Abstract
Despite supporting the highest biodiversity on Earth, tropical rainforests are undergoing intensive economic development. In particular, the island of Borneo has lost over 56 % of original lowland forest to resource extraction, fires, and illegal logging. Its rainforests contain 16 primate species, which serve as excellent ‘umbrella’ taxa for conservation initiatives. The genus Presbytis (Colobinae) is well represented across Borneo by four endemic species (P. chrysomelas; P. frontata; P. hosei, and P. rubicunda), but remains relatively understudied. Using ecological niche modelling, I calculated the distributions of the 12 Bornean Presbytis subspecies; evaluated habitat loss between 2000 and 2010, and examined the current land-use policies across remnant distributions. Subspecies experienced a mean 12.7 % (N = 12 sp.) habitat reduction over the 10 year period. 12.5 % of all habitats were allocated for conversion to oil palm and industrial tree plantations, while logging concessions accounted for a mean 26.3 % across distributions. While the current protected area networks encompassed an average 33.4 % of distributions, most PAs are underfunded, degraded and threatened by logging and mining operations. I therefore recommend priority gazetting of unallocated lands to new PAs within the distribution of Presbytis chrysomelas and Presbytis hosei sabana, which have experienced the highest forest loss in the last 10 years (22–50 %) and are critically endangered. Logging concessions appear to be at least as effective in maintaining forest cover as PAs and have the economic advantage for effective management, but may have detrimental effects to monkey populations. I recommend an urgent assessment of the effects of selective logging practices on species’ persistence, and further recommend population surveys to quantify the populations of critically endangered and data deficient subspecies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical rainforests are undergoing intensive economic development (Carlson et al. 2012; Hansen et al. 2008; Page et al. 2002), despite supporting the highest biodiversity on Earth (Pimm and Sugden 1994). The island of Borneo in particular has lost more than 56 % of its original lowland forest, due to resource extraction, fires, and illegal logging (Curran et al. 2004; Geist and Lambin 2002; Langner et al. 2007; Miettinen et al. 2011). Since the 1980s, ~80 % of the forests of Kalimantan (Indonesian Borneo) alone have been allocated as federally-managed industrial timber-logging concessions (Curran et al. 2004). Additionally, in the last 10 years, the rate of forest clearance for oil-palm production has increased by 212 % to account for 31,640 km2 of Kalimantan’s total area; 90 % of such clearances occurred on forested land (Carlson et al. 2012).
Borneo also represents one of the global top 25 biodiversity hotspots under threat (Myers et al. 2000) and is home to 16 primate species, ten of which are endemic (Groves 2001; Munds et al. 2013). Given the high rate of habitat loss, much action has been taken to conserve the Bornean orangutan (Pongo pygmaeus), including efforts to stabilize populations and moratoriums on logging throughout known distributions (Soehartono et al. 2007), and comprehensive studies on its ecology (e.g. Morrogh-Bernard et al. 2009; Wich et al. 2008), distribution (Husson et al. 2009) and conservation status (Wich et al. 2012). Such actions in turn serve as useful conservation surrogates for biodiversity within orang-utan distributions (Bibby 1998; Margules et al. 2002).
Indeed, in addition to protection for their own sake, the primates, as a large, well-studied (Rowe 1996) and charismatic order (Mittermeier et al. 2012), make excellent ‘umbrella’ species for conservation initiatives (Soemarna et al. 1995; Kiester et al. 1996; Bibby 1998; Sowa et al. 2007). Primates of the genus Presbytis are more extensively distributed than the orangutan across Borneo, and are represented by 4 endemic species: P. chrysomelas; P. frontata; P. hosei, and P. rubicunda, but remain relatively understudied (but see Ampang and Zain 2012; Davies et al. 1988; Marshall 2010; Nijman 2010; Ehlers Smith and Ehlers Smith 2013). Locality data for P. chrysomelas and P. frontata in particular are scarce, and the taxonomic status of the genus is still disputed and in a state of flux (c.f. Groves 2001; Brandon-Jones et al. 2004; Brandon-Jones 2006; Meyer et al. 2011; Table 1).
Basic knowledge of a species’ occurrence and distribution is an essential starting point for predicting extinctions as a result of habitat loss, and to subsequently devise strategies to focus conservation efforts (Margules and Pressey 2000; Groves 2003). Three types of species occurrence data exist: point localities, at which a species has been observed; extent of occurrences, as broad geographic ranges within which are all the localities that a species is recorded; and predicted distributions, in which the suitability of environmental conditions within a known extent of occurrence are assessed for the likelihood of species’ occupancy (Corsi et al. 1999; Guisan and Zimmermann 2000; Rondinini et al. 2006). If environmental and habitat requirements are known, ecological niche models can refine extent of occurrence data by omitting areas that are unsuitable (Rondinini et al. 2005, 2006), as these data representations often overestimate species’ distributions (commission errors). In species for which an abundance of locality data-points are available, more sophisticated predictive niche modelling techniques are known to perform well, in which computer algorithms predict species’ distributions based on a variety of environmental variables at multiple locations (e.g. Maximum Entropy, Phillips et al. 2006).
The high rate of habitat destruction, agricultural expansion and multiple land-use policies on Borneo is impetus for assessing the anthropogenic threats faced by the endemic and scarcely-documented Presbytis monkeys. In this study I aim to (1) present the distributions of the 4 species of Bornean Presbytis monkeys, and their respective subspecies, based on ecological niche modelling and a consensus of the literature; (2) evaluate the proportion of habitat loss for each subspecies over the 10 year period between 2000 and 2010; (3) calculate the current land-use policies throughout remnant distributions, and investigate the likely effects of these on the conservation status of each subspecies, and (4) make recommendations for conservation strategies to safeguard the persistence of each subspecies.
Methods
Study species
Genus Presbytis is a monophyletic taxon comprising at least 11 species restricted to the Greater Sunda Islands and the Malayan Peninsula (Meijaard and Groves 2004; Meyer et al. 2011). The genus is included in the colobine sub-family, which is distinct in the adaptation of its forestomach morphology, which facilitates folivory and granivory (seed eating; Chivers 1994; Kay and Davies 1994). Presbytis monkeys are among the most frugivorous and granivorous colobines, and typically consume between 25 and 65 % fruit parts (Davies et al. 1988; Marshall 2010; Nijman 2010). Body sizes are relatively gracile and range from 5.6 to 8.2 kg (Rowe and Myers 2010). The genus is almost entirely arboreal, and, with the exception of P. potenziani, maintains groups of single-adult males and multiple-adult females and their offspring. Extra-group males form all-male bands or range alone (Davies and Oates 1994).
Presbytis chrysomelas
Presbytis chrysomelas was recently elevated from a subspecies of P. femoralis (Groves 2001), and now comprises two of its own subspecies (Table 1). It has the most restricted distribution of the Presbytis monkeys and inhabits lowland tropical wet evergreen and swamp forests below 500 m above sea level (asl) in groups of 3–13 individuals (Ampang and Zain 2012; Boitani et al. 2006; Nijman et al. 2008a; Table 1). P. chrysomelas is considered critically endangered due to its low population size, restricted distribution and the high conversion rate of its habitat (Nijman et al. 2008b; Table 1).
Presbytis frontata
Presbytis frontata is monotypic (Groves 2001; Table 1) and inhabits tropical wet evergreen forests on mineral soils in a broad distribution across the centre of the island at relatively low densities up to an elevation of 2,000 m asl (Meijaard and Nijman 2008; Table 1). It lives in relatively small groups of >6 individuals (Nijman 2001) and is classified as vulnerable due to hunting and habitat loss (Meijaard and Nijman 2008). Presbytis frontata is particularly cryptic and under-studied, and is known to freeze upon contact with humans (Nijman and Nekaris 2012).
Presbytis hosei
The taxonomy of Presbytis hosei is under dispute and may actually contain up to three separate species (c.f. Nijman and Meijaard 2008a; Nijman 2010), but for simplicity it is treated here as one species comprising four subspecies (Groves 2001; Table 1). Presbytis hosei occurs in tropical wet evergreen forests on mineral soils up to 1,700 m asl in the north and east of Borneo. Above this altitude, densities become much lower (Nijman 2010). Average group sizes range from 7 to 10 individuals, at variable densities from >1 to over four groups km−2. The threat assessment for the respective subspecies ranges from vulnerable to endangered, as a result of hunting and habitat loss (Nijman et al. 2008a; Table 1). Presbytis hosei canicrus is noteworthy as its status and distribution is currently under review. Lhota et al. (2012) confirmed the species’ persistence in the Wehea Forest of the West Kutai district, and Brandon-Jones (1997) established that its range extends west beyond this region. Setiawan et al. (2009) suggested that the species may be absent in the Kutai National Park, its former population stronghold in the south of its range.
Presbytis rubicunda
Presbytis rubicunda represents the most comprehensively-studied of the endemic Presbytis monkeys (c.f. Supriatna et al. 1986; Davies et al. 1988; Davies 1991; Marshall 2010; Hanya and Bernard 2012; Ehlers Smith and Ehlers Smith 2013; Ehlers Smith et al. 2013a, b), contains five subspecies, and is considered the least threatened due to its broad distribution (Nijman and Meijaard 2008b; Table 1). It occurs at a range of densities, dependent on the availability of high-quality foods (Marshall 2010; Ehlers Smith and Ehlers Smith 2013), and typically comprises 3–10 individuals. Presbytis rubicunda occupies tropical swamp and wet evergreen forests on mineral soils up to 2,000 m asl, although it is likely populations above 700–800 m asl are at such low densities that they are non-viable (Marshall 2010).
Species’ distribution modelling
Given the state of flux that exists within the taxonomy of the genus, historical locality records assigned to the subspecies treated here are of dubious accuracy and practicality. Furthermore, sufficient locality data were lacking for the subspecies to perform advanced predictive niche modelling. Therefore, I used ecological niche modelling on the known extent of occurrence data-sets for each primate subspecies, which represents a consensus of the available locality data.
I used ArcGIS v10 (ESRI 2011) for all modelling. I accessed primate species’ extent of occurrence data from “All The World’s Primates” (Rowe and Myers 2010), land cover maps of Insular Southeast Asia from 2000 to 2010 produced by the CRISP project (Miettinen et al. 2011), and elevation data from the World Database on Protected Areas Consortium (WDPA 2006). I extracted by attribute the appropriate forest cover classes from the land cover maps of 2000 and 2010 for each subspecies according to its habitat requirements, as listed in the Southeast Asian Mammal Databank (Boitani et al. 2006; Table 1) which represents a consensus of the literature. For each subspecies I merged all habitat classes as appropriate, and then clipped the resultant layer by its recorded altitudinal limit. I then clipped each extent of occurrence layer by its species-specific habitat layer to produce a distribution layer, from which I then erased all rivers that bisected habitats as natural geographic barriers. I projected all data layers into the appropriate WGS 1984 UTM Zones of Borneo to facilitate accurate area calculations. The dissolve and multi-part to single-part functions were used to assign a unique identification to each polygon, and I then calculated the area of each polygon and subsequently deleted those smaller than 10 km2 from the distribution model as too small to be a viable habitat patch.
Two of the 12 subspecies treated here warrant specific treatment: given the uncertainty of the distributional boundary of P. h. canicrus, I performed two boundary scenarios thus: (1) a minimum scenario based on the data provided by Lhota et al. (2012), and (2) a maximum scenario based on the data provided by Brandon-Jones (1997). The interim distributional region between the known boundary of P. h. everetti and P. h. canicrus is ascribed to an “unconfirmed” subspecies that is thought to exist in the area and may be one or the other subspecies (Nijman 2010). Similarly, taking into account the likelihood that populations of P. rubicunda are non-viable above 700–800 m asl (Marshall 2010), I performed two ecological niche model scenarios for each subspecies for whom distributions exceeded 700 m asl thus: (1) the maximum altitudinal limit recorded (2,000 m asl), and (2) the viable altitudinal limit (700 m asl).
Land use projections
To assess the impacts of the current land-use allocations on the distributions of the 12 Presbytis subspecies, I used the data-set compiled by Wich et al. (2012) detailing the current major land uses including: (1) oil palm plantations (IOPP); (2) industrial tree plantations (ITP); (3) logging concessions, and (4) the protected area (PA) networks. A comprehensive overview of these land-use allocations and how the data were compiled can be found in Wich et al. (2012). I overlaid each subspecies’ distribution with each land-use data-layer to calculate the area and proportion that each allocation contributed to each overall distribution (Fig. 1).
Industrial oil palm plantation concessions (IOPP)
Oil palm plantation concessions are granted by the Indonesian and Malaysian governments at the local level for the conversion of natural forests and subsequent production of oil palm monocultures. In Malaysia, a minority of concessions are allocated from de-gazetted commercial forest reserves; the majority are issued in ‘conversion’ or ‘production’ forests, as is the case for Indonesian concessions. These data were unavailable for the Malaysian state of Sabah, and as such are not included in the analysis of land-use impacts on subspecies’ whose ranges (P. rubicunda chrysea, P. hosei sabana) are located within that state (Wich et al. 2012).
Industrial tree plantation concessions (ITP)
The conversion of natural forested lands to ITPs is not considered deforestation in the United Nations Framework Convention on Climate Change, as they are legally defined as ‘forest’ (Sasaki and Putz 2009; Wich et al. 2012). In Indonesia, ITP are granted by the Ministry of Forestry and converted on lands classified as ‘production forests’; in Malaysia the Forestry Department grants concessions on the equivalent ‘commercial forest reserves’ (Wich et al. 2012).
Logging concessions in natural forests
Logging concession licenses permit companies to extract natural timber products from the rainforests, although mandates of sustainable resource extraction prohibit deforestation through clear-felling (Wich et al. 2012).
Protected areas (PAs)
The gazetting of natural habitats into national parks; nature and wildlife sanctuaries; game and virgin jungle reserves; protection forests, and recreational parks prohibits logging and degradation of PAs (Wich et al. 2012). However, in reality, deforestation occurs in a large proportion of PAs on Borneo (Nellemann et al. 2007; Gaveau et al. 2013).
Results
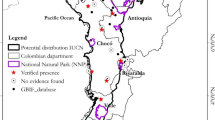
Taken at the specific level, P. rubicunda was distributed across the largest range of the Presbytis monkeys found on Borneo (>278,000 km2; Fig. 2), followed by P. frontata (>160,000 km2) and P. hosei (~130,000 km2). Presbytis chrysomelas had the most restricted distribution (~21,000 km2). Presbytis rubicunda carimatae, found only on the adjacent island of Karimata, had the smallest distribution at the subspecific level (130 km2). Of the mainland subspecies, P. chrysomelas cruciger and P. hosei hosei occupied the smallest area (3,780 and 5,790 km2, respectively; Fig. 3). Presbytis r. rubicunda (>98,000 km2), P. r. rubida (>92,000 km2) and P. r. ignita (~66,000 km2) had the largest distributions (Table 2; Fig. 3).
Distribution of (a) all Presbytis hosei subspecies including an unconfirmed subspecies between the known ranges of P. hosei everetti and P. hosei canicrus, based on the maximum scenario (Brandon-Jones 1997) and (b) Distribution of P. frontata
Each of the 12 subspecies lost natural habitat through forest conversions between 2000 and 2010 (range 4.6–53.7 %) with the exception of P. r. carimatae, whose range is entirely gazetted as a PA (Table 2; Fig. 4). A third of the subspecies lost over 10 % of their natural habitat over the 10 year period. Presbytis chrysomelas cruciger experienced the highest rate of habitat loss (>50 %) habitat since 2000. The subspecies located in Sabah (P. r. chrysea, P. h. sabana) also lost substantial proportions of forested habitat in the 10 year period (21.1 % and 22.8 %, respectively; Table 2).
For most subspecies, logging concessions represented the largest allocation of land-use throughout distributions, followed by PAs (Table 2). Unallocated land accounted for >10 % of all allocations in 10 subspecies, and in the case of P. h. hosei as much as 50 % of land within its distribution was unallocated. For the majority of subspecies, ITPs were a minor allocation throughout distributions. In Central Kalimantan and Sarawak, IOPPs also comprised a substantial proportion of land use, but were not assessable for Sabah (Table 2).
The overall distributions of P. rubicunda under 700 m asl were substantially lower in most cases, resulting in a general increase in the proportion of economic land-use allocations within distributions, and a complete decrease across all subspecies in PAs (with the exception of P. r. carimatea, for whom the entire distribution is protected), as much of the PA network spans the central mountainous region (Table 3; Fig. 1). The maximum scenario for modelling the distribution of P. h. canicrus increased the area by ~4,000 km2.
Discussion
Distribution model caveats
The distributional modelling presented here represented a consensus of the known locality data and environmental and habitat requirements of the Presbytis monkeys on Borneo, and is the most comprehensive description of their distributions to date. Given the lack of locality data and the under-studied nature of the subject species, it is likely that both commission and omission errors featured in the model (Rondinini et al. 2006). Commission errors may have occurred within areas of occupancy that have not been surveyed, despite the removal of habitat known to be unsuitable. For example, large Bornean mammals exhibit irregular distributional patterns with large gaps in apparently suitable habitats, as a result of mutual exclusion through intra-genus competition and hunting pressures (Meijaard et al. 2005). Similarly, it is possible omission errors occurred in unsurveyed regions that lie beyond species’ known geographical boundaries. However, given their under-studied nature and the rapid habitat destruction occurring throughout their ranges, such an assessment of the anthropogenic threats facing Bornean Presbytis monkeys is urgently required and potential errors in the modelling process may be justified to this end.
Land-use impacts on the distribution of Bornean Presbytis monkeys
Oil palm plantations and ITPs have replaced vast tracts of lowland forest and fragmented remnant forest blocks (Carlson et al. 2012). Given their arboreal nature (Davies and Oates 1994), Presbytis monkeys cannot persist in deforested and converted habitats, and are unlikely to be able to cross denuded patches to disperse. Thus, land conversions for agricultural expansion likely pose the largest threat to the persistence of Presbytis monkeys.
As clear-felling within logging concessions is prohibited, it is possible that with effective management, concessions can contribute to species’ persistence and appear to be as successful as PAs in preventing total forest clearance (Putz et al. 2012; Wich et al. 2012; Gaveau et al. 2013). However, the actual response of Bornean Presbytis monkeys to the effects of logging within concessions is largely uncertain. For example, P. rubicunda has been variously reported as “neutral” (Bennett and Dahaban 1995) and intolerant to logging (Meijaard et al. 2008), with declines in population density as a result (Johns 1992; Blouch 1997). The tallest trees with the largest DBHs, which are targeted for selective logging practices, are important within the ecology of P. rubicunda and are used for feeding (Ehlers Smith et al. 2013a), predator avoidance (Nijman and Nekaris 2012) and sleeping sites (DA Ehlers Smith, unpublished data).
Johns and Skorupa (1987) predicted that a primate species’ ability to persist in disturbed habitats increases with its degree of folivory, although the most important factor in resistance to disturbance appears to be the ability to exploit leaves when, as a result of logging activities, fruits are scarce (Johns 1986; Meijaard et al. 2005). In this way, Presbytis monkeys have an adaptive advantage. However, infant mortality due to starvation, lactational stress and abandonment often follows logging practices (Meijaard et al. 2005). Indeed, population density of P. h. canicrus appears to decline after logging events (Nijman 2000), with a possible time-lag between the onset of logging and population declines (Howell 2003). Meijaard et al. (2005) also noted that logging often results in an increase in hunting, which has a dramatic effect on P. hosei populations in Borneo’s interior where traditional hunting practices persist (Nijman 2005). No data exist for the responses of P. frontata and P. chrysomelas to logging, but Meijaard et al. (2005) concluded that Presbytis ssp. would benefit from a more wildlife conscientious concession management policy (Fig. 5).
The long-term viability of habitats may be more secure if designated as PAs, as they are less likely to be de-gazetted than other land-use allocations (Wich et al. 2012; Gaveau et al. 2013). However, while current PA allocations also account for substantial areas within each distribution (28–38 %, Table 2), up to 80 % of all established PAs in Indonesia are degraded (Nellemann et al. 2007). Indeed, clearance of forests in PAs occurred at a similar rate as that in logging concessions (1.2 and 1.5 %, respectively) during the 10 year period (Gaveau et al. 2013), and severe logging occurs in as much as half of all PAs, in particular the Gunung Palung; Tanjung Puting; Danau Sentarum, and Kutai National Parks (Curran et al. 2004; Ministry of Forestry 2006). While PAs in Malaysia appear to be subject to lower rates of degradation and deforestation, they are subject to small-scale deforestation across the region where insufficient management resources are available (Wich et al. 2012).
While not a land-use policy per se, it is also worth discussing the detrimental effect that hunting has on Presbytis monkeys, as all species on Borneo are hunted for their meat and bezoar stones (visceral excretions of high economic value, used in traditional Asian medicines). For example, Nijman (2005) documented declines of up to 80 % in P. hosei in the Kayan Mentarang National Park following hunting for bezoar stones, and highlighted that although the protected forest remained in pristine condition, a lack of protection enforcement can result in drastic declines. Such hunting pressures can have profound influences on species’ distributions, with local extinctions occurring within even remote and protected environments (Meijaard et al. 2005).
Conservation implications
At the species level, each of the four endemic Presbytis monkeys lost substantial areas of natural habitat over the 10 year period between 2000 and 2010. Given the current land-use allocations, this trend will continue into the future. Indeed, if all economic allocations are exploited, between 45 and 65 % of remaining habitat will be under concession, and a minimum of 10 % will be destroyed outright (Table 2; Fig. 1).
Presbytis frontata, as a monotypic species with a broad distribution across the centre of the island where the largest concentration of PAs are situated, experienced the smallest habitat reduction in the 10 year period, and is the least affected by IOPPs (given the requirements of low elevation and slope for oil palm cultivation [Wakker 2004] ). However, logging concessions accounted for over one-third of its remnant habitat (Table 2). At the subspecific level, only the habitat size of P. r. carimatae maintained stasis, suggesting efficient management of the PA of Karimata Island is in operation. However, populations of P. r. carimatae have not been conducted in 20 years (Yanuar et al. 1993) and the subspecies is considered data deficient on the IUCN Threat List (Nijman et al. 2008a), suggesting a resurvey should be considered a priority. Similarly, the uncertainty surrounding the area of occupancy of P. h. canicrus is impetus for further surveys to establish the subspecies’ distribution patterns. The minimum modelling scenario predicts an area of occupancy of 7,200 km2, while the maximum predicts 11,300 km2, which would increase if the unconfirmed species that occupies the area toward the northern border with P. h. everetti is in fact P. h. canicrus. Indeed, the subspecies occurs in the south-eastern portions of one of the largest, unfragmented forest-blocks remaining on Borneo (>55,000 km2) with few major geographic boundaries.
The largest habitat declines occurred in the subspecies with the most restricted distributions, particularly those in Sabah (Table 2). The most extreme case of distribution reduction occurred in the critically endangered P. c. cruciger, which lost over 50 % of its habitat in 10 years, while Presbytis hosei sabana and P. r. chrysea each lost over 20 % of their habitat in the same period, suggesting rapid population declines. Current economic land allocations are likely to have a particularly strong negative impact on the already threatened habitat of the critically endangered P. c. chrysea, as a further 30 % is due to be converted for OIPP and TIPs. Likewise, the huge logging concession allocations within the distributions of P. h. sabana and P. r. chrysea (60–65 % of total distributions; Table 2) may have negative impacts on their restricted populations (Meijaard et al. 2005; Howell 2003). Data for IOPPs in Sabah are unavailable, but given the large percentages of IOPP allocations in Sarawak (Table 2) and the large reduction in habitat over the last 10 years that has occurred in the Sabahan Presbytis subspecies, it is not unreasonable to assume that habitat conversion for current IOPPs is likely to be high, to the detriment of P. h. sabana and P. r. chyrsea populations.
The current PA network accounts for over 20 % of subspecies’ distributions in all but those that occur in Sabah. However, data show that the majority of PAs are too degraded and under-resourced to provide effective protection (Nellemann et al. 2007; Gaveau et al. 2013). Furthermore, the location of the majority of PA allocations is concentrated in the highlands, where in some cases, population densities are lower (P. rubicunda) or distributions do not occur (P. chrysomelas; Figs. 1, 2 and 4). These findings are consistent with the conclusions of Wich et al. (2012) that the current PA network is not optimally located and thus is likely insufficient for the long-term persistence of Bornean Presbytis monkeys.
Instead, huge proportions of each subspecies’ distributions are currently not designated for economic allocation (Table 2). While the gazetting of these areas would, of course, increase the PA network (for example, ~30 % of the remnant 3,800 km2 of habitat for P. c. chrysea is unallocated; if gazetted, the PA network of this critically endangered primate would increase to approximately 58 %), this does not guarantee species protection and persistence, due to the ineffective way PAs are currently managed (Nellemann et al. 2007). It is, in fact, possible that increasing the number of PAs within an already insufficiently funded PA network may be diluting, counterproductive and ultimately detrimental to species conservation. However, it is particularly clear that urgent action is required for the critically endangered species; the designation of a small PA on the unallocated lands to safeguard their persistence is likely to be more beneficial than no action at all. While logging concessions are better able to generate revenue than PAs, and appear at least as effective in preventing forest loss (Gaveau et al. 2013), the actual effects of logging on Presbytis populations living within concessions remain unclear, and may well have a negative effect (Nijman 2000; Howell 2003; Meijaard et al. 2005, 2008).
Conclusions and recommendations
The threat to Borneo’s endemic Presbytis monkeys through habitat destruction, disturbance and degradation is severe and sustained, and the trend is set to continue given current land-use allocations (Wich et al. 2012). Between 2000 and 2010, the Presbytis subspecies of Borneo experienced a mean 12.7 % (N = 12; mean of each value for both scenarios of P. h. canicrus contributes to grand mean) habitat reduction, and 12.5 % of all habitats were allocated for conversion to IOPP and ITPs. While the current protected area networks encompassed an average 33.4 % of distributions, the majority of PAs are degraded and threatened by logging and mining operations (Nellemann et al. 2007). I therefore recommend priority gazetting of unallocated lands to PAs within the distribution of critically endangered Presbytis chrysomelas and P. h. sabana, which have experienced the highest forest loss in the last 10 years (22–50 %). Logging concessions, which may have detrimental effects to Presbytis monkey populations (Meijaard et al. 2005), accounted for a mean 26.3 % across distributions but appear to be at least as effective in maintaining forest cover as PAs (Gaveau et al. 2013). I therefore recommend an urgent assessment of the effects of logging practices on species’ persistence, as concessions have an economic advantage for effective management over PAs. I further recommend population surveys to quantify the populations of the critically endangered P. c. chrysea and the data deficient P. r. carimatae and P. r. chrysea, and further surveys to establish the distributional boundaries of P. h. canicrus and P. h. everetti.
References
Ampang A, Zain BM (2012) Ranging patterns of critically endangered colobine, Presbytis chrysomelas chrysomelas. Sci World Journ. doi:10.1100/2012/594382
Bennett EL, Dahaban Z (1995) Wildlife responses to disturbances in Sarawak and their implications for forest management. In: Primack RB, Lovejoy TE (eds) Ecology, conservation and management of South-East Asian rainforests. Yale University Press, New Haven, pp 66–86
Bibby CJ (1998) Selecting areas for conservation. In: Sutherland WJ (ed) Conservation science and action. Blackwell Science, Oxford, pp 176–201
Blouch RA (1997) Distribution and abundance of orangutans (Pongo pygmaeus) and other primates in the Lanjak Entimau Wildlife Sanctuary, Sarawak, Malaysia. Trop Biod 4:259–274
Boitani L, Catullo I, Marzetti M, Masi M, Rulli M, Savini S (2006) The southeast Asian mammal databank: a tool for conservation and monitoring of mammal diversity in Southeast Asia. Instituto di Ecologia Applicata, Roma
Brandon-Jones D (1997) The zoogeography of sexual dichromatism in the Bornean grizzled surili, Presbytis comata (Desmarest 1822). Sarawak Mus J 50:177–200
Brandon-Jones D (2006) The pros and cons of a consensus list of Asian primate subspecies. Primate Conserv 20:89–93
Brandon-Jones D, Eudey AA, Geissmann T, Groves CP, Melnick DJ, Morales JC, Shekelle M, Stewart C (2004) Asian primate classification. Int J Primatol 25:97–164
Carlson KM, Curran LM, Asner GP, Pitner AM, Trigg SN, Adney JM (2012) Carbon emissions from forest conversion by Kalimantan oil palm plantations. Nat Clim Change 3:283–287
Chivers DJ (1994) Functional anatomy of the gastrointestinal tract. In: Davies AG, Oates JF (eds) Colobine monkeys: their ecology, behaviour and evolution. Cambridge University Press, Cambridge, pp 205–257
Corsi F, de Leeuw J, Skidmore AK (1999) Modelling species distributions with GIS. In: Boitani L, Fuller TK (eds) Research techniques in animal ecology: controversies and consequences. Columbia University Press, New York, pp 389–434
Curran LM, Trigg SN, McDonald AK, Astiani D, Hardiono YM, Siregar P, Caniago I, Kasischke E (2004) Lowland forest loss in protected areas of Borneo. Science 303:1000–1003
Davies AG (1991) Seed-eating by red leaf monkeys (Presbytis rubicunda) in dipterocarp forest of northern Borneo. Int J Primatol 12:119–144
Davies AG, Oates J (1994) Colobine monkeys: their ecology, behaviour and evolution. Cambridge University Press, Cambridge
Davies AG, Bennett EL, Waterman PG (1988) Food selection by two Southeast Asian colobine monkeys (Presbytis rubicunda and Presbytis melalophos) in relation to plant chemistry. Biol J Linn Soc 34:33–56
Ehlers Smith DA, Ehlers Smith YC (2013) Population density of red langurs in Sabangau tropical peat-swamp forest, Central Kalimantan, Indonesia. Am J Primatol 75:837–847
Ehlers Smith DA, Husson SJ, Ehlers Smith YC, Harrison ME (2013a) Feeding ecology of red langurs in Sabangau tropical peat-swamp forest, Indonesian Borneo: extreme granivory in a non-masting forest. Am J Primatol 75:848–859
Ehlers Smith DA, Ehlers Smith YC, Cheyne SM (2013b) Home-range use and activity patterns of Presbytis rubicunda in Sabangau tropical peat-swamp forest, Central Kalimantan, Indonesian Borneo. Int J Primatol 34:957–972
ESRI (2011) ArcGIS desktop: release 10. Environmental Systems Research Institute, Redlands
Gaveau DLA, Kshatriya M, Sheil D, Sloan S, Wich S, Ancrenaz M, Hansen M, Broich M, Molidena E, Wijaya A, Guariguata MR, Pacheco P, Potapov P, Turubanova S, Meijaard E (2013) Reconciling forest conservation and logging in Indonesian Borneo. PLoS One 8:e69887 69881–69811
Geist HJ, Lambin EF (2002) Proximate causes and underlying driving forces of tropical deforestation. Bioscience 52:143–150
Groves CP (2001) Primate taxonomy. Smithsonian Institute Press, Washington
Groves CR (2003) What to conserve? Selecting conservation targets. In: Groves CR (ed) Drafting a conservation blueprint: a practitioner’s guide to planning for biodiversity. Island Press, USA
Guisan A, Zimmermann NE (2000) Predictive habitat distribution models in ecology. Ecol Model 135:147–186
Hansen MC, Stehman SV, Potapov PV, Loveland TR, Townshend JRG, Defries RS, Pittman KW, Arunarwati B, Stolle F, Steininger MK, Carroll M, Dimiceli C (2008) Humid tropical forest clearing from 2000 to 2005 quantified by using multitemporal and multiresolution remotely sensed data. Proc Nat Acad Sci USA 105:9439–9444
Hanya G, Bernard H (2012) Fallback foods of red leaf monkeys (Presbytis rubicunda) in Danum Valley, Borneo. Int J Primatol 332:322–337
Howell, D (2003) The effects of human activity on primates and other large mammals in East Kalimantan, Indonesia. MSc Thesis. Central Washington University, USA
Husson SJ, Wich SA, Marshall AJ, Dennis RD, Ancrenaz M, Brassey R, Gumal M, Hearn AJ, Meijaard E, Simorangkir T, Singleton I (2009) Orangutan distribution, density, abundance and impacts of disturbance. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (eds) Orangutans: geographic variation in behavioural ecology and conservation. Oxford University Press, Oxford, pp 77–96
Johns AD (1986) Effects of selective logging on the behavioural ecology of west Malaysian Primates. Ecology 67:684–694
Johns AD (1992) Species conservation in managed forests. In: Whitmore TC, Sayer AJ (eds) Tropical deforestation and species extinction. Chapman and Hall, London, pp 15–53
Johns AD, Skorupa JP (1987) Responses of rainforest primates to habitat disturbance: a review. Int J Primatol 8:157–191
Kay RNG, Davies AG (1994) Digestive physiology. In: Davies AG, Oates JF (eds) Colobine monkeys: their ecology, behaviour and evolution. Cambridge University Press, Cambridge, pp 229–249
Kiester AR, Scott JM, Csuti B, Noss RF, Butterfield B, Sahr K, White D (1996) Conservation prioritization using Gap data. Conserv Biol 10:1332–1342
Langner A, Miettinen J, Siegert F (2007) Land cover change 2002–2005 in Borneo and the role of fire derived from MODIS imagery. Glob Change Biol 13:2329–2340
Lhota S, Loken B, Spehar S, Fell E, Pospěch A, Kasyanto N (2012) Discovery of Miller’s grizzled langur (Presbytis hosei canicrus) in Wehea forest confirms the continued existence and extends known geographical range of an endangered primate. Am J Primatol 74:193–198
Margules CR, Pressey RL (2000) Systematic conservation planning. Nature 405:242–253
Margules CR, Pressey RL, Williams PH (2002) Representing biodiversity: data and procedures for identifying priority areas for conservation. J Biosci 27(4 Suppl. 2):309–326
Marshall A (2010) Effects of habitat quality on primate populations in Kalimantan: gibbons and leaf monkeys as case studies. In: Supriatna J, Gursky-Doyen S (eds) Indonesian primates. Developments in primatology: progress and prospects. Springer Science, Chicago, pp 157–177
Meijaard E, Groves C (2004) The biogeographical evolution and phylogeny of the genus Presbytis. Primate Rep 68:71–90
Meijaard E, Nijman V (2008) Presbytis frontata. In: IUCN 2012. IUCN red list of threatened species. Version 2012.2. www.iucnredlist.org. 20 Feb 2013
Meijaard E, Sheil D, Nasi R et al (2005) Life after logging: reconciling wildlife conservation and production forestry in Indonesian Borneo. Center for International Forestry Research (CIFOR), Bogor, Indonesia
Meijaard E, Sheil D, Marshall AJ, Nasi R (2008) Phylogenetic age is positively correlated with sensitivity to timber harvest in Bornean mammals. Biotropica 40:76–85
Meyer D, Rinaldi D, Ramlee H, Perwitasari-Farajallah D, Hodges JK, Roos C (2011) Mitochondrial phylogeny of leaf monkeys (genus Presbytis, Eschscholtz, 1821) with implications for taxonomy and conservation. Mol Phylogenet Evol 59:311–319
Miettinen J, Shi C, Tan W, Liew S (2011) 2010 land cover map of insular Southeast Asia in 250-m spatial resolution. Remote Sens Lett 3:11–20
Ministry of Forestry (2006) Forest statistics of Indonesia 2005. Ministry of Forestry, Indonesia
Mittermeier RA, Schwitzer C, Rylands AB, Taylor LA, Chiozza F, Williamson EA, Wallis J (2012) Primates in peril: the world’s 25 most endangered primates 2012–2014. IUCN/SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Conservation and Science Foundation, Bristol
Morrogh-Bernard HC, Husson SJ, Knott CD, Wich SA, van Schaik CP, van Noordwijk MA, Lackman-Ancrenaz I, Marshall AJ, Kanamori T, Kuze N, Sakong RB (2009) Orangutan activity budgets and diet: a comparison between species, populations and habitats. In: Wich SA, Suci Utami Atmoko S, Tatang Mitra Setia, van Schaik CP (eds) Orangutans: geographic variation in behavioral ecology and conservation. Oxford University Press, Oxford, pp 119–133
Munds RA, Nekaris KAI, Ford SM (2013) Taxonomy of the Bornean slow loris, with new species Nycticebus kayan (Primates, Lorisidae). Am J Primatol 75:46–56
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Nellemann C, Miles L, Kaltenborn BP, Virtue M, Ahlenius H (2007) The last stand of the orangutan: state of emergency: illegal logging, fire and palm oil in Indonesia’s national parks. United Nations Environment Programme, GRID-Arendal
Nijman V (2000) Geographical distribution of ebony leaf monkeys Trachypithecus auratus (Geoffrey Saint Hilaire 1812) (Mammalia: Primates: Cercopithecidae). Contrib Zool 69:157–177
Nijman V (2001) Forest (and) primates: conservation and ecology of the endemic primates of Java and Borneo. Tropenbos Foundation, Wageningen
Nijman V (2005) Decline of the endemic Hose’s langur in Kayan Mentarang National Park, East Borneo. Oryx 39:223–226
Nijman V (2010) Ecology and conservation of the Hose’s langur group (Colobinae: Presbytis hosei, P. canicrus, P. sabana): a review. Indonesian primates. Developments in primatology: progress and prospects. Springer Science, Chicago, pp 269–284
Nijman V, Meijaard E (2008a) Zoogeography of primates in insular Southeast Asia: species-area relationships and the effects of taxonomy. Contrib Zool 77:117–126
Nijman V, Meijaard E (2008b) Presbytis rubicunda. In: IUCN 2012. IUCN red list of threatened species. Version 2012.2. www.iucnredlist.org. 20 Feb 2013
Nijman V, Nekaris KAI (2012) Loud calls, startle behaviour, social organisation and predator avoidance in arboreal langurs (Cercopithecidae: Presbytis). Folia Primatol 83:274–287
Nijman V, Hon J, Richardson M (2008a) Presbytis chrysomelas. In: IUCN 2012. IUCN red list of threatened species. Version 2012.2. www.iucnredlist.org. 19 Feb 2013
Nijman V, Meijaard E, Hon J (2008b) Presbytis hosei. In: IUCN 2012. IUCN red list of threatened species. Version 2012.2. www.iucnredlist.org. 20 Feb 2013
Page SE, Siegert F, Rieley JO, Boehm HDV, Jaya A, Limin S (2002) The amount of carbon released from peat and forest fires in Indonesia during 1997. Nature 420:61–65
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modelling of species geographic distributions. Ecol Model 190:231–259
Pimm SL, Sugden AM (1994) Tropical diversity and global change. Science 263:933–935
Putz FE, Zuidema PA, Synnott T, Pena-Claros M, Pinard MA et al (2012) Sustaining conservation values in selectively logged tropical forests: the attained and the attainable. Conserv Lett 5:296–303
Rondinini C, Stuart S, Boitani L (2005) Habitat suitability models reveal shortfall in conservation planning for African vertebrates. Conserv Biol 19:1488–1497
Rondinini C, Wilson KA, Boitani L, Grantham H, Possingham HP (2006) Tradeoffs of different types of species occurrence data for use in systematic conservation planning. Ecol Lett 9:1136–1145
Rowe N (1996) The pictorial guide to the living primates. Pogonias Press, Rhode Island
Rowe N, Myers M (2010) All the world’s primates. http://www.alltheworldsprimate.com. 21 Nov 2012
Sasaki N, Putz FE (2009) Critical need for new definitions of ‘‘forest’’ and ‘‘forest degradation’’ in global climate change agreements. Cons Lett 2:226–232
Setiawan A, Nugroho TS, Djuwantoko T, Pudyatmoko S (2009) A survey of Miller’s grizzled surili, Presbytis hosei canicrus, in East Kalimantan, Indonesia. Primate Conserv 24:139–143
Soehartono T, Susilo HD, Andayani N, Atmoko SSU, Sihite J et al (2007) Strategi dan rencana aksi konservasi orangutan Indonesia 2007–2017. Ministry of Forestry of the Republic of Indonesia, Jakarta
Soemarna K, Ramono W, Poniran S, van Schaik CP, Rijksen HD, Leighton M, Sajuthi D, Lelana A, Karesh W, Griffiths M, Seal US, Traylor-Holzer K, Tilzen R (1995) Conservation action plans for orang-utans in Indonesia. In: Nadler RD et al (eds) The neglected ape. Plenum Press, New York, pp 123–128
Sowa SP, Annis G, Morrey ME, Diamond DD (2007) A Gap analysis and comprehensive conservation strategy for riverine ecosystems of Missouri. Ecol Monogr 77:301–334
Supriatna J, Manullang BO, Soekara E (1986) Group composition, home range, and diet of the maroon leaf monkey (Presbytis rubicunda) at Tanjung Puting Reserve, Central Kalimantan, Indonesia. Primates 27:185–190
Wakker E (2004) Greasy palms: the social and ecological impacts of large-scale oil palm plantation development in Southeast Asia. A Report for Friends of the Earth, London, UK. Available at http://www.foe.co.uk/sites/default/files/downloads/greasy_palms_impacts.pdf
Wich S, Meijaard E, Marshall AJ, Husson S, Ancrenaz M, Lacy R et al (2008) Distribution and conservation status of the orangutan (Pongo spp.) on Borneo and Sumatra: how many remain? Oryx 42:329–339
Wich SA, Gaveau D, Abram N, Ancrenaz M, Baccini A et al (2012) Understanding the impacts of land-use policies on a threatened species: is there a future for the Bornean orang-utan? PLoS One. doi:10.1371/journal.pone.0049142
World Database on Protected areas (WDPA) Consortium (2006) CD-ROM. Managed by the WCMC-UNEP
Yanuar A, Bekti D, Saleh C (1993) The status of the Karimata primates Presbytis rubicunda carimatae and Macaca fascicularis carimatensis in Karimata Island, Indonesia. Trop Biodivers 1:157–162
Acknowledgments
I thank the Indonesian State Ministry of Research and Technology (RISTEK), the Directorate General of Forest Protection and Nature Conservation (PHKA) and CIMTROP for research permissions. Funding was provided by Chester Zoo and the North of England Zoological Society; Columbus Zoo and Aquariums, and Primate Conservation, Inc. Thanks also to Professors Vincent Nijman and KAI Nekaris, and Dr. Susan M. Cheyne and Dr. Suwido H. Limin. I am grateful to Professor Serge Wich and Dr. David Gaveau for providing the land-use data upon which much of this research was based and to Editor Professor David Hawksworth and two anonymous reviewers for their constructive feedback on the manuscript. This research was conducted as part of the OuTrop multi-disciplinary research project, conducted in collaboration with CIMTROP-University of Palangka Raya.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David Westcott.
Rights and permissions
About this article
Cite this article
Ehlers Smith, D.A. The effects of land-use policies on the conservation of Borneo’s endemic Presbytis monkeys. Biodivers Conserv 23, 891–908 (2014). https://doi.org/10.1007/s10531-014-0639-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-014-0639-0