Abstract
Feeding conditions, competitive regime, and female social relationships of Japanese macaques (Macaca fuscata) on Yakushima were compared between the two habitats at two different altitudes (coniferous forest, 1,000–1,200 m and coastal forest, 0–200 m). Fruit availability was higher in the coastal forest. There was no consistent difference in the frequency of agonistic interactions within a group during feeding between the two habitats. The coastal forest evoked stronger inter-group contest competition compared to the coniferous forest as evidenced by a higher inter-group encounter rate and a higher proportion of aggressive encounters to non-aggressive ones. Birth rate was higher in larger groups compared to smaller ones in the coastal forest, but did not differ in the coniferous forest. In spite of these differences in competitive regime, no variation in female social relationships was observed, such as direction and concentration on particular individuals in grooming, linearity in dominance rank, counter-attack, and support of juvenile kin during agonistic interactions. The present results indicate that the female social relationships of Japanese macaques are robust and do not change according to changes in the current environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecological conditions, such as predation, food abundance (Schülke 2003), food distribution (Weir and Grant 2004; Johnson et al. 2004) and food quality (Shopland 1987) can determine anti-predatory strategy of primates and the competitive regime they face, which in turn affect the social relationships and dispersal patterns that maximize individual fitness (van Schaik and van Hooff 1983). For example, clumped food can be monopolized by a few individuals, which promotes contest-type (direct) competition (van Schaik 1989). High-quality foods are worth fighting for, and thus animals may compete for high-quality foods but not necessarily for low-quality foods (Enquist et al. 1985).
In group-living primates, competition over foods occurs at two levels: within and between groups. The models by van Schaik and colleagues predict that female primates form the following social relationships depending on the intensity and type of within- and between-group competition (van Schaik 1989; Sterck et al. 1997). Under strong within-group contests, dominance relations among females are despotic and nepotistic (resident-nepotistic type). Under strong between-group and within-group contests, high-ranking females risk losing the support of low-ranking females in between-group contests if they enforce dominance too strongly. Therefore, dominance rank is linear and nepotistic, but tolerant (resident-nepotistic-tolerant type). Under strong between-group contests but weak within-group contests, females do not form decided agonistic dominance relationships and therefore the dominance relationships become more relaxed or egalitarian (resident-egalitarian type). When both within- and between-group contests are weak, females are not philopatric and their relationships are egalitarian (dispersal-egalitarian type). Models by Isbell (1991) also dealt with the effect of within- and between-group competition. She predicted that under strong within- and between-group feeding competition, females form highly expressed, stable, linear dominance hierarchies, and inherit their rank maternally. When only between-group contest is evident and within-group contest is lacking, they form weakly expressed, unstable, non-linear dominance hierarchies, and do not inherit their rank maternally. When both between- and within-group contests are lacking, female primates form weakly expressed, unstable, non-linear dominance hierarchies, and females transfer between groups. In this study, we basically focused on the predictions by Sterck et al. (1997).
These socioecological models have been proposed to explain the inter-species difference in primate social systems, thus the changes in evolutionary time scale. The models propose that ecological factors are proximate causes of variations in social behaviors and also assume that if these factors were constant throughout the evolutionary scale, individual strategies to cope with these ecological conditions would have been selected, leading to adaptive evolved patterns of social organization typical of each species (Koenig 2002). It remains unclear whether the principle could explain within-species variation and thus the changes in ecological time scale. Primate socioecological studies often use current environmental differences between species to infer environments over evolutionary time; although this may not always be applicable. Knowledge of the breadth of behavioral flexibility is important in order to assess how much they can match their social behavior to the current environment. Social behaviors may not necessarily be adaptive to the current environment if the environment has changed recently (van Schaik and Kappler 1996).
Examination of intra-specific variation is important because it clarifies whether the behavior is genetically fixed or flexible according to changes in habitat (Sterck et al. 1997; Isbell and Young 2002). Examples of within-species comparisons include Koenig et al. (1998), who compared two Hanuman langur populations; this study indicated that in areas where the main food was superabundant, females dispersed more often and dominance rank was unimportant. On the contrary, in other populations where the distribution of the main food was clumped, females were philopatric and formed linear dominance hierarchies. Likewise, female social relationships among patas monkeys vary from despotic to egalitarian, in accordance to the distribution of main foods (Nakagawa 2007). These studies, however, compare distantly located populations, and thus they cannot rule out the possibility that these differences are genetically fixed. The extent of flexibility of primate social behaviors without genetic differentiation remains unknown.
This study compares the food conditions, competitive regimes, and female social relationships of Japanese macaques (Macaca fuscata) on Yakushima, between groups living at two different altitudes. We used both our original data and the re-analyzed published data. This is an ideal place to examine the socioecological model and behavioral flexibility of this species because: (1) the two habitats are only 7 km apart without any geographic borders, so the effect of genetic difference is negligible (Hayaishi and Kawamoto 2006). (2) The habitat and diet vary considerably with altitude (Hanya 2004a). In the coastal forest, fruit availability is higher and macaques are more frugivorous. On the other hand, in the fruit-poor coniferous forest, they are more folivorous (Hanya 2004a). (3) Predation, one of the two most important ecological factors, is negligible, so we can isolate the effect of foods. No fossil or historic record of carnivores or large raptors exists on this island.
First, we compared the competitive regime. Since the number of food trees is more limited in the coastal forest (see Methods) and both population and group density is three times higher in the coastal forest (Hanya et al. 2004, 2006), we predict that both within- and between-group contests are more severe in the coastal forest (van Schaik 1989). In addition, macaques in the coastal forest rely more heavily on high-quality fruits/seeds, and fruit production is higher in the coastal forest (Hanya et al. 2003a). Assuming that macaques tend to defend high-quality foods/home ranges but do not defend low-quality foods/home ranges (food: Enquist et al. 1985; home range: Sugiura et al. 2000, 2002), within-and between-group contests are also expected to be more intense in the coastal forest. We used the frequency of agonistic interactions as an index of within-group contests (Mitchell et al. 1991). To assess the cause and effects of between-group competition, we compared frequency of agonistic inter-group encounters, behavior during encounters and economic defendability of home range, and the relation between group size and birth rate.
Then, we examined whether female social relationships varied in the way predicted by the socioecological model (Sterck et al. 1997). The model predicts that under intense within-group contest, female social relationships are resident-nepotistic or resident-nepotistic-tolerant type. In both of these types, female dominance rank is despotic and nepotistic, and thus female grooming is directed higher up the hierarchy, compared to the other two types (dispersal-egalitarian and resident-egalitarian). Consequently, high-ranking individuals receive the most grooming (Henzi and Barrett 1999). Grooming tends to be mostly concentrated on kin (Sterck et al. 1997), and females often support their juvenile kin. Although kin relationship was not known in the study groups, we regarded that they are less nepotistic if they groom evenly among the females in the groups. It is known that female Japanese macaques concentrate their grooming to kin (Furuichi 1984). Concerning the effect of between-group contest, the model predicts that the type of female social relationships under intense between-group contest differs between when within-group contest is strong (resident-nepotistic-tolerant) and weak (resident-egalitarian). However, considering that female Japanese macaques form a linear dominance hierarchy without exception (Yamagiwa and Hill 1998), including the present study groups (see Results), we assume that the “resident-egalitarian” type is not applicable to our study subjects. Therefore, we test only the prediction that the female Japanese macaques form more tolerant relationships under strong between-group contests. For example, counter-attack is predicted to be frequent under strong between-group contests. If higher-ranking individuals use grooming as a currency to gain coalitional support (Seyfarth and Cheney 1984) from lower-ranking ones in between-group contest, it is also predicted that more grooming will be directed toward lower-ranking ones.
Methods
Study site
Yakushima is an island in southwestern Japan (30°N, 131°E) that occupies an area of 503 km2. We conducted this study in the coniferous and coastal forests with an altitude of 1,000–1,200 m and 0–300 m a.s.l., respectively.
Mean annual fruit production in the coastal forest was about three times as large as that in the coniferous forest (coniferous forest: 28 kg/ha; coastal forest: 116 kg/ha, in terms of fresh weight of fleshy parts; Hanya et al. 2004). Basal area of trees that provide fruit and/or seeds as food for macaques in the coastal forest was 2.5 times larger than that in the coniferous forest (coniferous forest: 21.7 m2/ha; coastal forest: 53.8 m2/ha; Hanya et al. 2004). These data on food availability were gained from two 0.25-ha plots at 280 m and 1,050 m a.s.l. in 1999, 2000, and 2001. The plots were within the home ranges of the macaque study groups. The mean density of food trees that comprised at least 1% of the annual feeding time was 1,493 trees/ha in the coniferous forest (Hanya 2004b), which is much larger than that in the coastal forest (4.16; Agetsuma 1995).
Study subjects and behavioral observation
Since the coniferous forest is the new study site, behavioral data were available for only one group (HR group). In the coastal forest, however, we use both our original data of H group and published data from various groups. Group size, study period, observation time, type of data analyzed, source of the data, and sampling methods are summarized in Table 1. In all of the groups, mother–infant relations were known at least for mothers and juveniles under 2 years old, because suckling was observed during or before the study period. We included only juveniles under 2 years old in the analysis of agonistic support.
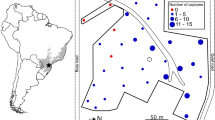
Our original behavioral data (HR and H groups) were taken as follows. Behavioral sampling protocol for other groups was identical to the one applied to these groups, at least concerning the behaviors analyzed here. In both groups, we conducted focal animal sampling of all adult females, except for two less habituated individuals, which were difficult to follow when they were alone, but they permitted the observers when they rested with other group members. Thus, the level of habituation of non-focal animals was sufficient to collect reliable data on the interactions we observed between them and the focal females, so their social relationships were at least partially reflected in the data presented here. Because female Japanese macaques change their social behaviors when they are estrous (Matsubara and Sprague 2004), we analyzed only anestrous females to minimize mating-related aggression. During observation, we recorded feeding, grooming (start and end time and partner) and agonistic interactions by continuous sampling. When the focal animal stayed in or touched the feeding tree, it was regarded as “arboreal feeding”. Otherwise, it was regarded as terrestrial feeding. Agonistic interactions include not only overt attack, threat, and chase but also silent supplanting because both affect food intake (Hill and Okayasu 1995). We regarded supplanting occurred when an individual approached another individual within 3 m, and the approached individuals moved away while the approaching animal was still moving. Results did not change if we analyzed only overt attack between the HR and H groups. For the P group, data of only overt attacks were not available (not written in the Hill and Okayasu 1995). We considered that an inter-group encounter occurred when the two groups approached and the members of the study group were regarded to have recognized the other group, for example gazing of members of the other group or responses to vocalization, such as flee. This definition was in accordance with the previous study of this species (Saito et al. 1998). Approximate distance between the two groups was less than 50 m for most cases. Agonistic behaviors toward the other group’s members, such as rush, flee, line-up, aggression, displace (listed by Saito et al. 1998) were recorded when they occurred. The frequency of inter-group encounters did not change much with respect to the age–sex classes (H group: following males, 0.031 times/h; following females, 0.026 times/h), so we combined the data.
We conducted census in the 7.5-km2 area of the coniferous forest in August of every year from 2000 to 2006 for 6–11 days. We followed four identified groups (HR, PE, OM and SY groups) and recorded their composition when they crossed open places (usually roads). The home range of the HR group was in the primary forest, and the other groups were in the disturbed forest. We used data only when the composition was confirmed by counting more than twice. Data on 24 groups-years were available. We calculated infant carrying rate (number of infants/number of females) for each group/year. All the censuses were conducted about 4–5 months after the birth period (Fooden and Aimi 2003), so they are comparable as an index of crude birth rate. In the coastal forest, the actual birth rate was calculated based on long-term observations of 30 groups-years.
Data analysis
In the analysis of grooming, we divided data into mating (September–November) and non-mating seasons (other months). This was because in the coastal forest, data of the two study groups were available only for one of the seasons (mating season for H and non-mating seasons for M groups). In the analysis of direction of grooming, we used all the data since the difference of the total amount of observation time does not seem to bias the results. However, in the analysis of number of grooming partners and concentration of grooming to particular individuals, observation time needed to be controlled. Therefore, only the data of the first 10 h for each focal female during the period from April (non-mating) or September (mating) were used.
Day-journey length was calculated as (observed ranging distance on that day) × (day length)/(observation time), when the observation time of the day was 8 h or more. Home range size was calculated as minimum convex polygon.
In the analysis of grooming, we calculated mean values in the number of female grooming partners or the coefficient of variation in grooming time to each female (including non-focal female partner) for each individual and examined the effect of habitat (coniferous vs. coastal) and season (mating vs. non-mating) by two-way ANOVA. In the analysis of the direction of the grooming, we calculated grooming index from individual A to individual B and compared with the opposite direction by paired t-test. Grooming index from A to individual B was defined as: [f B(A)*100]/[F(A) + F(B)], whereas f B(A) is the time A groomed B, and F(A) is the observation time of A (Furuichi 1984). We also included the grooming index when one of the pair was a non-focal animal in this analysis. In the analysis of agonistic interactions during feeding, we calculated the frequency for each individual and compared between groups by t-test. In the analysis of counter-attack, data were pooled for all the individuals and the frequency was compared by G-test or Fisher’s exact probability test because it was rare.
Results
In this section, we label each group by its habitat, e.g., conifHR, coastH.
Frequency of agonistic interactions during feeding
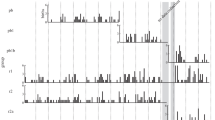
There was no consistent difference between coniferous and coastal groups in the frequency of agonistic interactions during feeding. During feeding, frequency of agonistic interaction was significantly higher in the coastP than coastH, but the difference between the conifHR and the coastal groups was not significant (Fig. 1c). When feeding was divided into arboreal and terrestrial feeding, the order of the frequency of aggression was coastP > conifHR > coastH, although the differences were not significant (Fig. 1a, b).
Frequency of agonistic interactions during feeding. a Terrestrial feeding. b Arboreal feeding. c All feeding. Means + SD are shown. Numbers in parentheses are sample size (number of focal animals). Data of the P group were cited from Hill and Okayasu (1995)
Inter-group relationships
Between-group contests were more intense in the coastal forest than in the coniferous forest. The frequency of inter-group encounters was 5.08 times higher in the coastH group (37/1,260 = 0.029 encounters/h) than that in the conifHR group (4/703 = 0.0057 encounters/h). Agonistic behaviors toward other group members were observed for all of the cases in the coastH group but no such behavior was observed in the conifHR group. In the coastH group, these agonistic behaviors were one-directional for 11 cases (30%) and they were always subordinate to the larger-sized coastNA group, which means dominant-subordinate relations exist between groups. The result was the same even when we compared with the more extensive published data in the coastal forest. The frequency of aggressive inter-group encounters during the group follows of seven groups in the coastal forest (120/3,742 = 0.032 encounters/h; Sugiura et al. 2000) was almost the same as that of the coastH group. Dominance-subordinate relationship was apparent in 66% (100/151) of encounters in the extensive dataset and the larger groups were dominant over smaller groups.
Home range was more defendable in the coastal forest than in the coniferous forest. Although the day journey length was longer in the conifHR group (2.19 km) than in the coastH group (1.33 km; comparisons of monthly average; t = 4.10, df = 8, P < 0.01), defendability index (day journey length/diameter of home range; Mitani and Rodman 1979) was higher in the coastH group (1.54) than in the conifHR group (1.18).
Birth rate was higher for larger groups in the coastal forest but did not differ with respect to group size in the coniferous forest. In the coastal forest, birth rate of the groups sized less than 15 was significantly smaller than larger groups (Takahata et al. 1998a). In the coniferous forest, on the other hand, infant carrying rate of the larger conifHR and conifSY groups (number of females = 6–9; 25 infant-year/82 female-year = 30.5%) was not significantly different from the smaller conifOM and conifPE groups (number of females: 3–5; 20/59 = 33.9%; G = 0.18, P = 0.671). The infant carrying rate of the conifHR group (16/55 = 29%) living in the primary forest was not significantly different from that of the SY group (9/27 = 33%) which lived in the disturbed forest but had comparable group size (G = 0.14, P = 0.700).
Female social relationships
Grooming relationships did not differ between the two habitats. The grooming time from dominants to subordinates and from subordinates to dominants did not differ both in the coniferous and coastal forests in both mating and non-mating seasons (Fig. 2). Two-way ANOVA revealed that the effect of habitat (coniferous vs. coastal) on the number of female grooming partners was not significant in both seasons (habitat: F = 0.69; season: F = 13.5, P = 0.0013; habitat*season: F = 2.08. Mean ± SD = 1 ± 0.8 (coniferous, mating); 2.6 ± 1.4 (coniferous, non-mating), 1.4 ± 0.8 (coastal, mating); 2.9 ± 0.6 (coastal, non-mating)). In the case of the coefficient of variation in grooming time to each female, the effect of the habitat and season was not significant (habitat: F = 1.41, NS; season: F = 2.08, NS; habitat × season: F = 0.099, NS. Mean ± SD = 2.6 ±0.4 (coniferous, mating); 2.3 ± 0.6 (coniferous, non-mating); 2.3 ± 0.3 (coastal, mating); 2.1 ± 0.2 (coastal, non-mating)). Time spent being groomed by females (% to the observation time) did not correlate to the dominance rank in any of the groups or seasons (mating, conifHR: τ = 0.33, P = 0.29; non-mating, conifHR: τ = 0.52, P = 0.10; mating, coastH: τ = 0.048, P = 0.88; non-mating, coastM: τ = 0.20, P = 0.62).
Directions of grooming among females. Only those dyads that were observed to groom together were included. Means + SD are shown. Numbers in parentheses are sample size (number of dyads). Data of the M group were cited from Furuichi (1984)
Female agonistic relationships also did not differ between the two habitats. Female dominance relationships were linear in all the groups (Table 2). The proportion of counter-attack among the observed female–female agonistic interactions was small in all the groups; 0/96 = 0% in the conifHR group in the coniferous forest, 1/22 = 4.5% in the coastH group and 1/291 = 0.3% in the coastP group in the coastal forest (conifHR vs. coastH: Fisher’s exact probability test, P = 0.227; conifHR vs. coastP: P = 0.755). The frequency of support to focal females’ infants was also low in all the groups and there was no consistent difference between habitats (2/310 = 0.0065/h in the conifHR group in the coniferous forest, 0/254 = 0/h in the coastH group and 8/475 = 0.017/h in the coastP group in the coastal forest). Since the difference in the number of juvenile kin per female was small among the groups (conifHR: 0.88, coastH: 1.0, coastP: 1.2), the results were not affected by the number of juvenile kin.
Discussion
As summarized in Table 3, our results indicate that competitive regime (between-group contests) varies between forests in a manner which can be predicted from food condition (fruit availability and quality of food). In the coastal forest, macaque foods were of higher quality, which is worth competing for, and the foods were distributed in a few food trees. Consequently, it was more likely that one group could monopolize foods than in the coniferous forest. These food conditions are liable to intensify between-group contest. However, little variation in female social relationships was observed between the groups and we found no difference in the within-group contest between the two habitats, although we assessed it only by indirect measures.
Food condition and competitive regime
Between-group contests were more intense in the coastal forest, considering the facts that the rate of aggressive encounter was higher, and that displacements and dominant-subordinate relationships were frequently observed. Neighboring groups around the conifHR group can also be followed for all day (Hanya et al. 2003b), so the difference in encounter rate was not the result of the difference in habituation of neighboring groups. There was no evidence that within-group contest was more intense in one of the habitats than in the other.
The difference in between-group contests can be explained by the economic defendability of home ranges (Sugiura et al. 2000) and difference in group density (van Schaik 1989). Group density in the coastal forest is three times as large as that in the coniferous forest (Hanya et al. 2004), so macaque groups in the coastal forest encounter other groups more often, which increases the likelihood of between-group direct interactions. Defendability index was higher in the coastal forest than in the coniferous forest. Fruit production and total basal area of fruit/seed-food trees were higher in the coastal forest, so the value of home ranges per unit area was higher, making it worthwhile to defend the ranges against other groups. In the coniferous forest, on the other hand, defending their home ranges is not economical because the group density is low, and also because their home ranges are not valuable enough to defend. When food availability is too high and does not limit population density, the intensity of between-group contest is low (Isbell 1991). However, this is apparently not the case because the density of Japanese macaques is limited by food availability in Yakushima (Hanya et al. 2004).
There were a number of differences in food conditions between the two habitats which might affect the within-group contest. For example, fewer food trees may cause monopolization of food patch by a few individuals in one group, thus promoting within-group contest. Coastal macaques depend more on fruits (Hanya 2004a), which are contestable resources. This might promote the aggression during feeding. However, we could not find consistent difference in the frequency of agonistic interactions during feeding between the two habitats. The effect of diet may differ within the coastal forest or year by year, which may have influenced the different frequency of agonistic interactions during feeding in the coastH and coastP groups. In fact, macaques in the coastal forest change their ranging pattern with respect to the supra-annual variations in fruiting of major food fruits (Hill and Agetsuma 1995). Since our data on within-group contest were based on data of fewer groups/years than those of between-group contest, the results were more likely to be affected by the supra-annual variations in food conditions. Another possibility is that the within-group contest was also intense in the coastal forest, but its effect was concealed due to the strong between-group contest. Dominants may have tolerated subordinates under strong between-group contest in the coastal forest, and thus we could not detect any difference in the intensity of within-group contest by the frequency of agonistic interactions. Future studies to measure within-group contest directly, for example by demographic parameters, will be necessary. To properly assess the effect of food competition among females on reproductive success, we also need data on infant mortality. In the coastal forest, there is no difference in infant mortality with respect to group size or mothers’ rank (Takahata et al. 1998a, 1998b). Therefore, we do not need to alter our conclusions, at least in the coastal forest, even when infant mortality is considered. However, data in the coniferous forest are still deficient.
Flexibility and stability of female social relationships in response to ecological conditions
In spite of the differences in food conditions and competitive regimes, female social relationships were quite similar between the coniferous and coastal groups. Under severe between-group contest, females are predicted to be tolerant (see Introduction), and thus the direction of grooming would not be related to dominance and counter-attack would be frequent. However, in spite of the difference in the intensity of between-group contest, there was no difference in the direction of grooming or counter-attack between the two habitats. We believe that basic female social relationships are robust and do not change according to ecological conditions within populations of Japanese macaques.
An alternative explanation might be possible, which assumes that the difference in the intensity of competition between the groups is negligible when competition is measured by a more direct parameter, such as food intake or birth rate. The mismatches between the observed levels of aggression and competitive regimes may be explained by the fact that competition is seldom measured in fitness units (Koenig 2002). Although it has been reported that female rank does not affect birth rate and the number of lifetime offspring produced in the coastal forest (Takahata et al. 1998b), data in the coniferous forest are still lacking. Therefore, our conclusion on the difference in within-group contest remains tentative. In addition, frequency of agonistic interaction might not always be a correct indicator of between-group contest, since groups contesting over the same resources might avoid each other (Koenig 2002). However, our conclusion on between-group contest can also be supported by direct evidence (crude birth rate). The data were derived from various groups for 7 and 21 years in the coniferous and coastal forests, respectively. The effect of habitat heterogeneity on birth rate was not supported by the comparison between the groups living in different habitats with similar group size. The data in the coastal forest included 20 different groups, so the result cannot be explained by habitat heterogeneity. All of these facts deny the possibility that there was no variation in female social relationships only because the difference in between-group contest between the two habitats is negligible.
The present results point out that the socioecological models are not necessarily applicable to the changes in ecological time scale. Socioecological models have explained many of the inter-species variations, thus changes in evolutionary time scale, of primate social relationships (Mitchell et al. 1991; Barton et al. 1996; Boinski et al. 2002; Cords 2002); however, some studies have revealed that socioecological models are not sufficient to fully explain the social relationships of female primates (Korstjens et al. 2002; Izar 2004; this study). Izar (2004) pointed out that group size, female dispersal, and linearity of dominance of capuchin monkeys are explained by ecological conditions, but detailed female relationships within groups, such as support during aggression, are not. In addition, flexibility and stability may differ among taxonomic groups. Phylogenetic analysis of the entire Order Primates indicates that some lineages, especially the cercopithecids, strongly conserve social organization (Di Fiore and Rendall 1994). Macaques of the fascicularis group (M. fascicularis, M. fuscata, M. mulatta and M. cyclopis) are distributed within an enormous range of ecological conditions, including tropical rainforest and snowy cool-temperate forest. However, their female social relationships are quite similar (Matsumura 1999). On the other hand, genus Saimiri includes species of both female-bonded and non-female-bonded species (Mitchell et al. 1991). In future study of socioecology, it is important to distinguish flexible and stable social characteristics or taxonomic groups. Flexible social character can be explained by the current ecological conditions; however, evolution of a stable social character or taxonomic group would be explained not only by the current environment but also by the past environment or geological changes. To clarify how flexible or stable the social characters and/or taxonomic groups are, comparisons at various taxonomic levels are essential. Intra-specific comparison, such as this study, is the first step to understand the flexibility of the species.
Change history
26 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10329-021-00893-y
References
Agetsuma N (1995) Foraging strategies of Yakushima macaques (Macaca fuscata yakui). Int J Primatol 16:595–609
Barton RA, Byrne RW, Whiten A (1996) Ecology, feeding competition and social structure in baboons. Behav Ecol Sociobiol 38:321–329
Boinski S, Sughrue K, Selvaggi L, Quatrone R, Henry M, Cropp S (2002) An expanded test of the ecological model of primate social evolution: competitive regimes and female bonding in three species of squirrel monkeys (Saimiri oerstedii, S. boliviensis, and S. sciureus). Behaviour 139:227–261
Cords M (2002) Friendship among adult female blue monkeys (Cercopithecus mitis). Behaviour 139:291–314
Di Fiore A, Rendall D (1994) Evolution of social organization: a reappraisal for primates by using phylogenetic methods. Proc Natl Acad Sci USA 91:9941–9945
Enquist M, Plane E, Röed J (1985) Aggressive communication in fulmars (Fulmarus glacialis) competing for food. Anim Behav 33:1007–1020
Fooden J, Aimi M (2003) Birth-season variation in Japanese macaques, Macaca fuscata. Primates 44:109–117
Furuichi T (1984) Symmetrical patterns in non-agonistic social interactions found in unprovisioned Japanese macaques. J Ethol 2:109–119
Hanya G (2004a) Diet of a Japanese macaque troop in the coniferous forest of Yakushima. Int J Primatol 25:55–71
Hanya G (2004b) Seasonal variations in the activity budget of Japanese macaques in the coniferous forest of Yakushima: effects of food and temperature. Am J Primatol 63:165–177
Hanya G, Noma N, Agetsuma N (2003a) Altitudinal and seasonal variations in the diet of Japanese macaques in Yakushima. Primates 44:51–59
Hanya G, Yoshihiro S, Zamma K, Kubo R, Takahata Y (2003b) New method to census primate groups: estimating group density of Japanese macaques by point census. Am J Primatol 60:43–56
Hanya G, Yoshihiro S, Zamma K, Matsubara H, Ohtake M, Kubo R, Noma N, Agetsuma N, Takahata Y (2004) Environmental determinants of the altitudinal variations in relative group densities of the Japanese macaques on Yakushima. Ecol Res 19:485–493
Hanya G, Kiyono M, Yamada A, Suzuki K, Furukawa M, Yoshida Y, Chijiiwa A (2006) Not only annual food abundance but also fallback food quality determines the Japanese macaque density: evidence from seasonal variations in home range size. Primates 47:275–278
Hayaishi S, Kawamoto Y (2006) Low genetic diversity and biased distribution of mitochondrial DNA haplotypes in the Japanese macaque (Macaca fuscata yakui) on Yakushima Island. Primates 47:158–164
Henzi SP, Barrett L (1999) The value of grooming to female primates. Primates 40:47–59
Hill DA, Agetsuma N (1995) Supra-annual variation in the influence of Myrica rubra fruit on the behavior of a troop of Japanese macaques in Yakushima. Am J Primatol 35:241–250
Hill DA, Okayasu N (1995) Absence of ‘youngest ascendancy’ in the dominance relations of sisters in wild Japanese macaques (Macaca fuscata yakui). Behaviour 132:367–379
Isbell LA (1991) Contest and scramble competition: patterns of female aggression and ranging behavior in primates. Behav Ecol 2:143–155
Isbell LA, Young TP (2002) Ecological models of female social relationships in primates: similarities, disparities, and some directions for future clarity. Behaviour 139:177–202
Izar P (2004) Female social relationships of Cebus apella nigritus in a southeastern Atlantic forest: an analysis through ecological models of primate social evolution. Behaviour 141:71–99
Johnson CA, Grant JWA, Giraldeau L-A (2004) The effect of patch size and competitor number on aggression among foraging house sparrows. Behav Ecol 15:412–418
Koenig A, Beise J, Chalise MK, Ganzhorn JU (1998) When females should contest for food: testing hypotheses about resource density, distribution, size, and quality with Hanuman langurs (Presbytis entellus). Behav Ecol Sociobiol 42:225–237
Koenig A (2002) Competition for resources and its behavioral consequences among female primates. Int J Primatol 23:759–783
Korstjens AH, Sterck EHM, Noë R (2002) How adaptive or phylogenetically inert is primate social behaviour? A test with two sympatric colobines. Behaviour 139:203–225
Matsubara M, Sprague DS (2004) Mating tactics in response to costs incurred by mating with multiple males in wild females Japanese macaques. Int J Primatol 25:901–917
Matsumura S (1999) The evolution of “egalitarian” and “despotic” social systems among macaques. Primates 40:23–31
Mitani JC, Rodman PS (1979) Territoriality: the relation of ranging pattern and home range size to defendability, with an analysis of territoriality among primate species. Behav Ecol Sociobiol 5:241–251
Mitchell CL, Boinski S, van Schaik CP (1991) Competitive regimes and female bonding in two species of squirrel monkeys (Saimiri oerstedi and S. sciureus). Behav Ecol Sociobiol 28:55–60
Nakagawa N (2007) Despotic wild patas monkeys (Erythrocebus patas) in Kala Maloue, Cameroon. Am J Primatol: doi:10.1002/ajp.20481 (in press)
Saito C, Sato S, Suzuki S, Sugiura H, Agetsuma N, Takahata Y, Sasaki C, Takahashi H, Tanaka T, Yamagiwa J (1998) Aggressive intergroup encounters in two populations of Japanese macaques (Macaca fuscata). Primates 39:303–312
van Schaik CP (1989) The ecology of social relationships amongst female primates. In: Standen V, Folley RA (eds) Comparative socioecology. Blackwell, Oxford, pp 195–218
van Schaik CP, Kappler PM (1996) The social systems of gregarious lemurs:lack of convergence with anthropoids due to evolutionary disequilibrium? Ethology 102:915–941
van Schaik C, van Hooff JARAM (1983) On the ultimate causes of primate social systems. Behaviour 85:91–117
Schülke O (2003) To breed or not to breed-food competition and other factors involved in female breeding decisions in the pair-living nocturnal fork-marked lemur (Phaner furcifer). Behav Ecol Sociobiol 55:11–21
Seyfarth RM, Cheney DL (1984) Grooming, alliances and reciprocal altruism in vervet monkeys. Nature 308:541–543
Shopland JM (1987) Food quality, social deployment, and the intensity of feeding interference in yellow baboons (Papio cynocephalus). Behav Ecol Sociobiol 21:149–156
Sterck EHM, Watts DP, van Schaik CP (1997) The evolution of female social relationships in nonhuman primates. Behav Ecol Sociobiol 41:291–309
Sugiura H, Saito C, Sato S, Agetsuma N, Takahashi H, Tanaka T, Furuichi T, Takahata Y (2000) Variation in intergroup encounters in two populations of Japanese macaques. Int J Primatol 21:519–535
Sugiura H, Agetsuma N, Suzuki S (2002) Troop extinction and female fusion in wild Japanese macaques in Yakushima. Int J Primatol 23:69–84
Takahata Y, Suzuki S, Okayasu N, Sugiura H, Takahashi H, Yamagiwa J, Izawa K, Agetsuma N, Hill D, Saito C, Sato S, Tanaka T, Sprague D (1998a) Does troop size of wild Japanese macaques influence birth rate and infant mortality in the absence of predators? Primates 39:245–251
Takahata Y, Suzuki S, Agetsuma N, Okayasu N, Sugiura H, Takahashi H, Yamagiwa J, Izawa K, Furuichi T, Hill DA, Maruhashi T, Saito C, Sato S, Sprague DS (1998b) Reproduction of wild Japanese macaque females of Yakushima and Kinkazan islands: a preliminary report. Primates 39:339–349
Weir LK, Grant JW (2004) The causes of resource monopolization: interaction between resource dispersion and mode of competition. Ethology 110:63–74
Yamagiwa J, Hill D (1998) Intraspecific variation in the social organization of Japanese macaques: past and present scope of field studies in natural habitats. Primates 39:257–273
Acknowledgments
This study would not have been possible without help from the members of “Yakuzaru-Chosatai” (Yakushima Macaque Research Group) who voluntarily joined the censuses. We would like to thank our friends and colleagues in Yakushima for their hospitality and help. Dr. C. P. van Schaik, reviewers and the editor of this journal and the staff at the Primate Research Institute, Kyoto University (KUPRI), gave us helpful suggestions on the manuscript. The Yakushima Forest Environment Conservation Center gave us permission to conduct the research. The Sarugoya Committee and Field Research Center of KUPRI offered us excellent facilities. We are grateful to these people and organizations. This study was financed by the Cooperation Research Program of KUPRI, MEXT Grant-in-Aid for JSPS Fellows, 21st Century COE Program (A14) and Global COE Program “Formation of a Strategic Base for Biodiversity and Evolutionary Research: from Genome to Ecosystem”.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hanya, G., Matsubara, M., Hayaishi, S. et al. Food conditions, competitive regime, and female social relationships in Japanese macaques: within-population variation on Yakushima. Primates 49, 116–125 (2008). https://doi.org/10.1007/s10329-007-0073-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-007-0073-y