Abstract
Damping off of tomato caused by Athelia rolfsii causes serious losses in the Benin Republic despite the use of chemical fungicides. To reduce pesticide dependency and provide better control, we evaluated the activity of soil isolate GT4041 against A. rolfsii in Japan for use in Benin. Isolate GT4041, applied in its PC1 culture broth, strongly inhibited A. rolfsii mycelial growth in dual culture and completely inhibited sclerotial germination. In growth chamber tests, sclerotia were pretreated with GT4041 or GT4041 was preincubated in soil before application or applied directly on sclerotia on the soil; GT4041was always used in its culture broth. Its direct application on the soil reduced disease incidence the most in the growth chamber and in the greenhouse. GT4041 inhibited mycelial growth of five other tomato fungal pathogens and also increased tomato root and shoot dry mass. GT4041 produced proteases and indole acetic and it grew optimally at 28 °C. Scanning electron microscopy of GT4041 showed cylindrical, smooth spores in flexuous chains. This is the first report of S. sasae as a biocontrol agent for tomato damping off caused by A. rolfsii.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato is among the most appreciated vegetable fruits grown in the Benin Republic (James et al. 2010), where production nearly doubled from approximately 2.4 million tons in 2011 to approximately 5 million tons in 2020 (FAO 2020). In 2020, worldwide tomato production was estimated to be 186 million tons. Pests, especially fungal pathogens such as Athelia rolfsii, however, are a serious threat to tomato cultivation. First reported by Rolfs on tomato in 1892, A. rolfsii is a telluric, omnipathogenic, and facultative, soil-borne parasite of many crops and causes damping off and stem rot, and an economically important tomato pathogen in the Benin Republic (Aycock 1966; James et al. 2010). Sikirou et al. (2015) studied the geographical distribution and prevalence of the main tomato fungal pathogens in the Benin Republic and concluded that A. rolfsii is the most disseminated and devastating. A. rolfsii also infects many other agricultural crops such as peanut and cowpea and more than 500 species of horticultural crops in warm tropical and subtropical areas (Muthukumar and Venkatesh 2014; Punja 1985), where it thrives in rainy and humid environments (Adandonon et al. 2004; Bhuiyan et al. 2012; Mukherjee and Raghu 1997; Punja 1985). Fungicides and tillage, the main control strategies, have limited efficacy because the fungus has a wide host range, grows rapidly and produces a high number of sclerotia that remain viable in the soil for many years (Adandonon et al. 2004; Sennoi et al. 2013). In addition, the numerous fungicides that have been developed against A. rolfsii must be applied in large quantities (Punja 1985). Although chemical fungicides such as flutolanil, pentachloronitrobenzene (PCNB) prothioconazole, pyraclostrobin and tebuconazole have been effective in the control of A. rolfsii (Khatri et al. 2017; Scinos 1989; You et al. 2021), the fungus has developed resistance to PCNB and tebuconazole (Franke et al. 1998; Shim et al. 1998), and its sensitivity decreases to fungicides after frequent use (Franke et al. 1998; Le et al. 2012). Fungicides also have undesireable side effects; they can inhibit soil enzymatic activity, decrease microbial populations and change soil biological activity (Baćmaga et al. 2016, 2019; Ratna Kumar et al. 2015; Roman et al. 2021). As an alternative to fungicides, biological control agents (BCAs) such as Bacillus amyloliquefaciens, Bacillus halotolerans, Bacillus safensis, Bacillus subtilis, Bacillus velezensis, Ganoderma spp., Pseudomonas fluorescens, Trichoderma harzianum, and Trichoderma sp. inhibited the growth of A. rolfsii (Adandonon et al. 2006; Chen et al. 2019; Dutta et al. 2022; Farhaoui et al. 2022; Mukherjee and Raghu 1997; Osemwegie et al. 2010). However, Streptomyces species have not been tested (Sahu et al. 2019).
Streptomyces species are gram-positive, spore-producing actinobacteria and main bioactive compound producers among prokaryotic species, producing 39% of all microbial metabolites and 80% of the bioactive metabolites known to be synthesized by species belonging to the order Streptomycetales (Bérdy 2012). Streptomyces species also produce numerous antifungal compounds that suppress the growth of diverse fungal pathogens (Taechowisan et al. 2003). For instance, antibiotics and antimicrobial enzymes produced by cells of S. griseus or present in cell-free extracts of S. plumbeus suppress the growth of Fusarium oxysporum f. sp. cubense (Abdullah et al. 2021; Zacky and Ting 2013). S. blastmyceticus also inhibits powdery mildew caused by Podosphaera xanthii (Ganphung et al. 2019). In the Benin Republic, banana column juice was found to inhibit A. rolfsii in vitro and in tomato plants, but biocontrol agents against A. rolfsii on tomato plant have not yet been reported (Sikirou et al. 2010). Toward eventual use against tomato damping-off in the Benin Republic, candidate BCAs were isolated from soils in Gotsu City (Japan), tested for inhibitory activity, and the best candidate was further evaluated in vitro and on tomato seedlings in the growth chamber and greenhouse.
Materials and methods
Plant and pathogen
Seedlings of tomato cv. Reika were used for growth chamber and greenhouse experiments. Seeds were sown in nursery plastic trays (80 × 70 mm) containing commercial soil (Kumiai Nippi Engeibaido No. 1; Nihon Hiryo, Fujioka, Japan). Seedlings were transplanted to 7-cm-diameter vinyl pots and grown using standard cultural practices in a growth chamber (26 ± 2 °C) with 12 h of fluorescent light and sunlight from the chamber window. Seedlings were used 21 days later in the same pot for growth chamber experiments. For greenhouse experiments, 21-day-old seedlings were transplanted to 10-l plastic pots containing 8 l of Sakata Super Mix A soil (Sakata Seed, Yokohama, Japan) and 40 g of Magamp K fertilizer (HYPONeX JAPAN, Osaka, Japan). For all plant experiments, plants were watered according to standard practices.
Two strains of Athelia rolfsii were used in this study: MAFF103050 from the Japan NARO Genebank and SHIMANE002 isolated from an experimental field of Shimane University. Each strain was preserved on potato sucrose agar (PSA; 200 g/l potatoes, 2.0% w/v sucrose, 2.0% w/v agar) slants for further use. A mycelial disc (6 mm diameter) or one sclerotium was placed on the center of potato dextrose agar (PDA; 200 g/l potatoes, 2.0% w/v dextrose, 2.0% w/v agar) plates and maintained at 26 ± 2 °C with 12 h light/12 h dark for 7 days to generate mycelia and for 14 days to produce sclerotia.
Antagonist GT4041 isolation and culture
In a study by the plant pathology laboratory of Shimane University, 166 soil microbial isolates were collected from Gotsu City (Kawahira), Shimane Prefecture, Japan, isolated as described by Lemtukei et al. (2016), then screened for inhibtion of mycelial growth of A. rolfsii. Among 25 isolates that inhibited the fungus, strain GT4041 was the most inhibitory and tested further in the present study. It was stored in 15–20% v/v glycerol solution at −80 °C until used. From this stock, isolate GT4041 was grown on Luria-Bertani (LB) agar (0.5% w/v yeast extract, 1% w/v bactotryptone, 1% w/v NaCl, 2% w/v agar) in an incubator for 3 days at 28 °C. Individual colonies were transferred to separate culture tubes containing 5 ml of PC1 broth (1% w/v each of starch, polypeptone, molasses, and beef extract in 1000 ml deionized water at pH 7.2). The cultures were incubated at 26 ± 2 °C for 3 days with constant shaking at 180 rpm. For all experiments, the cultures had an OD600nm > 2 when used. Unless otherwise specified, these PC1 broth cultures were used in all experiments.
In vitro assays of antifungal activity of GT4041
GT4041 was tested for inhibition of mycelial growth and sclerotial germination of Athelia rolfsii. Each experiment was done three times, with five Petri plates per treatment. For tests of mycelial growth inhibition, a 6-mm-diameter mycelial plug of each strain of A. rolfsii was taken from the edge of 5-day-old PDA cultures and placed on soil agar (SA) (SA; 4.0% w/v soil, 2.0% w/v agar) at one edge of separate plates. A paper disc (8-mm diameter, Advantec, Tokyo, Japan) was placed 5 cm from the mycelial plug on the same plate, then 50 µl of GT4041 or 50 µl of PC1 broth (control) was dropped onto the paper disc. Plates were then incubated at 26 ± 2 °C in the dark for 10 days, and mycelial area of inhibition (mm2) was determined using LIA 32 software (http://www.agr.nagoya-u.ac.jp/~shinkan/LIA32/index-e.html). For tests of sclerotial inhibition, 10 sclerotia were incubated with 3 ml of GT4041 cultured in PC1 broth or in PC1 broth only (control) in tubes at 26 ± 2 °C for 24 h with constant shaking at 180 rpm. One sclerotium was then placed on the center of a PDA plate, then incubated and mycelial area measured as described for the mycelial assay.
Growth chamber and greenhouse tests of GT4041 disease suppression
Three types of applications of GT4041 were tested in growth chamber experiments; either sclerotia were preincubated with a GT4041 culture (treatment 1) or GT4041 cultures were mixed with soil (treatments 2 and 3). (1) Twenty sclerotia of each pathogen strain were incubated in GT4041 broth cultures for 24 h (10 sclerotia per 3 ml), then placed on the soil near the base of the stem of a 3-week-old tomato seedling and lightly covered with untreated soil. (2) GT4041 in its PC1 culture broth (5 ml) was mixed with 15 g of soil in small plastic pots, covered and incubated for 3 days in the dark before being applied over 20 sclerotia that had just been placed around the base of the tomato stem. (3) GT4041 in its PC1 culture broth (5 ml) was mixed with 15 g of soil in small plastic pots, then the mixture was immediately applied in the same manner as the second application. For each strain of A. rolfsii, 20 sclerotia were incubated in PC1 broth for 24 h, then placed on the soil near the basal stem of a 3-week-old tomato seedling and covered with previously mixed soil. As a positive control, incubated sclerotia were placed on the soil near the tomato stem and covered with untreated soil. Two weeks after inoculation with A. rolfsii, the percentage of plants that developed damping off was calculated as disease incidence (DI): (Number of diseased plants / Total number of Plants) × 100. Five plants were used per treatment per fungal strain, and the completely randomized experiment was done three times.
In experiments in a vinyl greenhouse (< 27 ± 5 °C) from June to September 2021 at Shimane University, 20 sclerotia were placed on soil near the plant stems as in the growth chamber experiments, 10 ml of GT4041 was either mixed with 10 g of soil or not and directly applied on the sclerotia. After application of GT4041-only, it was lightly covered by untreated soil. Five pots of plants were used per treatment, and the completely randomized experiment was done three times. After 9 weeks, DI was determined as described in the previous paragraph.
In vitro activity of GT4041 against other tomato fungal pathogens
GT4041 was also tested in dual culture, as described above, with a mycelial plug from a 14-day-old PDA culture of six other tomato fungal pathogens: Alternaria alternata, Botrytis cinerea, Stemphylium lycopersici, Fusarium oxysporum f. sp. lycopersici, and Sclerotinia sclerotiorum. The area of mycelial inhibition was determined after 3 weeks.
Growth chamber tests of tomato growth promotion by GT4041
GT4041 (3 ml; or PC1 broth as the control) was applied on the soil around the base of 3-week-old tomato seedlings. Five plants were used per treatment, and the experiment was done three times. After 3 weeks, the shoot and the roots of each plant were separated, oven-dried at 70 °C until constant mass, and weighed separately.
Identification of GT4041
DNA was extracted from a GT4041 colony as described by Suzuki et al. (2006), and the 16 S rDNA region was amplified by PCR using the primers described by Huong et al. (2007), Matsui et al. (2009) and Nakagawa et al. (2001) in Table S1. PCR amplification, DNA sequencing, and sequence homology search using BLAST were done as described by Abdullah et al. (2021). A phylogenetic tree was generated using CLUSTAL W for multiple sequence alignments (Thompson et al. 1994). Genetic distances between the aligned DNA were determined using Kimura’s two-parameter model (Kimura 1980) and the neighbor-joining method (Saitou and Nei 1987) in MEGA X (https://www.megasoftware.net/). The pathogen Actinoalloteichus cyanogriseus NBRC14455 was used as the outgroup.
Scanning electron microscopy
GT4041 was grown on LB agar plates for 5 days, then 3–4 pieces (8 mm diameter) of the colony were cut from the colony and placed in a 2 ml microtube, 1 ml of 6.25% (v/v) glutaraldehyde in 0.2 M phosphate buffer was added, and the tube held at 4 °C for 24 h. The glutaraldehyde was then removed with a pipette, and samples were washed three times for 10 min each with 2 ml of 0.2 M phosphate buffer. Samples were then dehydrated with an ethanol series (2 times × 10 min for each step): 50%, 70%, 90%, 100%. The ethanol was then removed, 2 ml of tert-butyl-alcohol was added (2 times), and then the sample was freeze-dried in a tert-butyl-alcohol freeze dryer (VDF-21 S: Vacuum Device, Mito, Japan). The freeze-dried sample was coated with platinum using ion sputtering (E-101, Hitachi, Tokyo, Japan). Mycelia and spores were observed for morphology using a Hitachi S-4800 field-emission SEM at 5 kV (Hitachi High-Tech, Tokyo, Japan).
Production of protease, indole-3-acetic acid (IAA), and optimal growth temperature for GT4041
Protease production was tested on skim milk agar (skim milk powder 5 g; agar 15 g; 1 l water); an 8-mm-diameter paper disc (Advantec, Tokyo, Japan) was placed in the center of the plate and inoculated with 50 µl of GT4041; for the control, 50 µl of PC1 broth was added to the disc. The clear area (mm2) on the agar was measured after 7 days using LIA 32 software (http://www.agr.nagoya-u.ac.jp/~shinkan/LIA32/index-e.html). For the test for IAA production using colorimetry and Salkowski’s reagent (Gordon and Weber 1951), GT4041 was grown in nutrient broth (peptone, 5 g; yeast extract, 1.5 g; beef extract, 1.5 g; and NaCl, 5 g; per liter water) with 0.1% l-tryptophan (w/v), which is a known precursor for IAA synthesis by Streptomyces sp (Datta and Basu 2000). The tubes were maintained at 26 ± 2 °C for 4 days with constant shaking at 180 rpm. One milliliter of supernatant was added to 2 ml of Salkowski’s reagent and incubated for 30 min at ambient temperature. Absorption was measured at 530 nm using a spectrometer (UV mini-1240: Photometric Mode, Shimandzu co., Kyoto, Japan). A 3-indoleacetic acid standard (Wako Chemical Pure Industries Ltd, Osaka, Japan) was used at 5, 10, 15, 20, 25, and 30 µg/ml to calculate the amount of IAA produced by GT4041 using a regression line (Sarker and Al-Rashid 2013). GT4041 was grown at 4, 20, 28, and 38 °C to test for optimal growth using the method of Haidary et al. (2018), then the colonies on each plate were counted after 7 days.
Statistical analyses
Significant differences (P < 0.05) among means for treatment groups in each set of experiment were calculated using Tukey-Kramer’s test or a t test in SPSS ver. 22.0 for Windows (IBM, Armonk, NY, USA).
Results
In vitro activity of GT4041 against A. rolfsii
In dual culture, GT4041 significantly inhibited mycelial growth of A. rolfsii with an inhibition zone of 2162.6 ± 622.6 mm2 (mean ± SD) for strain MAFF103055 and 2910.2 ± 352.4 mm2 for strain SHIMANE002 compared to 0 mm2 for the control (Fig. 1). GT4041 also completely inhibited germination of the sclerotia of both strains compared to the control (Fig. 2).
GT4041 disease suppression in growth chamber and greenhouse
In the growth chamber tests of the first application type, sclerotia were incubated with GT4041 in its PC1 culture broth before being placed on soil near the plant stems. The mean disease incidence (DI) ± SD caused by strain MAFF103055 was 0% and 23.3 ± 25.2% for SHIMANE002 compared to 100% for the broth-treated controls (Fig. 3A). The pretreatment of the sclerotia thus inhibited disease by pathogenic strain MAFF103055 but not for strain SHIMANE002. In the second application type, GT4041 in its culture broth was mixed with soil, held in the dark for 3 days, then applied on the sclerotia near plant stems. In this case, control of the two strains was not as effective as the pretreatment with the antagonist; DI was 46.7 ± 11.5% MAFF103055 and 53.3 ± 11.5% for SHIMANE002 compared to 100% for the controls (Fig. 3B). In the third application, soil mixed with GT4041 in its culture broth was placed on sclerotia near the stems. DI was 13.3 ± 11.5% with strain MAFF103055 and 26.6 ± 11.5% with SHIMANE002 compared to 100% for the controls (Fig. 3C). The third treatment, in which the soil was mixed with GT4041 in its culture broth and immediately applied, DI was significantly reduced more than in the second treatment, which was applied after a 3-day incubation of the antagonist in the soil.
Disease incidence (DI, % of diseased plants) caused by Athelia rolfsii (strain MAFF103055 and SHIMANE002) on tomato seedlings (cv. Reika) after different types of GT4041 application and 2 weeks of incubation in a growth chamber. In A, 20 sclerotia for each strain of A. rolfsii was incubated with GT4041 in PC1 broth for 24 h with constant shaking at 180 rpm, then placed on the soil near the base of 3-week-old seedlings and lightly covered with untreated soil. In B, 5 ml of GT4041 in its PC1 culture broth was mixed with 15 g of soil, held in the dark for 3 days, then applied directly on 20 sclerotia on soil near the stem. In C, 5 ml of GT4041 in its PC1 culture broth was mixed with 15 g of soil in small plastic pots, then immediately applied on 20 sclerotia on soil near of the stem. Means and standard deviations are shown; an asterisk indicates a significant difference compared to the control (Student’s t test, P < 0.05)
In the greenhouse experiment, GT4041 in its culture broth was directly applied to sclerotia on soil near plant stems, DI was 6.7 ± 11.5% with MAFF103055 and 0% with SHIMANE002 compared to 100% for the controls (Fig. 4). However, when GT4041 in its culture broth was first mixed with soil, then applied on the sclerotia, DI was 60.0 ± 20.0% with MAFF103055 and 6.7 ± 11.5% with SHIMANE002 compared to 100% for the controls (Fig. 4). Thus, direct application of GT4041 in its culture broth provided better control than when mixed with soil.
Disease incidence (DI, % of diseased plants) caused by Athelia rolfsii (strain MAFF103055 and SHIMANE002) on tomato seedlings (cv. Reika) after different types of GT4041 application and 2 weeks of incubation in a greenhouse. GT4041 in its PC1 culture broth was mixed with soil (10 ml of GT4041 in 10 g of soil) or not (10 ml of “GT4041 only”), then directly applied on sclerotia on soil near of the stems. “GT4041 only” application was lightly covered with soil. Means and standard deviations are shown. Different letters above the means for a strain of A. rolfsii indicates a significant difference compared to the control (Tukey-Kramer’s test, P < 0.05). The plants in (B) are results from the experiment in (A)
In vitro inhibition of other tomato fungal pathogens by GT4041
In dual culture tests, GT4041 inhibited mycelial growth of five of the six tomato pathogens: Alternaria alternata, Botrytis cinerea, Fusarium oxysporum f. sp. lycopersici, Stemphylium lycopersici and Sclerotinia sclerotiorum. The respective mean (± SD) inhibition areas were 438.6 ± 242.7 mm2, 1368.4 ± 333.3 mm2, 165.1 ± 216.1 mm2, 578.4 ± 351.6 mm2, and 1685.7 ± 596.9 mm2 (Fig. 5).
Tomato growth promotion by GT4041 in a growth chamber
By 3 weeks after GT4041 was applied to the soil, the mean (± SD) dry mass of tomato shoots and roots was 0.32 ± 0.06 g and 0.11 ± 0.05 g, respectively, compared to the control mass of 0.12 ± 0.04 g for shoots and 0.03 ± 0.04 g for roots (Fig. 6).
Identification of GT4041
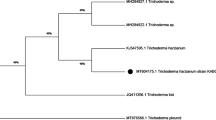
Phylogenetic analysis of the 16 S rDNA indicated 99.0% similarity between isolate GT4041 and Streptomyces sasae NBRC109809 (Fig. 7).
Scanning electron microscopy of GT4041
GT4041 had flexuous chains of cylindrical, smooth spores (Fig. 8).
GT4041 production of protease and IAA, optimal growth temperature
GT4041 was positive for protease and IAA production. The clear zone area indicating the presence of protease was 1226.6 ± 153.7 mm2 (mean ± SD) compared to 0 mm2 for the control (Fig. 9), and 42.3 ± 4.1 µg/ml of IAA (mean ± SD) was measured compared to 0 µg/ml for the control (data not shown). The mean (± SD) number of colonies at 20 °C, 28 °C, and 38 °C differed significantly (268.2 ± 41.3, 297.2 ± 73.2, and 244.1 ± 64.7, respectively) compared to 4 °C (Fig. 10). Growth was optimal at 28 °C.
Discussion
Isolate GT4041 was identified as S. sasae (Fig. 7), which was first reported 7 years ago in South Korea, and its smooth, cylindrical spores in flexuous chains (Fig. 8) and optimal growth temperature agree with previous studies (Lee and Whang 2015; Srivastava et al. 2021). GT4041 can thus be more effective in warm ecosystems. GT4041 in its PC1 culture broth inhibited in vitro mycelial growth of both A. rolfsii strains by (Fig. 1), indicating that it produced antifungal compounds. These results are similar with those of other studies in which A. rolfsii mycelia were inhibited by Pseudomonas cf. monteilii strain 9 and Bacillus subtilis strain MZ488846 (Farhaoui et al. 2022; Rakh et al. 2011), and the inhibition of the mycelium on soil agar in our dual culture assay confirmed the efficacy of GT4041 on soil. Additionally, the total inhibition of sclerotial germination after the 24-h incubation with GT4041 in its culture broth (Fig. 2), confirmed the strong antifungal properties of GT4041. GT4041 also produced protease, which can degrade cell walls to contribute to its antifungal activity. These results are similar to those for the biological control properties of Trichoderma harzianum, which produces protease and inhibits hydrolytic enzymes of the pathogen Botrytis cinerea (Elad and Kapat 1999). In our growth chamber experiment, in contrast to the total inhibition of sclerotial germination in vitro, strain SHIMANE002 infected tomato in the first treatment (Fig. 3A), which could be related to soil factors such as moisture and temperature, which might enhance sclerotial germination despite the antagonistic effect of GT4041, similar to the combined effect of moisture and temperature on Sclerotium cepivorum sclerotia found by Crowe and Hall (1980). Immediate application of soil mixed with GT4041 (Fig. 3C) was more efficient than applying the same mixture after 3 days (Fig. 3B). In addition, in the greenhouse, application of GT4041 in its culture broth provided better disease control efficient than mixing GT4041 with soil (Fig. 4). These results could indicate that the concentration of antifungal compounds was higher when GT4041 was applied directly in its culture broth. Applying GT4041 before and after soils are inoculated with sclerotia should help us understand whether the biocontrol mechanism involves priming of resistance-related genes. Based on the experiments with tomato plants, we recommend direct application of the GT4041 culture on plant stems for the best efficacy.
Additionally, GT4041 also inhibited the mycelial growth of five other fungal pathogens (Fig. 5), including soil- and airborne fungi. These results are consistent with those of other studies using S. sasae. For example, S. sasae strain St-3 strongly inhibits mycelial growth of Fusarium oxysporum f. sp. lactucae (KACC 42795) and Pythium ultimum (KACC 40705) on plates and on radish and pepper plants. (Santhanarajan et al. 2021) and S. sasae strain TG01 inhibits growth of Fusarium solani (InaCCF76) and F. oxysporium (InaCCF78) (Sudiana et al. 2020). An analysis of the secondary metabolites produced by S. sasae TG01 revealed the presence of a bioactive compound, 2-methyl-1,3-dioxolane (Sudiana et al. 2020). Perhaps this compound is also responsible for the antifungal property of GT4041 because it also inhibits the growth of Candida albicans ATCC 10231 a fungi (yeast) and gram-positive and gram-negative bacteria such as Enterobacter faecalis ATCC 29212, Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 4352, Proteus mirabilis ATCC 14153, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 29213 and Staphylococcus epidermidis ATCC 12228 (Küçük et al. 2011). The acetyl acetate extract of the culture of another Streptomyces sp. UK-201, which shares 96.88% similarity with S. sasae (JR-39, Hq267987), inhibited diverse microorganisms such as Bacillus subtilis, Micrococcus luteus, Staphylococcus epidermis, Brevibacterium linens, Escherichia coli, Pseudomonas fluorescens and Saccharomyces cerevisiae. GC-MS analysis of the secondary metabolites produced by Streptomyces sp. UK-201 showed a diverse range of compounds such as undecane, 2-propyl-1-pentanol, tetra-butylbenzene, 1,2,4,5-tetramethyl benzene, (E)-3-tetradecane, 2-methyl-1,3-dioxolane (Srivastava et al. 2021). We will also analyze the secondary metabolites produced by GT4041 to determine whether they contribute to fungal inhibition and disease control.
The growth promotion of tomato by isolate GT4041 (Fig. 6) can be explained by its production of IAA. In addition to synthesizing IAA, Streptomyces sp. can also synthesize gibberellic acid and zeatin (Solans et al. 2011); however, IAA production by S. sasae has not yet been reported. IAA is known to stimulate plant growth through the production of long roots with more root hairs and lateral roots, which enhances nutrient uptake (Datta and Basu 2000). IAA can be synthesized through the precursor tryptophan (Trp) via the Trp-dependent IAA biosynthetic pathway (Mohite 2013; Lee and Whang 2016). In the presence of Trp, S. fradiae NKZ-259 produces a maximum IAA yield of up to 82.363 µg/ml (Myo et al. 2019), which is two times higher than our result (42.3 ± 4.1 µg/ml). Here, we only confirmed that IAA was produced on medium amended with Trp, but for a better understanding of requirements for IAA production by S. sasae, further studies with and without Trp are needed.
In conclusion, the selected soil isolate S. sasae strain GT4041, which is not pathogenic to tomato plants, was inhibitory to A. rolfsii (in vitro and in planta) and stem rot and damping of tomato while also promoting tomato growth promotion. It thus has promise as a good biological control agent and source for the development of a new microbial fungicide.
References
Abdullah ZK, Kihara J, Gondo Y, Ganphung R, Yokoyama Y, Ueno M (2021) Suppressive effect of secondary metabolites from Streptomyces plumbeus isolate F31D against Fusarium oxysporum f. sp. lycopercisi, the causal agent of Fusarium wilt of tomato. J Gen Plant Pathol 87:335–343. https://doi.org/10.1007/s10327-021-01020-x
Adandonon A, Aveling TAS, Tamo M (2004) Occurrence and distribution of cowpea damping-off and stem rot and associated fungi in Benin. J Agric Sci 142:561–566. https://doi.org/10.1017/S0021859604004629
Adandonon A, Aveling TAS, Labuschagne N, Tamo M (2006) Biocontrol agents in combination with Moringa oleifera extract for integrated control of Sclerotium-caused cowpea damping-off and stem rot. Eur J Plant Pathol 115:409–418. https://doi.org/10.1007/s10658-006-9031-6
Aycock R (1966) Stem rot and other diseases caused by Sclerotium rolfsii. North Carolina Agric Exp Stn Tech Bull 174
Baćmaga M, Wyszkowska J, Kucharski J (2016) The effect of the Falcon 460 EC fungicide on soil microbial communities, enzyme activities and plant growth. Ecotoxicology 25:1575–1587. https://doi.org/10.1007/s10646-016-1713-z
Baćmaga M, Wyszkowska J, Kucharski J (2019) The biochemical activity of soil contaminated with fungicides. J Environ Sci Health B 54:252–262. https://doi.org/10.1080/03601234.2018.1553908
Bérdy J (2012) Thoughts and facts about antibiotics: where we are now and where we are heading. J Antibiot 65:385–395. https://doi.org/10.1038/ja.2012.27
Bhuiyan MAH, Rahman MT, Bhuiyan KA (2012) In vitro screening of fungicides and antagonists against Sclerotium rolfsii. Afr J Biotechnol 11:14822–14827. https://doi.org/10.5897/AJB12.1693
Chen L, Wu YD, Chong XY, Xin QH, Wang DX, Bian K (2019) Seed-borne endophytic Bacillus velezensis LHSB1 mediate the biocontrol of peanut stem rot caused by Sclerotium rolfsii. J Appl Microbiol 128:803–813. https://doi.org/10.1111/jam.14508
Crowe FJ, Hall DH (1980) Soil temperature and moisture effects on sclerotium germination and infection of onion seedlings by Sclerotium cepivorum. Phytopathology 70:74–78. https://doi.org/10.1094/Phyto-70-74
Datta C, Basu PS (2000) Indole acetic acid production by a Rhizobium species from root nodules of a leguminous shrub, Cajanus cajan. Microbiol Res 155:123–127. https://doi.org/10.1016/S0944-5013(00)80047-6
Dutta P, Kaman PK, Kumari A, Saikia B, Deb L (2022) Management of Sclerotium rolfsii causing basal rot of Piper longum through organic approaches. Indian Phytopathol 75:267–271. https://doi.org/10.1007/s42360-021-00428-x
Elad Y, Kapat A (1999) The role of Trichoderma harzianum protease in the biocontrol of Botrytis cinerea. Eur J Plant Pathol 105:177–189. https://doi.org/10.1023/A:1008753629207
FAO (2020) Crop and livestock products. FAOSTAT. Food and Agriculture Organization of the United Nations. http://www.fao.org/faostat/en/#data/QCL
Farhaoui A, Adadi A, Tahiri A, Alami NE, Khayi S, Mentag R, Ezrari S, Radouane N, Mokrini F, Belabess Z, Lahlali R (2022) Biocontrol potential of plant growth-promoting rhizobacteria (PGPR) against Sclerotiorum rolfsii diseases on sugar beet (Beta vulgaris L). Physiol Mol Plant Pathol 119:101829. https://doi.org/10.1016/j.pmpp.2022.101829
Franke MD, Brenneman TB, Stevenson KL, Padgett GB (1998) Sensitivity of isolates of Sclerotium rolfsii from peanut in Georgia to selected fungicides. Plant Dis 82:578–583. https://doi.org/10.1094/PDIS.1998.82.5.578
Ganphung R, Kihara J, Ueno M (2019) Biological control of powdery mildew caused by Podosphaera xanthii in cucumber by Streptomyces blastmyceticus strain STS1 isolated in Shimane Prefecture. J JSATM 26:61–68
Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26:192–195. https://doi.org/10.1104/pp.26.1.192
Haidary M, Tamura T, Ueno M (2018) Inhibitory activity of Paenibacillus sp. isolated from soil in Gotsu city, Shimane Prefecture, against Xanthomas oryzae pv. Oryzae, the causal agent of rice bacterial leaf blight. J Adv Microbiol 8:197–210. https://doi.org/10.4236/aim.2018.83014
Huong NL, Itoh K, Suyama K (2007) Diversity of 2,4-diclorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetiacid (2,4,5-T)-degrading bacteria in vietnamese soils. Microb Environ 22:243–256. https://doi.org/10.1264/jsme2.22.243
James B, Atcha-Ahowé C, Godonou I, Baimey H, Goergen G, Sikirou R, Toko M (2010) Integrated pest management in vegetable production: a guide for extension workers in West Africa. IITA, Ibadan. https://hdl.handle.net/10568/63650
Khatri K, Kunwar S, Barocco RL, Dufault NS (2017) Monitoring fungicide sensitivity levels and mycelial compatibility groupings of Sclerotium rolfsii isolates from Florida peanut fields. Peanut Sci 44:83–92. https://doi.org/10.3146/PS17-7.1
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. https://doi.org/10.1007/BF01731581
Küçük HB, Yusufoglu A, Mataracı E, Dösler S (2011) Synthesis and biological activity of new 1,3-dioxolanes as potential antibacterial and antifungal compounds. Molecules 16:6806–6815. https://doi.org/10.3390/molecules16086806
Le CN, Mendes R, Kruijt M, Raaijmakers JM (2012) Genetic and phenotypic diversity of Sclerotium rolfsii in groundnut fields in central Vietnam. Plant Dis 96:389–397. https://doi.org/10.1094/PDIS-06-11-0468
Lee H, Whang K (2015) Streptomyces sasae sp. nov., isolated from bamboo (Sasa borealis) rhizosphere soil. Int J Syst Evol Microbiol 65:3547–3551. https://doi.org/10.1099/ijsem.0.000454
Lee J, Whang K (2016) Optimization of indole-3-acetic acid (IAA) production by Bacillus megaterium BM5. Korean J Soil Sci 49:461–468. https://doi.org/10.7745/KJSSF.2016.49.5.461
Lemtukei D, Tamura T, Nguyen TQ, Kihara J, Ueno M (2016) Antagonistic potential of isolated microorganisms from soil in Shimane Prefecture against rice blast disease caused by Magnaporthe oryzae. Bull Fac Life Environ Sci Shimane Univ 21:9–12
Matsui T, Kato K, Namihira T, Shinzato N, Semba H (2009) Stereospecific degradation of phenylsuccinate by actinomycetes. Chemosphere 76:1278–1282. https://doi.org/10.1016/j.chemosphere.2009.06.021
Mohite B (2013) Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J Soil Sci Plant Nutr 13:638–649. https://doi.org/10.4067/S0718-95162013005000051
Mukherjee PK, Raghu K (1997) Trichoderma sp. as a microbial suppressive agent of Sclerotium rolfsii on vegetables. World J Microbiol Biotechnol 13:497–499. https://doi.org/10.1023/A:1018501006122
Muthukumar A, Venkatesh A (2014) Biological inductions of systemic resistance to collar rot of peppermint caused by Sclerotium rolfsii. Acta Physiol Plant 36:1421–1431. https://doi.org/10.1007/s11738-014-1520-1
Myo EM, Ge B, Ma J, Cui H, Liu B, Shi L, Jiang M, Zhang K (2019) Indole-3-acetic acid production by Streptomyces fradiae NKZ-259 and its formulation to enhance plant growth. BMC Microbiol 19:155. https://doi.org/10.1186/s12866-019-1528-1
Nakagawa Y, Tamura T, Kawasaki H (2001) Genetic analysis method (in japanese). (ed) Identification manual of actinomycetes. Business Center for Academic Societies Japan, Tokyo, Japan, pp 249–257. The Society for Actinomycetes Japan
Osemwegie OO, Oghenekaro AO, Owolo LO (2010) Effects of pulverized Ganoderma spp., on Sclerotium rolfsii Sacc and post-harvest tomato (Lycopersicon esculentum Mill.) Fruits preservation. J Appl Sci Res 6:1794–1800
Punja ZK (1985) The biology, ecology, and control of Sclerotium rolfsii. Annu Rev Phytopathol 23:97–127. https://doi.org/10.1146/annurev.py.23.090185.000525
Rakh RR, Raut LS, Dalvi SM, Manwar AV (2011) Biological control of Sclerotium rolfsii, causing stem rot of groundnut by Pseudomonas cf monteilii . Recent Res Sci Technol 3:26–34. https://doi.org/10.25081/rrst.2017.9.3355
Ratna Kumar PK, Shiny Niharika P, Hemanth G (2015) Impact of fungicides on the growth and distribution of soil mycoflora in agriculture fields at Narasannapeta. Int J Sci Res 6:2337–2347. https://doi.org/10.21275/ART20164650
Roman DL, Voiculescu DI, Filip M, Ostafe V, Isvoran A (2021) Effects of triazole fungicides on soil microbiota and on the activities of enzymes found in soil: a review. Agriculture 11:893. https://doi.org/10.3390/agriculture11090893
Sahu KP, Singha S, Gupta A, Singha UB, Brahmaprakash GP, Saxena AK (2019) Antagonistic potential of bacterial endophytes and induction of systemic resistance against collar rot pathogen sclerotium rolfsii in tomato. Biol Control 137:104014. https://doi.org/10.1016/j.biocontrol.2019.104014
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Santhanarajan A, Han Y, Koh S (2021) The efficacy of functional compost manufactured using spent coffee ground, rice bran, biochar, and functional microorganisms. Appl Sci 11:7703. https://doi.org/10.3390/app11167703
Sarker A, Al-Rashid J (2013) Analytical protocol for determination of indole 3 acetic acid (IAA) production by plant growth promoting bacteria (PGPB). In: Technical report of quantification of IAA by microbes, pp 1–2. https://www.researchgate.net/publication/263818523
Scinos AS (1989) Targeting fungicides for control of southern stem rot on peanut. Plant Dis 73:723–726. https://doi.org/10.1094/PD-73-0723
Sennoi S, Jogloy S, Saksirirat W, Kesmala T, Patanothai A (2013) Genotypic variation of resistance to southern stem rot of Jerusalem artichoke caused by Sclerotium rolfsii. Euphytica 190:415–424. https://doi.org/10.1007/s10681-012-0813-y
Shim MY, Starr JL, Keller NP, Woodard KE, Lee TAJr (1998) Distribution of isolates of Sclerotium rolfsii tolerant to pentachloronitrobenzene in Texas peanut fields. Plant Dis 82:103–106. https://doi.org/10.1094/PDIS.1998.82.1.103
Sikirou R, Zannou A, Gbèhounou G, Tosso F, Komlan FA (2010) Fungicide effect of banana column juice on tomato southern blight caused by Sclerotium rolfsii: Technical and economic efficiency. Afr J Agric Res 5:3230–3238. https://doi.org/10.5897/AJAR10.513
Sikirou R, Ezin V, Beed F, Etchiha afoha SAP, Tosso FD, Ouessou Idrissou F (2015) Geographical distribution and prevalence of the main tomato fungal wilt diseases in Benin. Int J Biol Chem Sci 9:603–613. https://doi.org/10.4314/ijbcs.v9i2.3
Solans M, Vobis G, Cassan F (2011) Production of phytohormones by root-associated saprophytic actinomycetes isolated from the actinorhizal plant Ochetophila trinervis. World J Microbiol Biotechnol 27:2195–2202. https://doi.org/10.1007/s11274-011-0685-7
Srivastava N, Gupta S, Sarethy IP (2021) Characterization of Streptomyces sp. UK-201 from Lachhiwala Reserve Forest, a biodiversity hot spot of the Himalayas. Nat Prod J 11:207–220. https://doi.org/10.2174/2210315509666191113152549
Sudiana IM, Putri A, Napitupulu TP, Idris, Purnaningsih I, Kanti A (2020) Growth inhibition of Fusarium solani and F oxysporum by Streptomyces sasae TG01, and its ability to solubilize insoluble phosphate. Biodivers J Biol Divers 21:429–435. https://doi.org/10.13057/biodiv/d210201
Suzuki S, Taketani H, Kusumoto K, Kashiwagi Y (2006) High-throughput genotyping of filamentous fungus aspergillus oryzae based on colony direct polymerase chain reaction. J Biosci Bioeng 102:572–574. https://doi.org/10.1263/jbb.102.572
Taechowisan T, Peberdy JF, Lumyong S (2003) Isolation of endophytic actinomycetes from selected plants and their antifungal activity. World J Microbiol Biotechnol 19:381–385. https://doi.org/10.1023/A:1023901107182
Thompson J, Higgins D, Gibson T (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties, and weight matrix choice. Nucleic Acid Res 22:4673–4680. https://doi.org/10.1093/nar/22.22.4673
You J, Tang T, Wang F, Mao T, Yuan B, Guo J, Guo X, Duan Y, Huang J (2021) Baseline sensitivity and control efficacy of strobilurin fungicide pyraclostrobin against Sclerotium rolfsii. Plant Dis 105:3503–3509. https://doi.org/10.1094/PDIS-01-21-0176-RE
Zacky FA, Ting ASY (2013) Investigating the bioactivity of cells and cell-free extracts of Streptomyces griseus towards Fusarium oxysporum f. sp. cubense race 4. Biol Control 66:204–208. https://doi.org/10.1016/j.biocontrol.2013.06.001
Acknowledgements
The authors thank the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) and the Faculty of Life and Environmental Sciences of Shimane University for their financial support in the publication of this report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The nucleotide sequence presented in this paper is available in the DDBJ/EMBL/GenBank database under accession number LC720967.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adam Dade, G.M.A., Kihara, J. & Ueno, M. Control of tomato southern blight caused by Athelia rolfsii (syn. Sclerotium rolfsii) using the soil isolate Streptomyces sasae strain GT4041. J Gen Plant Pathol 89, 159–169 (2023). https://doi.org/10.1007/s10327-023-01122-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-023-01122-8