysian leaf monkeys Presbytis obscura and Presbytis melalophos. Am J Phys Anthropol 46:297–308
Fleagle JG (1978) Locomotion, posture, and habitat utilization in two sympatric Malaysian leaf-monkeys (Presbytis obscura and Presbytis melalophos). In: Montgomery GG (ed) The ecology of arboreal folivores. Smithsonian Institution Press, Washington, D.C.
Fleagle JG (1999) Primate adaptation and evolution. Academic Press, San Diego
Geissmann T, Nijman V (2008) Hylobates muelleri. In: IUCN red list threat. Species. http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T39888A10270564.en. Accessed 4 Apr 2018
Geissmann T, Nijman V (2008) Hylobates funereus. In: IUCN red list threat. Species. http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T39890A10271063.en. Accessed 4 Apr 2018
Geissmann T, Nijman V (2008) Hylobates abbotti. In: IUCN red list threat. Species. http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T39889A10270691.en. Accessed 4 Apr 2018
Gilhooly LJ, Rayadin Y, Cheyne SM (2015) A Comparison of Hylobatid Survey Methods Using Triangulation on Müller’s Gibbon (Hylobates muelleri) in Sungai Wain Protection Forest, East Kalimantan, Indonesia. Int J Primatol 36:567–582. https://doi.org/10.1007/s10764-015-9845-1
Giman B, Stuebing R, Megum N et al (2007) A camera trapping inventory for mammals in a mixed use planted forest in Sarawak. Raffles Bull Zool 55:209–215
Gordon CH, Stewart EA-M (2007) The use of logging roads by a solitary felid, the clouded leopard. Cat News 47:12–13
Harley D (1990) Aging and reproductive performance in langur monkeys (Presbytis entellus). Am J Phys Anthropol 83:253–261. https://doi.org/10.1002/ajpa.1330830213
Harrison M, Hendri DM et al (2010) Biodiversity of the mungku baru ulin forest, Central Kalimantan, Indonesia. Orangutan Tropical Peatland Project, Palangka Raya, Indonesia
Harrison ME, Cheyne SM, Husson SJ et al (2012) Preliminary assessment of the biodiversity and conservation value of the bawan forest, Central Kalimantan, Indonesia. Orangutan Tropical Peatland Project, Palangka Raya
Hart D (2007) Predation on primates: a biogeographical analysis. In: Gursky S, Nekaris KAI (eds) Primate anti-predator strategies. Springer, New York, pp 27–62
Hilser H (2011) An Assessment of Primate Health in the Sabangau Peat-Swamp Forest, Central Kalimantan, Indonesian Borneo. MSc Dissertation, Oxford Brookes University, UK
Loken B, Spehar S, Rayadin Y (2013) Terrestriality in the Bornean orangutan (Pongo pygmaeus morio) and implications for their ecology and conservation. Am J Primatol 75:1129–1138. https://doi.org/10.1002/ajp.22174
Loken B, Boer C, Kasyanto N (2015) Opportunistic behaviour or desperate measure? Logging impacts may only partially explain terrestriality in the Bornean orang-utan Pongo pygmaeus morio. ORYX. https://doi.org/10.1017/S0030605314000969
Macdonald WR, David Bothwell H, Hearn A, Cheyne A, Susan M et al (2018) Clouds over the hills: deforestation and large-scale plantations threaten an ambassador of Southeast Asian biodiversity (Neofelis diardi), as revealed by multi-scale modeling and camera trapping. J Anim Ecol
Marshall AJ (2010) Effect of habitat quality on primate populations in Kalimantan: gibbons and leaf monkeys as case studies. In: Gursky-Doyen S, Supriatna J (eds) Indonesian primates. Springer, New York, pp 157–177
Marshall AJ, Nardiyono Engstrom LM et al (2006) The blowgun is mightier than the chainsaw in determining population density of Bornean orangutans (Pongo pygmaeus morio) in the forests of East Kalimantan. Biol Conserv 129:566–578. https://doi.org/10.1016/j.bibcon.2005.11.025
Marshall AJ, Ancrenaz M, Brearley FQ et al (2009) The effects of forest phenology and floristics on populations of Bornean and Sumatran orangutans. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (eds) Orangutans: geographic variation in behavioral ecology and conservation. Oxford University Press, Oxford, pp 97–116
Matsuda I, Tuuga A, Higashi S (2008) Clouded leopard (Neofelis diardi) predation on proboscis monkeys (Nasalis larvatus) in Sabah, Malaysia. Primates 49:227–231. https://doi.org/10.1007/s10329-008-0085-2
Matsuda I, Tuuga A, Bernard H (2011) Riverine refuging by proboscis monkeys (Nasalis larvatus) and sympatric primates: implications for adaptive benefits of the riverine habitat. Mamm Biol 76:165–171
Matsuda I, Tuuga A, Bernard H et al (2013) Leaf selection by two Bornean colobine monkeys in relation to plant chemistry and abundance. Sci Rep 3:1873. https://doi.org/10.1038/srep01873
McConkey KR, Chivers DJ (2004) Low mammal and hornbill abundance in the forests of Barito Ulu. Central Kalimantan, Indonesia. Oryx 38:439–447
McKey DB, Gartlan PG, Waterman FL et al (1981) Food selection by black colobus monkeys (Colobus satanus) in relation to plant chemistry. Biol J Linn Soc 16:115–146
Medway L (1970) The monkey of Sundaland: ecology and systematic of the cercopithecids of a humid equatorial environment. In: Napier NH (ed) Old world monkeys: evolution, systematic and behaviour. Academic Press, New York, pp 513–554
Meijaard E, Sheil D, Nasi R et al (2005) Life after logging: reconciling wildlife forestry and production forestry in Indonesian Borneo. CIFOR, Bogor
Meijaard E, Wich S, Ancrenaz M, Marshall AJ (2012) Not by science alone: why orangutan conservationists must think outside the box. Year Ecol Conserv Biol 1249:29–44. https://doi.org/10.1111/j.1749-6632.2011.06288.x
Meittinen J, Hooijer A, Shi C et al (2012) Extent of industrial plantations on Southeast Asian peatlands in 2010 with analysis of historical expansion and future projections. GCB Bioenergy 4:908–918
Miller L (2002) Eat or be eaten: predator sensitivity foraging among primates. Cambridge University Press, Cambridge
Morino L (2011) Clouded leopard predation on a wild juvenile siamang 1414:362–368. https://doi.org/10.1159/000324303
Morrogh-Bernard H, Husson S, Page SE, Rieley JO (2003) Population status of the Bornean orang-utan (Pongo pygmaeus) in the Sebangau peat swamp forest, Central Kalimantan, Indonesia. Biol Conserv 110:141–152
Mourthé I, Guedes D, Fidelis J et al (2007) Ground use by northern muriquis (Brachyteles hypoxanthus). Am J Primatol 69:706–712
Munoz D, Estrada A, Naranjo E, Ochoa S (2006) Foraging ecology of howler monkeys in a cacao (Theobroma cacao) plantation in Comalcalco, Mexico. Am J Primatol 68:127–142. https://doi.org/10.1002/ajp.20211
Nijman V, Richardson M, Geissmann T (2008) Hylobates albibarbis. In: IUCN red list threat. Species 2008. http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T39879A10279127.en. Accessed 4 Nov 2015
O’Brien TG, Kinnaird MF, Nurcahyo A et al (2003) Fire, demography and the persistence of siamang (Sympgylangus syndactylus: Hylobatidae) in a Sumatran rainforest. Anim Conserv 6:115–121
O’Connell AF, Nichols JD, Karanth KU (2011) Camera traps in animal ecology: methods and analyses. Springer, New York
Oates JF, Davies AG, Delson E (1994) The diversity of living colobines. In: Davies GA, Oates JF (eds) Colobine monkeys: their ecology, behaviour and evolution. Cambridge University Press, Cambridge, pp 45–73
Oka T, Iskandar E, Ghozali DI (2000) Effects of forest fragmentation on the behaviour of Bornean gibbons. In: Guhardia E, Fatawi M, Sutisna M et al (eds) Ecological studies: rainforest ecosystems of east Kalimantan: el Nino, drought, fire and human impacts. Springer, London, pp 229–241
Rawson B, Tuong Bach L (2011) Preliminary observations of geophagy amongst Cambodia’s Colobinae. Vietnam J Primatol 5:41–46
Rodman PS (1977) Feeding behaviour of orang-utans of the Kutai Nature Reserve, East Kalimantan. In: Clutton-Brook TH (ed) Primate ecology: studies of feeding and ranging behaviour in lemurs, monkeys and apes. Academic Press, London, pp 171–209, 381-413
Rudran R (1973) Adult male replacement in one-male troops of purple-faced langurs (Presbytis senex senex) and its effect on population structure. Folia Primatol 19:166–192
Rustam YM, Tsuyuki S (2012) Comparison of mammalian communities in a human-disturbed tropical landscape in East Kalimantan, Indonesia. Mammal Study 37:299–311
Samejima H, Ong R, Lagan P, Kitayama K (2012) Camera-trapping rates of mammals and birds in a Bornean tropical rainforest under sustainable forest management. For Ecol Manag 270:248–256. https://doi.org/10.1016/j.foreco.2012.01.013
Sapari I, Sadikin LA, Santoso E, Sadranto M (2005) Biodiversity, social, economy and culture of communities in three villages of upstream Belantikan, Central Kalimantan. Pangkalan Bun, Indonesia
Seyfarth R, Cheney DL (1980) The ontogeny of vervet monkey alarm calling behavior: a preliminary report. Ethology 54:37–56
Shine R, Harlow PS, Keogh JS, Boeadi (1998) The influence of sex and body size on food habits of a giant tropical snake, Python reticulatus. Funct Ecol 12:248–258
Slik JWF, Verburg RW, Kessler PJA (2002) Effects of fire and selective logging on the tree species composition of lowland dipterocarp forest in East Kalimantan, Indonesia. Biodivers Conserv 11:85–98
Stanford CB (1989) Predation on capped langurs (Presbytis pileata) by cooperatively hunting jackals (Canis aureus). Am J Primatol 19:53–56
Stark DJ, Nijman V, Lhota S et al (2012) Modeling population viability of local proboscis monkey Nasalis larvatus populations: conservation implications. Endanger Species Res 16:31–43
Strasser E (1992) Hindlimb proportions, allometry, and biomechanics in Old World Monkeys (Primates, Cercopithecidae). Am J Phys Anthropol 87:187–213
Struhsaker T (2000) The effects of predation and habitat quality on the socioecology of African monkeys: lessons from the Islands of Bioko and Zanzibar. In: Whitehead PF, Jolly C (eds) Old world monkeys. Cambridge University Press, Cambridge, pp 393–430
Supriatna J, Manullang BO, Soekara E (1986) Group composition, home range, and diet of the maroon leaf monkey (Presbytis rubicunda) at Tanjung Puting Reserve, Central Kalimantan, Indonesia. Primates 27:185–190
The Nature Conservancy (2011) Berau Forest Carbon Programme: delivering practical solutions to support development of a national-level REDD Framework in Indonesia
Tsuji Y, Higuchi H, Suryobroto B (2014) A note on responses of juvenile Javan lutungs (Trachypithecus auratus mauritius) against attempted predation by crested goshawks (Accipter trivirgatus). Hum Nat 25:105–110
Tsuji Y, Prayitno B, Suryobroto B (2016) Report on the observed response of Javan lutungs (Trachypithecus auratus mauritius) upon encountering a reticulated python (Python reticulatus). Primates 57:149–153
Uhde NL, Sommer V (2002) Anti-predatory behaviour in gibbons. Eat or be eaten: predator sensitive foraging among primates, pp 268–291
van Niewstadt MGL, Shiel D, Kartawinata K (2001) The ecological consequences of logging in the burned forests of east Kalimantan, Indonesia. Conserv Biol 15:1183–1186
Wanelik K, Abdulazis Cheyne SM (2013) Note-, phase- and song-specific acoustic variables contributing to the individuality of male duet song in the Bornean Southern gibbon (Hylobates albibarbis). Primates 54:159–170
Wells K, Pfeiffer M, Lakim MB, Linsenmair KE (2004) Use of arboreal and terrestrial space by a small mammal community in a tropical rain forest in Borneo, Malaysia. J Biogeogr 31:641–652
Whitten AJ (1980) The Kloss Gibbon in Siberut. University of Cambridge, Cambridge
Wiens F, Zitzmann A (1999) Predation on a wild slow loris (Nycticebus coucang) by a reticulated python (Python reticulatus). Folia Primatol 70:362–364
Wilcove DS, Giam X, Edwards D et al (2013) Navjot’s nightmare revisited: logging, agriculture, and biodiversity in Southeast Asia. Trends Ecol Evol 28:531–540
Wilcox CH, Supiansyah S, Abdul Azis K et al (2017) Predator mobbing and interspecies cooperation: an interaction between gibbons, langurs and a clouded leopard. Asian Primates J 6:20–26
Wilson CC, Wilson WL (1975) The influence of selective logging on primates and some other animals in East Kalimantan. Folia Primatol 23:245–274
Wilting A, Fischer F, Bakar SA, Linsenmair KE (2006) Clouded leopards, the secretive top-carnivore of South–East Asian rainforests: their distribution, status and conservation needs in Sabah, Malaysia. BMC Ecol 6:1–27
Workman C, Schmitt D (2012) Positional behavior of Delacour’s langurs (Trachypithecus delacouri) in Northern Vietnam. Int J Primatol 33:19–37
Zuberbuhler K, Jenny D (2002) Leopard predation and primate evolution. J Hum Evol 43:873–886. https://doi.org/10.1006/jhev.2002.0605
Acknowledgements
This project involved collaboration between Borneo Nature Foundation and many individuals, organizations and universities. For logistical support and permits we thank Balai Lingkungan Hidup in Purak Cahu, Pak Herry Mulyadi Tuwan, Pak Purwanto and Pak Agusdin, The Nature Conservancy, Yayorin and the Bupati of Lamandau Regency. For their research collaboration, we thank the BRINCC team, Stan Lhota and Gabriella Fredriksson, the Centre for the International Cooperation in Management of Tropical Peatlands (CIMTROP), University of Palangka Raya and the Indonesian Ministry of Science and Technology (RISTEK) and Director General of Nature Conservation (PHKA). Funding for the behavioral work in Sabangau was provided by Chester Zoo and the North of England Zoological Society, Columbus Zoo and Aquariums, and Primate Conservation, Inc. The Robertson Foundation provided funding for the majority of the camera trap survey work presented here. For their additional financial support, we thank Panthera, International Animal Rescue (IAR) Indonesia, Borneo Nature Foundation, The Barito River Initiative for Nature Conservation and Communities (BRINCC), The Clouded Leopard Project/Point Defiance Zoo and Aquarium, Fresno Chafee Zoo and the Orangutan Foundation UK. We thank Erik Meijaard and Ikki Matsuda for their helpful suggestions and comments on an earlier draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Cheyne, S.M., Supiansyah, Adul et al. Down from the treetops: red langur (Presbytis rubicunda) terrestrial behavior. Primates 59, 437–448 (2018). https://doi.org/10.1007/s10329-018-0676-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-018-0676-5