Abstract
The efficacy of eight fungal and eight bacterial isolates was tested for their ability to inhibit the growth of Sclerotium rolfsii, the causal agent of collar rot of peppermint. In vitro studies revealed that Trichoderma harzianum (THA) and Pseudomonas fluorescens (PFM) showed the highest inhibition of mycelial growth (68.28; 74.25 %) of S. rolfsii. The antagonists T. harzianum and P. fluorescens were compatible with each other and they were tested alone and together in in vivo for the control of S. rolfsii. Besides, the induction of defense-related enzymes such as peroxidase, polyphenoloxidase, phenylalanine ammonia-lyase, and the accumulation of phenolics in peppermint plants due to the application of bioagents were also studied. Combined application of talc-based formulation of bioagents and challenge inoculation with S. rolfsii recorded maximum induction of defense-related enzymes, and accumulation of phenolics as compared with individual application. This study suggests that the increased induction of defense-related enzymes (two- to threefold) and phenolic content (threefold) due to the combination treatment of bioagents might be involved in the reduction of collar rot incidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peppermint (Mentha piperita L.) is an important aromatic perennial herb grown throughout the world, belonging to the family Lamiaceae. It is extensively cultivated in India and about 70 % of the international annual requirement is met from crops raised in the central region of the Indo-Gangetic plains (Anonymous 2004). Mentha is cultivated in Himalayan hills, Haryana, Uttar Pradesh, Punjab and Bihar. Of these, Uttar Pradesh is the largest producing state in the country contributing 80–90 % of the total production followed by Punjab, Haryana, Bihar and Himachal Pradesh. In India, peppermint is grown throughout the year (Shukla et al. 1998) and it is affected by several fungal diseases. Of these, collar rot caused by Sclerotium rolfsii is a major constraint in the peppermint cultivation in Tamil Nadu. It is a soil-borne plant pathogen which causes considerable damage to the crop and the disease intensity in the field ranged from 5 to 20 % (Anand and Bahadur 2004). The management of S. rolfsii, the causal agent of collar rot was a difficult task; attempts were made to manage the collar rot by using different fungicides. But the indiscriminate use of fungicides resulted in the accumulation of residual toxicity in soil, environmental pollution and altered the biological balance in the soil by decimating the non-target and beneficial microorganisms. Development of fungicides’ resistance in the pathogen has also been reported (Bharathi et al. 2004).
Recent efforts have focused on developing environmentally safe, long-lasting and effective biocontrol agents for the management of collar rot disease. Trichoderma species and plant growth promoting rhizobacteria, especially Pseudomonas fluorescens (Trevisan) Migula., have been developed commercially as a talc-based formulation and tested against several crop diseases (Niranjana et al. 2009). P. fluorescens mixed with other strains of fungi/bacteria increased the biocontrol efficacy (Duffy et al. 1996). Studies on fungal and bacterial biocontrol agents against peppermint collar rot have been attempted earlier under both greenhouse and field conditions (Kamalakannan et al. 2003; Anand and Bahadur 2004). However, no attempts have been made for the management of collar rot of peppermint by using combination of fungal and bacterial antagonist and to understand the mechanism of disease resistance induced by combination of biocontrol agents. The investigation of mechanisms of biological control by fungal and bacterial antagonists also revealed that biocontrol agents, which protect the plants from various pathogens in several crops, activate the defense-related enzymes including phenylalanine ammonia-lyase (PAL), peroxidase (PO), etc., which are involved in synthesis of phytoalexins (Van Peer and Schippers 1992). Besides, early and increased expression of defense-related genes in induced systemic resistance (ISR) is very important in protecting the crops against several pathogens (Xue et al. 1998). For the induction of ISR, strains of bacterial antagonists Pseudomonas spp., and fungal antagonists Trichoderma spp., appear to be promising. For example, increase in accumulation of phenolic compounds by application of bioagents against tea blister blight and damping-off of tomato and chilli (Saravanakumar et al. 2007; Ramamoorthy et al. 2002); increase in activities of PO in bioagent-treated cucumber seedlings against P. aphanidermatum (Yedidia et al. 1999); increase of PO and polyphenoloxidase (PPO) activities in cucumber roots treated with P. corrugate against P. aphanidermatum (Chen et al. 2000), and accumulation of phenolics and increase in PAL in groundnut plants treated with P. fluorescens against Cercospora personata (Meena et al. 1999) were observed by different researchers. ISR against different pathogens by microbial strains has also been achieved in tobacco (Maurhofer et al. 1994), cucumber (Liu et al. 1995), radish (Leeman et al. 1995), sugarcane (Viswanathan and Samiyappan 1999) and chilli (Muthukumar et al. 2011). However, in the case of biological control of soil-borne diseases, knowledge of ISR for the management of collar rot of peppermint disease is lacking. The objectives of the present study are: (1) to evaluate the antagonistic activity of fungal and bacterial isolates against S. rolfsii in in vitro, (2) to test the compatibility between effective fungal and bacterial antagonist, and (3) to study the potential for induction of systemic resistance in peppermint by combination of effective fungal and bacterial isolate.
Materials and methods
Peppermint cuttings were obtained from farmer’s field at Alangudi, Chidambaram, Tamil Nadu, India, and used for the greenhouse experiments in the entire period of investigation.
Isolation, maintenance and identification of pathogen
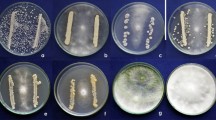
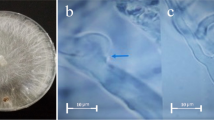
Diseased peppermint plants with collar rot symptoms (collar region) were collected from the field. They were washed, cut into 5 mm segments including the advancing margins of infection. The segments were surface-sterilized in 0.5 % sodium hypochlorite solution for 5 min and rinsed in three changes of sterile distilled water. The segments were separately dried in between sheets of sterile filter paper and placed (three pieces per plate) on fresh potato dextrose agar (PDA) medium (Ainsworth 1961) impregnated with streptomycin, and incubated for 7 days at 28 ± 2 °C. The fungal growth on the 5th day, which arose through the sclerotial bodies was cut by inoculation loop and transferred aseptically to the PDA slants and allowed to grow at room (28 ± 2 °C) temperature to obtain the pure culture of the fungus. The culture thus obtained was stored at 28 ± 2 °C in a biological oxygen demand (BOD) incubator. The purified isolates were identified based on morphological and colony characteristics (Punja and Damini 1996; Sarma et al. 2002; Watanabe 2002). They were identified as S. rolfsii.
Isolation and identification of Trichoderma species
Soil samples were collected from major peppermint (rhizosphere) growing areas of Tamil Nadu, viz, Coimbatore (Alangudi), Dindigul (Attupalam), Erode (Edappadi), Hosur (Kattuvalasu), Krishnagiri (Pochampalli), Namakkal (Vattur), Salem (Morepalayam) and Theni (Neeraganur) districts. One gram of rhizosphere soil near the root surface was collected and isolation was done as per the method described by Elad and Chet (1983) using Trichoderma selective medium. The colonies with light green colour, circular spots were individually purified and subcultured. Eight Trichoderma isolates were maintained. The visual observation on Petri plates was adopted for identification of Trichoderma species. For visual observation, the isolates were grown on PDA agar for 3–5 days. The mode of mycelial growth, colour, odour and changes of medium colour for each isolate were examined every day (Leahy and Colwell 1990). Further, isolates were compared with the taxonomic key for the genus Trichoderma (Samuels et al. 2004).
Screening of Trichoderma isolates against Sclerotium rolfsii
The antagonistic activity of Trichoderma isolates against S. rolfsii was tested by dual culture technique (Dennis and Webster 1971) using PDA medium. Each treatment was replicated four times with five plates per replication and experiment was repeated four times. The percent inhibition of mycelial growth of pathogen was calculated according to Vincent (1929).
where C is the radial growth in control, T is the radial growth in treatment, I is the inhibition percent.
Isolation and identification of bacteria
Soil samples were collected from major peppermint (rhizosphere) growing areas of Tamil Nadu. After removing the loosely adhering soil from freshly excised roots, root segments (1 g) were taken and isolation was done as per the method described by Elad and Chet (1983) using King’s B medium (King et al. 1954 ). Totally eight bacterial isolates were obtained. These isolates were identified according to Bergey’s Manual of Systematic Bacteriology.
Screening of bacterial isolates against Sclerotium rolfsii
The antagonistic activity of bacterial isolates against S. rolfsii was tested by dual culture technique (Dennis and Webster 1971) using PDA medium. Each treatment was replicated four times with five plates per replication and experiment was repeated four times. The plates were incubated at room temperature (28 ± 2 °C) for 48 h. The radial growth (in mm) of the pathogen was measured after incubation. The percent inhibition of mycelial growth was also calculated.
Based on the dual culture technique the most effective isolate of T. harzianum (THA) and P. fluorescens (PFM) was used for subsequent studies.
Compatibility of THA and PFM
The compatibility was determined for THA and PFM by using PDA and KB medium, respectively. The medium was mixed with T. harzianum and P. fluorescens each at 2/18 mL of PDA. Each milliliter of fungal and bacterial suspension contained 1 × 108 cfu mL−1. The medium containing biocontrol agents was poured into Petri plates and immediately after solidification, two sterile paper discs were placed at equidistance to each other on the seeded medium. For THA, 5 μL of spore suspension was added to the first sterile filter paper disc and 5 μL of 0.01 mL of sterile distilled water was added to the second filter paper disc as control, respectively. For PFM, 5 μL of spore suspension was added to the first sterile filter paper disc and 5 μL of 0.01 mL of sterile distilled water was added to the second filter paper disc as control, respectively. The plates were incubated at room temperature (28 ± 2 °C) for 5 days (THA) and 72 h (PFM) and observed for the inhibition zone. Absence of inhibition zone around the disc indicates the compatibility with respective bacterial and fungal isolates, and the presence of inhibition zone indicates the incompatibility.
Bioformulations of THA and PFM
The talc-based formulation of THA and PFM was prepared by following the method described by Papavizas et al. (1984), Vidhyasekaran and Muthamilan (1995). The bacterial strain was grown separately in conical flasks (250 mL) containing 100 mL of King’s broth (KB) and kept for 48 h on a rotary shaker (150 rpm) at (28 ± 2 °C). Bacteria were subsequently pelleted by centrifugation at 8,000×g for 10 min at 4 °C. The pellets were washed with sterile distilled water three times, and the concentration of cells adjusted to 3 × 108 cfu mL−1 by dilution to give the suspensions an optical density of 0.45 (A610 nm) using UV–visible spectrophotometer (Mortensen 1992). Ten grams of carboxy methyl cellulose (CMC) was added to 1 kg of talc and mixed well and the mixture was autoclaved for 30 min on each of two consecutive days. For PFM, 100 mL of 48 h grown bacterial suspension containing 3 × 108 cfu mL−1 was mixed with carrier mixture under aseptic conditions. For THA, 100 mL of molasses yeast broth autoclaved flasks was inoculated with 5-mm mycelial discs of THA and incubated at 28 ± 2 °C for 10 days. After 10 days, the mycelial mat was harvested and ground to form spore suspension. Then the spore concentration was adjusted to 1.5 × 108 cfu g−1 by using UV–visible spectrophotometer. The formulations thus prepared were allowed to dry aseptically (~35 % moisture content) and were then ground to powder. They were then packed in sterile polythene bags and stored at 4 °C.
Greenhouse studies
Seedling dip and soil application
Sterilized soil (1.0 kg) was mixed with the pathogen inoculum at 5 g (multiplied on sand-maize medium) and filled in 15 × 30 cm diameter earthen pots. Surface-sterilized mint cuttings were separately treated with the talc-based formulation of the antagonists and planted in pots. The treatment schedule followed is mentioned below (SD-seedling dip; SA-soil application).
Treatment schedule
- T1 :
-
T. harzianum SD at 8 × 108 cfu mL−1
- T2 :
-
T. harzianum SA at 2.5 kg ha−1
- T3 :
-
T1 + T2
- T4 :
-
P. fluorescens SD at 8 × 108 cfu mL−1
- T5 :
-
P. fluorescens SA at 2.5 kg ha−1
- T6 :
-
T4 + T5
- T7 :
-
T. harzianum SD + P. fluorescens SD
- T8 :
-
T. harzianum SA + P. fluorescens SA
- T9 :
-
Propiconazole at 0.1 % as soil drenching
- T10 :
-
Inoculated control
Seedling dip with Propiconazole at 0.1 % was used for comparison and pathogen-alone-inoculated pots served as control. The experiment was conducted with three replications in a randomized block design. The treated seedlings were sown in pathogen-inoculated soil at eight seedlings per pot and irrigated daily. The observations on the incidence of collar rot were recorded at 20 days after inoculation
Induction of defense-related proteins+
THA and PFM in single and in combination were used in the induction of defense reactions in peppermint. The bioformulation-treated cuttings were planted in earthen pots (15 × 30 cm diameter) filled with sterilized potting soil. In the first set, treated plants were challenge-inoculated with S. rolfsii and in the second set, treated plants were not challenged with the pathogen. Plants without prior treatment of biocontrol agents were inoculated with the pathogen. Three replications were maintained in each treatment and each replicate consisted of five pots. The experiments were conducted using completely randomized block design on a glasshouse bench. The humidity in the greenhouse was maintained at around RH 70 %. The temperature was adjusted to 28 °C (day)/22 °C (night).
Collection of leaf sample
Plant leaf tissues were collected at different time intervals (0, 3, 6, 9 and 12 days after pathogen inoculation). Four plants were sampled from each replication separately and were maintained for biochemical analysis. At different intervals, leaf samples were taken and analyzed for biochemical changes, viz., PO, PPO, PAL and total phenol content. Samples from each treatment were analyzed thrice. The enzyme activities were determined on days 0, 3, 6, 9 and 12 days of storage at 24 ± 1 °C. Microsoft Excel program was used to create all graphical representations.
Assay of peroxidase (PO)
The PO activity was assayed as described by Hammerschmidt et al. (1982). Extraction was carried out by homogenizing 1 g of the leaf sample in 2 mL of 0.1 M sodium phosphate buffer (pH 6.5) using pre-chilled pestle and mortar (4 °C). The homogenate was centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatant served as enzyme source and the reaction mixture consisted of 1.5 mL of 0.05 M pyrogallol, 0.5 mL of enzyme extract and 0.5 mL of 1 % H2O2. The reaction mixture was incubated at 28 ± 2 °C. At the start of enzyme reaction, the absorbance of the mixture was set to zero at 420 nm in the spectrophotometer and the change in the absorbance was recorded at 20-s intervals for 30 min. Boiled enzyme preparation served as control. The peroxidase activity was expressed as change in the absorbance of the reaction mixture min−1 mg−1 of fresh tissue.
Assay of polyphenoloxidase (PPO)
One gram of the sample was homogenized in 2 mL of 0.1 M sodium phosphate buffer (pH 6.5) in a pre-chilled pestle and mortar. The homogenate was centrifuged at 10,000 rpm for 15 min at 4 °C and the supernatant served as an enzyme source. PPO activity was determined as per the procedure given by Mayer et al. (1965). The reaction mixture consisted of 1.5 mL of 0.1 M sodium phosphate buffer (pH 6.5) and 200 μL of the enzyme extract. To start the reaction, 200 μL of 0.01 M catechol was added. The reaction mixture was incubated at room temperature and the absorbance was set to zero at 495 nm. The changes in absorbance were recorded at 30-s intervals for 2 min and the activity was expressed as change in absorbance min−1 mg−1 of fresh tissue.
Determination of phenylalanine ammonia-lyase (PAL) activity
One gram of leaf tissue was homogenized in 5 mL of 0.1 M sodium borate buffer, pH 7.0 containing 0.1 g insoluble polyvinyl pyrrolidone (PVP). The extract was filtered through muslin cloth and the filtrates were centrifuged at 12,000 g at 4 °C for 20 min. The supernatant served as an enzyme source. Samples containing 0.4 mL of enzyme extract were incubated with 0.5 mL of 0.1 M borate buffer, pH 8.8 and 0.5 mL of 12 mM l-phenylalanine in the same buffer for 30 min at 30 °C. PAL activity was determined as the rate of conversion of l-phenylalanine to trans-cinnamic acid at 290 nm as described by Dickerson et al. (1984) and was expressed as micromoles of cinnamic acid min−1 mg−1 of fresh tissue.
Estimation of phenolic content
Peppermint plant leaf tissues (1 g) were homogenized in 10 mL of 80 % methanol and shaken for 15 min at 70 °C. Phenol was assayed as described by Zieslin and Ben-Zaken (1993). One gram of leaf tissue was homogenized in 10 mL of 80 % methanol and agitated for 15 min at 70 °C. Then 1 mL of the methanolic extract was added to 5 mL of distilled water and 250 μL of Folin–Ciocalteu reagent (1 N) and incubated at 25 °C. After 3 min, 1 mL of the saturated solution of sodium carbonate and 1 mL of distilled water were added and the reaction mixtures were incubated further for 1 h at 25 °C. The absorption of the developed blue colour was measured using spectrophotometer at 725 nm. The total soluble phenol content was calculated according to a standard curve obtained from a Folin–Ciocalteu reagent with a phenol solution (C6 H6 O) and expressed as catechol equivalent μg g−1 of fresh tissue.
Experimental design and statistical analysis
All the experiments were carried out in a CRD. For the data on the effect of biocontrol agents on mycelial growth, percent reduction over control was calculated. The data on disease incidence were arcsine transformed before undergoing statistical analysis. The data were analyzed using the IRRISTAT version 92-1 program developed by the biometrics unit, International Rice Research Institute, Metro Manila, The Philippines. Data were subjected to analysis of variance (ANOVA). The treatment mean values were compared by Duncan’s multiple range test (DMRT) at 5 % significance level (Gomez and Gomez 1984).
Results
Isolation of antagonists
Eight Trichoderma isolates were obtained from peppermint rhizosphere soils of eight different locations. All the eight Trichoderma isolates were identified as T. viride (TVA), T. harzianum (THA), T. viride (TVE), T. viride (TVK), T. harzianum (THP), T. harzianum (THV), T. viride (TVM) and T. viride (TVN).
Eight bacterial isolates were obtained from peppermint rhizosphere soils of eight different locations. All the eight bacterial isolates were identified as P. fluorescens (PFA), B. subtilis (BSA), B. subtilis (BSE), P. fluorescens (PFK), B. subtilis (BSP), P. fluorescens (PFV), P. fluorescens (PFM) and P. fluorescens (PFN).
Compatibility between THA and PFM
The fungal antagonists THA and PFM were tested for their compatibility in in vitro. Absence of inhibition zone around the disc indicated that T. harzianum and P. fluorescens were compatible with pathogen (data not shown).
In vitro inhibition by Trichoderma isolates
All the Trichoderma isolates inhibited the mycelial growth of S. rolfsii (Table 1). Among the Trichoderma isolates tested, THA was the most effective in inhibiting the mycelial growth of S. rolfsii (68.28 %) as compared to control. The least growth inhibition of pathogen (54.48 %) was exhibited by the isolate TVM.
In vitro inhibition by bacterial isolates
Among the eight bacterial isolates tested, PFM recorded the maximum inhibition zone of 13.3 mm with a minimum of 23.00 mm mycelial growth of S. rolfsii accounting for 74.25 % reduction of the mycelial growth over control (Table 2), whereas the least growth inhibition of pathogen (64.17 %) was exhibited by the isolate BSE (32.00 mm).
Collar rot incidence
The combined application of T. harzianum SA + P. fluorescens SA recorded the minimum incidence of collar rot of 12.00 % and accounted for 87.14 % of reduction over control (Table 3).
Induction of biochemical defense mechanisms in peppermint plants by combination of biocontrol agents
THA and PFM formulation combinations differed in their ability to stimulate PO and PPO activities in peppermint. Increased PO and PPO activities were observed in combined application of biocontrol agents (THA SA + PFM SA) inoculated with S. rolfsii as compared to untreated control plants. The activities were found to increase from the 3rd day after inoculation and it reached the peak on the 9th day after inoculation and thereafter, a decline was noticed (Tables 4, 5). PAL activity was significantly higher in combined application of biocontrol agents inoculated with S. rolfsii than the untreated control. PAL accumulation reached a maximum at 9th day after inoculation and it was increased from 3rd day after sampling and was significantly higher in combination treatment (Table 6). Similarly, the activity of maximum accumulation of total phenol content was also observed in the combination treatment (Table 7). Inoculated control with pathogen alone did not show any remarkable change in the activity of phenolic substances.
Discussion
In the present study, we have evaluated the fungal and bacterial isolates against collar rot of peppermint pathogen. Trichoderma isolate THA recorded the maximum growth inhibition of S. rolfsii. Similarly, among the screened antagonists, the isolate TH-18 of T. harzianum showed the highest (83.06 %) inhibition of mycelial growth of S. rolfsii causing foot and root rot of soybean (Bhuiyan et al. 2012). Radwan et al. (2006) reported that Jn14 (T. harzianum) and T36 (T. hamatum) were the most effective isolates and inhibited the mycelial growth of S. rolfsii at 79 %. Control of S. rolfsii by using T. harzianum was reported by several researchers (Ganesan et al. 2003; Ganesan 2004; Ganesan and Sekar 2004). The inhibitory effect of T. harzianum against S. rolfsii might be due to the production of antibiotic substances including viridin, gliotoxin, glioviridin, dermin and trichodermin (Eziashi et al. 2007; Ghildiyal and Pandey 2008).
The present investigation indicates that the bacterial isolates of P. fluorescens PFM recorded the maximum inhibition on the mycelial growth of S. rolfsii in in vitro. Similar observations on variation in antagonistic efficacy between isolates were recorded by several workers. Kamalakannan et al. (2003) reported that P. fluorescens (PFMMP) recorded the highest inhibition zone against Rhizoctonia solani causing stem and stolon rot of peppermint. Pastor et al. (2010) reported that Pseudomonas spp. isolated from rhizosphere soil of groundnut plants showed highest antagonistic activity against S. rolfsii. Recently, Paramageetham (2013) reported that P. fluorescens isolate PATPT 6 was found to be a potential antagonist against S. rolfsii. The inhibitory effect of P. fluorescens against S. rolfsii might be due to the production of antibiotic substances. Several strains of Pseudomonas have been reported to produce a wide array of antifungal antibiotics such as 2,4-diacetylphloroglucinol, oligomycin, phenazine, pyoluteorin, pyrolnitrin and pyocyanin, responsible for their antifungal action (Nielson et al. 1998; Gupta et al. 2001; Muthukumar et al. 2010).
In the present study, the antagonists such as T. harzianum and P. fluorescens were compatible, which is an important prerequisite for co-inoculated microorganisms (Li and Alexander 1998). Compatible multiple strains might be advantageous when dealing with multiple diseases. Also, a single strain may not grow equally well in a variety of environmental conditions (Fukui et al. 1994).
Several biocontrol agents induce systemic resistance in plants and disease reduction and increased plant growth is seen in many crops (Nandakumar et al. 2001; Ramamoorthy et al. 2002). Plants are endowed with defense genes, but they are quiescent in normal healthy plants. When these defense genes are activated by various factors, they induce systemic resistance against diseases. One of the factors is antagonistic microbes, which show direct antagonistic activity against pathogens not only by producing various metabolites, but also by inducing defense-related enzymes. These have recently been found to be a new way whereby plants defend themselves from pathogen attack (Bharathi et al. 2004). This phenomenon called ISR has been demonstrated in several plants (Alstrom 1991; Van Peer et al. 1991; Wei et al. 1991; Leeman et al. 1995) and found to reduce disease symptoms of a wide range of pathogens. This bacterial antagonist mediated is generally associated with onset of defense mechanism including the early and increased expression of defense enzymes such as PO, PAL and accumulation of phenolics, phytoalexins, lignins, etc. (Chen et al. 2000). PO has been implicated in a number of physiological functions that may contribute to resistance including exudation of hydroxyl cinnamyl alcohol into free radical intermediates, which subsequently are coupled to lignin polymer (Gross 1980). In the present study, peppermint cuttings treated with combination of bioagents had higher levels of peroxidase and PPO enzymes as compared with cuttings treated with single bioagents, and also cuttings treated with fungicide treatment. Several workers have reported the association between higher levels of defense-related enzymes (PO and PPO) and greater disease resistance (Vivekanandhan et al. 2004; Vinothkumar et al. 2007). Suppression in the wilt incidence of cucumber and higher level of defense enzymes peroxidase and catalase were observed in plants treated with T. viride indicating that the production of phytoalexin or lignin might be involved in disease suppression (Zhuang et al. 2005). Liang et al. (2006) stated that cucumber seeds treated with Brevibacillus brevis showed a higher activity of PO at 12 days after pathogen inoculation. Later stages may contribute (PO) to cross linking of hydroxyl proline-rich glycoproteins (HRGPs), lignifications that act as barriers against pathogen entry. Rajendran and Samiyappan (2008) indicated that cotton seeds treated with endophytic Bacillus species showed a higher induction of peroxidase activity.
In the present study, up to threefold increase in PAL activity was observed in combination of bioagent-treated peppermint plants. Generally, induction of PAL enzyme is correlated with increased resistance to pathogenic infection (Bell et al. 1984). Induction of PAL by fluorescent pseudomonads was reported in tomato against Fusarium oxysporum f.sp. lycopersici (Ramamoorthy et al. 2002). Early and increased synthesis of PAL was observed in the T. viride, P. fluorescens and B. subtilis pre-treated peppermint plants challenged with R. solani (Kamalakannan et al. 2003). Nakkeeran et al. (2006) revealed that chilli seeds treated with P. chlororaphis strain PA23 resulted in increased activity of PAL when compared to uninoculated control. Sangeetha et al. (2010) reported that banana fruits treated with bacterial antagonists (individual and in combination) and challenge-inoculated with crown rot pathogens (banana) showed up to fourfold increase in PAL activity. Muthukumar et al. (2011) reported that combined application of talc-based formulation of bioagents and challenge-inoculated with P. aphanidermatum recorded maximum induction of defense-related enzymes such as PO, PPO and PAL as compared with individual application.
In addition to PAL and PO, the presence of phenolic compounds in plants or their synthesis in response to infection has often been associated with resistance (Ingham 1972). The increase in phenolic content in plant system has been correlated with increased resistance to pathogens (Velazhahan and Vidhyasekaran 1994). It is also well known that resistant plants contain more phenols or produce polyphenols more rapidly than susceptible ones. In the present study also, combinations of bioagents recorded maximum reduction of collar rot disease and also induced higher accumulation of phenolics; therefore, this increased phenolic content might have contributed to increased resistance to collar rot pathogen. The increase in the phenolics due to treatment with P. fluorescens and challenge inoculation with pathogen were reported in sugarcane against Colletotrichum falcatum (Viswanathan and Samiyappan 1999). Chilli plants pre-treated with B. subtilis and P. chlororaphis and challenge-inoculated with P. aphanidermatum triggered the defense-related enzymes in phenylpropanoid pathway and increased the accumulation of phenolics (Kavitha et al. 2005). Rangeshwaran et al. (2008) indicated that increase in phenol content of chickpea seedlings was due to the treatment with endophytic bacteria B. megaterium and maximum phenol content was observed on the 6th day after treatment. Sangeetha et al. (2010) reported that in banana fruits treated with bacterial antagonists (individual and in combination) and challenge-inoculated with crown rot pathogens (banana), 3.6-fold increase in phenolic content was observed as compared with control. Combined application of talc-based formulation of bioagents and challenge inoculation with P. aphanidermatum recorded higher level of phenol content (sixfold) in chilli plants as compared with individual application (Muthukumar et al. 2011).
In conclusion, the increased activities of defense-related enzymes such as PO, PPO and PAL and elevated accumulation of phenolics due to the application of biocontrol agents singly and in combination suggest that activation of host biochemical defense mechanism may be involved in the suppression of collar rot disease in peppermint.
Author contribution
Initially, the work was designed by A. Muthukumar followed by collections of plant and soil samples by A. Venkatesh. The collection of literature was done by A. Muthukumar. Then the in vitro experiment was carried out by A. Muthukumar and the statistical analysis was furnished by A. Muthukumar. Finally, A. Muthukumar and A. Venkatesh interpreted the data and wrote the manuscript.
Abbreviations
- THA:
-
Trichoderma harzianum Attupalam
- PFM:
-
Pseudomonas fluorescens Morepalayam
- PR:
-
Pathogenesis-related protein
- PO:
-
Peroxidase
- PPO:
-
Polyphenoloxidase
- PAL:
-
Phenylalanine ammonia-lyase
- PGPR:
-
Plant growth promoting rhizobacteria
- ISR:
-
Induced systemic resistance
References
Ainsworth GC (1961) Dictionary of the fungi. Can J Microbiol 34:157–161
Alstrom S (1991) Induction of disease resistance in common bean susceptible to halo blight bacterial pathogen after seed bacterization with rhizosphere pseudomonads. J Gen Appl Microbiol 37:495–501
Anand S, Bahadur Harikesh (2004) Control of collar rot in mint (Mentha spp.) caused by Sclerotium rolfsii using biological means. Curr Sci 87:362–366
Anonymous (2004) Essential oils association of India. http://www.nhb.gov.in/Horticulture
Bell JN, Dixon RA, Bailey JA, Rowell PM, Lamb CJ (1984) Differential induction of chalcone synthase mRNA activity at the onset of phytoalexin accumulation in compatible and in compatible plant–pathogen interactions. Pro Natl Acad Sci USA 81:3384–3388
Bharathi R, Vivekanandhan R, Harish S, Ramanathan A, Samiyappan R (2004) Rhizobacteria-based bio-formulations for the management of fruit rot infection in chillies. Crop Prot 23:835–843
Bhuiyan MA, Rahman HB, Bhuiyan KA (2012) In vitro screening of fungicides and antagonists against Sclerotium rolfsii. Afr J Biotechnol 11:14822–14827
Chen C, Belanger RR, Benhamou N, Paulitz TC (2000) Defense enzymes induced in cucumber roots by treatment with plant growth promoting rhizobacteria (PGPR). Physiol Mol Plant Pathol 56:13–23
Dennis L, Webster J (1971) Antagonistic properties of species group of Trichoderma I. Production of non-volatile antibiotics. Trans Br Mycol Soc 57:25–39
Dickerson DP, Pascholari SF, Hagerman AE, Butler LG, Niholson RL (1984) Phenylalanine ammonia lyase and hydroxyl cinnamate: Co A ligase in maize mesocotyls inoculated with Helminthosporium maydis or Hemlinthosporium carbonum. Physiol Mol Plant Pathol 25:111–123
Duffy BK, Simon A, Weller DM (1996) Combination of Trichoderma koningii with fluorescent pseudomonads for control of take-all on wheat. Phytopathology 86:188–194
Elad Y, Chet I (1983) Improved selective media for isolation of Trichoderma or Fusarium spp. Phytoparasitica 11:55–58
Eziashi EI, Omamor IB, Odigie EE (2007) Antagonism of Trichoderma viride and effects of extracted water soluble compounds from Trichoderma species and benlate solution on Ceratocystis paradoxa. Afr J Biotechnol 6:388–392
Fukui R, Schroth MN, Hendson M, Hancock JG (1994) Interaction between strains of pseudomonads in sugar beet spermospheres and the relationship to pericarp colonization by Pythium ultimum in soil. Phytopathology 84:1322–1330
Ganesan S (2004) Studies on the biocontrol of soil-borne plant pathogens. Ph.D. Thesis, Madurai Kamarajar University, Madurai
Ganesan S, Sekar R (2004) Biocontrol mechanism of groundnut (Arachis hypogea L.) diseases-Trichoderma systems. Biotechnological Applications in Environment and Agriculture, Jaipur, pp 312–327
Ganesan SP, Manimaran K, Ramesh, Sekar R (2003) Biocontrol of onion basal rot disease caused by Fusarium oxysporum f.sp.cepae. Microbiol Society, Maharastra, pp 119–124
Ghildiyal A, Pandey A (2008) Isolation of cold tolerant antifungal strains of Trichoderma species from glacial sites of Indian Himalayan region. Res J Microbiol 8:559–564
Gomez KA, Gomez AA (1984) Statistical procedure for agricultural research. Wiley, New York
Gross GG (1980) The biochemistry of lignification. Adv Bot Res 8:25–63
Gupta CP, Dubey RC, Kang SC, Maheshwari DK (2001) Antibiosis mediated necrotrophic effect of Pseudomonas GRC2 against two fungal plant pathogens. Curr Sci 81:91–94
Hammerschmidt R, Nuckles EM, Kuc J (1982) Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Plant Pathol 20:73–82
Ingham JL (1972) Phytoalexins and other natural products as factors in plant diseases. Bot Rev 38:343–424
Kamalakannan A, Mohan L, Kavitha K, Harish S, Radjacommare R, Nakkeeran S, Parthiban VK, Karuppiah R, Angayarkanni T (2003) Enhancing resistance to stem and stolen rot of peppermint (Mentha pipertia Linn.) using bio-control agents. Acta Phytopathol Entomol Hung 38:293–305
Kavitha K, Mathiyazhahan S, Sendhilvel V, Nakkeeran S, Chandrasekar G (2005) Development of bio formulations of antagonistic bacteria for the management of damping-off in chilli. Arch Phytopathol Plant Prot 38:19–30
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanine and fluorescein. J Lab Clin Med 44:301–307
Leahy JG, Colwell RR (1990) Microbial degradation of hydrocarbons in the environment. Microbiol Rev 54:305–315
Leeman M, Van Pelt JA, Den Ouwden FM, Heinsbroek M, Bakker PAHM, Schippers B (1995) Induction of systemic resistance against Fusarium wilt of radish by lipopolysaccharides of Pseudomonas fluorescens. Phytopathology 85:1021–1027
Li DM, Alexander M (1998) Co-inoculation with antibiotic-producing bacteria to increase colonization and nodulation by rhizobia. Plant Soil 108:211–219
Liang JG, Zhang BX, Chen ZY, Yu JQ (2006) Studies on the resistance to cucumber damping- off induced by plant growth promoting rhizobacteria CH1. Acta Hortic Sin 33:282–288
Liu L, Kloepper JW, Tuzun S (1995) Induction of systemic resistance in cucumber against bacterial angular leaf spot by plant growth promoting rhizobacteria. Phytopathology 85:843–847
Maurhofer M, Hase C, Meuwly P, Metraux JP, Defago G (1994) Induction of systemic resistance of tobacco to tobacco necrosis virus by the root-colonizing Pseudomonas fluorescens strain CHAO: influence of the gcaA gene and pyoverdine production. Phytopathology 84:139–146
Mayer AM, Harel E, Shaul RB (1965) Assay of catechol oxidase a critical comparison of methods. Phytochemistry 5:783–789
Meena B, Radhajeyalakshmi R, Marimuthu T, Vidhyasekaran P, Sabitha D, Velazhahan R (1999) Induction of pathogenesis related proteins, phenolics and phenylalanine ammonia-lyase in groundnut by Pseudomonas fluorescens. J Plant Dis Prot 107:524–527
Mortensen CN (1992) Seed bacteriology laboratory guide. Danish Government Institute of Seed Pathology for Developing Countries, Copenhagen
Muthukumar A, Nakkeeran S, Eswaran A, Sangeetha G (2010) In vitro efficacy of bacterial endophytes against the chilli damping-off pathogen Pythium aphanidermatum. Phytopathol Mediterr 49:179–186
Muthukumar A, Eswaran A, Sangeetha G (2011) Induction of systemic resistance by mixtures of fungal and endophytic bacterial isolates against Pythium aphanidermatum. Acta Physiol Plant 33:1933–1944
Nakkeeran S, Kavitha K, Chandrasekar G, Renukadevi P, Fernando WGD (2006) Induction of plant defence compounds by Pseudomonas chlororaphis PA 23 and Bacillus subtilis BSCBE 4 in controlling damping-off of hot pepper caused by Pythium aphanidermatum. Biocontrol Sci Technol 16:403–416
Nandakumar R, Babu S, Viswanathan R, Sheela J, Raguchander T, Samiyappan R (2001) A new bio-formulation containing plant growth promoting rhizobacterial mixture for the management of sheath blight and enhanced grain yield in rice. Biol Control 46:493–510
Nielson MN, Sorensen J, Fels J, Pedersen HC (1998) Secondary metabolite and endo chitinase dependent antagonism towards plant pathogenic micro fungi of Pseudomonas fluorescens isolates from sugar beet rhizosphere. Appl Environ Microbiol 64:563–3569
Niranjana SR, Lalitha S, Hariprasad P (2009) Mass multiplication and formulations of biocontrol agents for use against Fusarium wilt of Pigeonpea through seed treatment. Int J Pest Manag 55:317–324
Papavizas GC, Dunn MT, Lewis JA, Beagle-Ristaino J (1984) Liquid fermentation technology for experimental production of biocontrol fungi. Phytopathology 74:1171–1175
Paramageetham CH (2013) Bio control of Sclerotium rolfsii a polyphagous plant pathogen by Pseudomonas aeruginosa isolated from forest liter. Int J Res Plant Sci 3:1–4
Pastor NA, Reynoso MM, Tonelli ML, Masciarelli O, Rosaso SB, Tonelli ML, Masciarelli D, Rosas SB, Rovera M (2010) Potential bio control Pseudomonas sp. Pc12 against damping-off of tomato caused by Sclerotium rolfsii. J Plant Pathol 92:737–745
Punja ZK, Damini A (1996) Comparative growth, morphology and physiology of three Sclerotium species. Mycologia 88:694–706
Radwan M, Baraka Fadel AL, Mahareeq I, Mohammad IAL (2006) Biological control of Sclerotium rolfsii by using indigenous Trichoderma spp. isolates from Palestine. Hebron Univ Res J 2:27–47
Rajendran L, Samiyappan R (2008) Endophytic Bacillus species confer increased resistance in cotton against damping-off disease caused by Rhizoctonia solani. Plant Pathol 7:1–12
Ramamoorthy V, Raguchander T, Samiyappan R (2002) Enhancing resistance of tomato and hot pepper to Pythium disease by seed treatment with fluorescent pseudomonads. Eur J Plant Pathol 108:429–441
Rangeshwaran R, Raj J, Sreeramakumar P (2008) Identification of endophytic bacteria in chickpea (Cicer arietinum L.) and their effect on plant growth. J Biol Control 22:13–23
Samuels GJ, Chaverri P, Farr DF, McCray EB (2004) USDA, Beltsville, USA. Trichoderma online systematic Botany and Mycology Laboratory, ARS, USDA. Retrieved September 20, 2004, from http://nt.arsgrin.gov/taxadescriptions/keys/TrichodermaIndex.cfm
Sangeetha G, Thangavelu R, Usha Rani S, Muthukumar A, Udayakumar R (2010) Induction of systemic resistance by mixtures of antagonist bacteria for the management of crown rot complex on banana. Acta Physiol Plant 32:1177–1187
Saravanakumar D, Vijayakumar C, Kumar N, Samiyappan R (2007) PGPR-induced defense responses in the tea plant against blister blight disease. Crop Prot 26:556–565
Sarma BK, Singh UP, Singh KP (2002) Variability in Indian isolates of Sclerotium rolfsii. Mycologia 94:1051–1058
Shukla PK, Haseeb AS, Sharma S (1998) Soil texture, root lesion nematodes and yield of peppermint (Mentha piperita). J Herbs Spices Med Plants 6:1–8
Van Peer R, Schippers B (1992) Lipopolysaccharides of plant growth promoting Pseudomonas spp. strain WCS417r induce resistance in carnation to Fusarium wilt. Neth J Plant Pathol 98:129–139
Van Peer R, Niemann GJ, Schippers B (1991) Induced resistance and phytoalexin accumulation in biological control of Fusarium wilt of carnation by Pseudomonas sp. Strain WCA417r. Phytopathology 81:728–734
Velazhahan R, Vidhyasekaran P (1994) Role of phenolic compounds, peroxidase and polyphenol-oxidase in resistance of groundnut to rust. Acta Phytopathol Entomol Hung 29:23–29
Vidhyasekaran P, Muthamilan M (1995) Development of formulations of Pseudomonas fluorescens for control of chickpea wilt. Plant Dis 79:782–786
Vincent JM (1929) Distribution of fungal hyphae in the presence of certain inhibitors. Nature 159:850
Vinothkumar A, Kumar VC, Verma SK, Kharwar RN (2007) Induction of defense enzymes in Pseudomonas fluorescens treated chickpea roots against Macrophomina phaseolina. Indian Phytopathol 60:289–295
Viswanathan R, Samiyappan R (1999) Induction of systemic resistance by plant growth promoting rhizobacteria against red rot disease caused by Colletotrichum falcatum in sugarcane. Proc Sugar Technol Assoc 61:24–39
Vivekanandhan R, Ravi M, Sible GV, Prakasam V, Samiyappan R (2004) Pseudomonas fluorescens (FP7) amended with chitin bioformulation for the management of anthracnose pathogen in mango cultivar Alphonso. Madras Agric J 91:475–782
Watanabe T (2002) Sclerotium sp. morphologies of cultured fungi and key species: pictorial atlas of soil and seed fungi, 2nd edn. CRC Press, New York
Wei G, Kloepper JW, Tuzun S (1991) Induction of systemic resistance of cucumber to Colletotrichum orbiculare by selected strains of plant growth-promoting rhizobacteria. Phytopathology 41:1508–1512
Xue L, Charest PM, Jabaji-Hare SH (1998) Systemic induction of peroxidases, β-1, 3-glucanases, chitinases and resistance in bean plants by binucleate Rhizoctonia species. Phytopathology 88:359–365
Yedidia I, Benhamou N, Chet I (1999) Concomitant induction of systemic resistance to Pseudomonas syringea pv. lachrymans in cucumber by Trichoderma asperellum and accumulation of phytoalexins. Appl Environ Microbiol 69:7343–7353
Zhuang J, Gao Z, Yang C, Chen J, Xue Y, Lianxiao M (2005) Biocontrol of Fusarium wilt and induction of defense enzyme activities on cucumber by Trichoderma viride strain T23. Acta Phytopathol Sin 35:179–183
Zieslin N, Ben-Zaken R (1993) Peroxidase activity and presence of phenolic substances in peduncles of rose flowers. Plant Physiol Biochem 31:333–339
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Krolicka.
Rights and permissions
About this article
Cite this article
Muthukumar, A., Venkatesh, A. Biological inductions of systemic resistance to collar rot of peppermint caused by Sclerotium rolfsii . Acta Physiol Plant 36, 1421–1431 (2014). https://doi.org/10.1007/s11738-014-1520-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-014-1520-1