Abstract
Sugar beet is widely grown throughout the world and represents the second largest crop used to produce sugar. Root rot in sugar beet, caused by Fusarium, significantly reduces yield, juice purity, and sugar concentration. Here, 307 Fusarium isolates were collected from sugar beet roots exhibiting typical root rot symptoms in eight provinces or autonomous regions of China from 2009 to 2012. Based on morphological characteristics and sequence data of the internal transcribed spacer (ITS) region of ribosomal DNA (rDNA) and the translation elongation factor 1α (EF-1α), Fusarium oxysporum (38.4%) was identified as the most prevalent species, followed by F. solani (20.9%), and F. equiseti (18.9%). These three species were widely distributed in all eight of the provinces and autonomous regions. F. tricinctum (5.9%), F. brachygibbosum (4.6%), F. redolens (3.3%), F. proliferatum (3.3%), F. graminearum (2.3%), F. verticillioides (1.6%), F. nygamai (0.7%), and F. culmorum (0.3%) were less frequently obtained. Of the 307 Fusarium isolates, 117 representing different species and geographic locations were demonstrated to cause tip rot and vascular discoloration in sugar beet roots, with disease incidence ranging from 84.2 to 100.0% and disease index ranging from 41.94 to 75.83. This is the first detailed report of Fusarium species, in particular F. tricinctum, F. brachygibbosum, F. redolens, F. proliferatum, F. nygamai, and F. culmorum, causing sugar beet root rot in China.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugar beet, Beta vulgaris L., is grown in countries as diverse as Russia, France, United States, Germany, and Turkey (FAOSTAT 2016). Approximately 166,000 ha in China are devoted yearly to sugar beet production, The main sugar beet production areas include Xinjiang Uygur Autonomous Region, Heilongjiang Province, Inner Mongolia Autonomous Region, Hebei Province, Shanxi Province, Gansu Province, Jilin Province, and Liaoning Province, with more than 90% of the gross production of sugar beets in China in Xinjiang, Heilongjiang, Inner Mongolia, and Hebei.
Root rot of sugar beet, caused by Fusarium spp. is an economically important fungal disease globally, including China (Hanson and Jacobsen 2006; Hanson and Lewellen 2007; Harveson and Rush 1998; Liu et al. 1997; Ruppel 1991; Zhao et al. 2002). Sugar beet root rot was first reported in Texas, United States by Martyn et al. (1989), with F. oxysporum f. sp. radicis-betae listed as the causal agent. Foliar symptoms of the disease in the field include yellowing, chlorosis, wilting, and an eventually severely scorched appearance, which gradually spread from the margins of the leaves to the center. Root symptoms include tip rot, vascular discoloration, and increased lignification of the taproot, as well as secondary roots (Harveson et al. 2009).
Symptoms of root rot disease in sugar beet were initially thought to be confined just to roots; however, recent studies have demonstrated that other sugar beet diseases can also cause root rot symptoms. For example, Fusarium yellows of sugar beet in the United States, caused by F. solani, F. avenaceum, F. acuminatum, and F. roseum can also exhibit root rot symptoms (Ruppel 1991). Hanson et al. (2017) reported that the ability of Fusarium oxysporum f. sp. betae, typically associated with Fusarium yellows of sugar beet, to cause root rot symptoms may vary in different sugar beet cultivars. In addition, Fusarium yellowing decline, caused by F. secorum, in North and Central America, can induce vascular necrosis in roots and petioles, as well as half- and full-leaf yellowing foliar symptoms in sugar beet (Arabiat et al. 2017; Secor et al. 2014).
F. oxysporum has been reported globally to be the most prevalent species causing sugar beet root rot (Hanson and Jacobsen 2006; Harveson and Rush 1998; Liu et al. 1997; Nitschke et al. 2009; Zhao et al. 2002). The first report of Fusarium species associated with root rot of sugar beet other than F. oxysporum was that published by Francis and Luttebacher (2003) in which they indicated that F. culmorum was also a causal agent of root rot in sugar beets in the United Kingdom. Hanson and Lewellen (2007) also reported F. solani as a causal organism of Fusarium root rot of sugar beet in the United States. Until recently, reports on the occurrence of root rot of sugar beet caused by Fusarium spp. have been limited to the United Kingdom and United States and to these three Fusarium species.
Several Fusarium species have been consistently isolated from sugar beet plants in field plantings in China, including F. oxysporum, F. solani, F. moniliforme, F. equiseti, F. graminearum, and F. lateritium in the Xinjiang Uygur Autonomous Region (Zhao et al. 2002), and F. solani, F. moniliforme, and F. avenaceum in Heilongjiang Province (Liu et al. 1997). These two studies, however, were only based on morphological characteristics and thus only partially definitive.
Morphological identification of Fusarum species is time-consuming, requires expertise, and can often be problematic. Thus, molecular analyses are needed for reliable identification. Various genes and /or genomic regions, such as translation elongation factor-1 alpha (EF-1α), calmodulin, α/β-tubulin, and the internal transcribed spacer (ITS) region of ribosomal DNA (rDNA) have been evaluated as molecular diagnostic tools to identify Fusarium isolates (Geiser et al. 2004; Hill et al. 2011; Mulè et al. 2004).
A lack of information exists in China about Fusarium species, their abundance, and geographical distribution in China. This information is crucial for epidemiological studies, developing a better understanding of the distribution and importance of individual species and designing effective management strategies to control their associated diseases. Therefore, the objectives of the present study were to identify Fusarium species associated with sugar beet root rot and to characterize their distribution and frequency throughout China.
Materials and methods
Sampling and fungal isolation
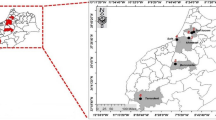
Sugar beet roots with rot symptoms were collected from 2009 to 2012 in eight different provinces or autonomous regions located in northern China throughout most of the main production areas of sugar beets. Five infected sugar beet roots were randomly selected from each surveyed field. Roots were rinsed under running tap water to remove soil and debris, and then small pieces (about 5 × 5 mm2) were removed from the border between necrotic and apparently healthy tissue. The sampled root tissues were surface sterilized in 70% ethanol for 40 s and then in 1% NaOCl for 3 min, rinsed three times with sterile distilled water and then dried on sterilized filter paper. Five pieces of tissue from each root were placed on potato dextrose agar (PDA) (20 g dextrose, 20 g agar, and the broth from 250 g white potatoes made up to 1 l with distilled water) amended with 50 µg/ml streptomycin sulfate and incubated at 25 °C in the dark for 72–96 h. Multiple fungal isolates were obtained from each plant tissue piece, but only those with a different morphology were selected as individual isolates. Fungal colonies that were microscopically confirmed to be Fusarium species were transferred to fresh PDA plates for single-spore purification (Leslie and Summerell 2006). A total of 307 single-spored Fusarium isolates were recovered after sufficient growth and maintained by serial transfer on PDA. In addition, mycelia from the Fusarium isolates were placed on sterile filter paper at − 20 °C for long-term storage (Fong et al. 2000).
Morphological characterization
Pure cultures of 117 representative isolates (Supplementary Table 1) exhibiting different morphologies and representing different species and geographic locations, were incubated on PDA plates at 25 °C in the dark for 7 days. The mycelial appearance, pigmentation, and colony margin of isolates were then recorded. The isolates were also cultured on carnation leaf agar (CLA) (Nelson et al. 1983) to microscopically examine the shape and size of conidia or confirm the existence of chlamydospores as described by Leslie and Summerell (2006).
DNA extraction and PCR amplification
Cultures of each isolate were grown for 1 week on a cellophane disc that was placed on PDA before DNA extraction. Mycelia were scraped from the surfaces of cultures and transferred to Eppendorf tubes. DNA from each of the collected samples was separately extracted as described by White et al. (1990). UV spectrophotometry (Nanodrop 2000, Thermo Fisher Scientific, Beverly, MA, USA) was used to determine the concentration and purity of DNA before use in the polymerase chain reaction (PCR).
The internal transcribed spacer region of ribosomal DNA (rDNA ITS) and a portion of the coding region of the translation elongation factor-1 alpha (EF-1α) gene were amplified by PCR. The ITS region was amplified using ITS1 and ITS4 primers (White et al. 1990), and the EF-1α gene was amplified using EF1F and EF2R primers (O’Donnell and Cigelnik 1997). The 25 µl PCR mixture used in each amplification consisted of 11 µl ddH2O, 12.5 µl Premix Taq (Ex Taq version 2.0, containing 0.625 U DNA polymerase, 200 µM dNTP and 1.5 mM Mg2+) (Takara Bio, Otsu, Shiga, Japan), 0.5 µl each of primer (10 µM), and 0.5 µl DNA (100 µg/ml). Negative controls containing the same reagents but without DNA were included with all PCR sets.
Amplification was performed in a Mastercycler gradient thermal cycler (Eppendorf, Hamburg, Germany) using the following protocol: initial denaturation at 94 °C for 3 min; 30 cycles of denaturing at 94 °C for 40 s, annealing at 56 °C for 40 s, and extension at 72 °C for 1 min; one cycle of extension at 72 °C for 10 min; and a final incubation at 4 °C.
DNA sequencing and phylogenic analysis
PCR products were purified with an AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) and ligated to the T-vector pMD19 (Takara Bio) according to the manufacturers’ instructions. The ligation reaction mixture was used to transform high-efficiency competent cells of Escherichia coli MC1022 (kindly provided by Dr. Salah Bouzoubaa, Université de Strasbourg, France) by heat shock at 42 °C for 90 s. The competent cells were then cultured in Luria–Bertani broth (10 g tryptone, 5 g yeast extract, and 10 g NaCl made up to 1 l with distilled water and the pH adjusted to 7.0 with 1 N NaOH), 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-gal, 100 µg/ml), and isopropyl-β-d- thiogalactopyranoside (IPTG, 100 µg/ml). White colonies possessing the target DNA insertion were verified by PCR and sent to Beijing Sunbiotech (Beijing, China) for sequencing. BLAST searches were performed against DNA sequences at NCBI (http://ncbi.nlm.nih.gov) (Altschul et al. 1997) and the FUSARIUM-ID/FUNCBS database at Fusarium MLST (http://www.westerdijkinstitute.nl/Fusarium/) (Geiser et al. 2004).
Manually editing of the individual data sets of rDNA ITS and EF-1α gene sequences was conducted using DNAMAN software (version 8) (Lynnon Biosoft, San Ramon, CA, USA), and obvious errors were corrected. Phylogenetic analysis based on a combination of two sequences (rDNA ITS and EF-1α) was carried out with MEGA 5 (version 5.2.2) (http://www.megasoftware.net/) software using the maximum likelihood (ML) method. Fusarium begoniae (GenBank accessions: NR_111864 and KC514054) was used as an outgroup. Bootstrap support was estimated based on 1000 pseudoreplicates.
Pathogenicity tests
The 117 representative Fusarium isolates (Supplementary Table 1) were also used in pathogenicity tests on sugar beet plants according to the revised procedure of Hill et al. (2011). Representative isolates were transferred to Spezieller Nährstoffarmer broth (SNB) (Leslie and Summerell 2006) and rocked on a shaker at 25 °C in the dark for 7 days to generate a conidial suspension. After filtering through four layers of sterile cheesecloth, the conidial suspension was adjusted to 105 spores/ ml using a hemacytometer. Two sugar beet seeds (cv. HI0305) were placed in a sterile plastic pot (1 l) filled with a combination of nutrient soil and sawdust (1:1, v/v) that had been sterilized with dry heat at 161 °C for 4 h before use. At least 60 seeds were sown for use with each isolate to obtain a sufficient number of plants at the same growth stage. The pots were arranged in a randomized block design in a greenhouse maintained at 25 to 27 °C, with a 12-h photoperiod, and watered daily to maintain vigorous growth. Thirty healthy sugar beet plants were selected 6 weeks after sowing for use with each isolate. Plants were gently removed from the soil, rinsed under running tap water, and roots were immersed in a spore suspension (105 spores/ml) for 15 min with intermittent agitation and then replanted in pots. Sugar beet roots serving as controls were dipped in sterile SNB media that did not contain conidia. The pathogenicity test utilized three replicates per isolate, as well as controls where each replicate consisted of 10 plants. Plants were held under a shade cloth for 3 days to reduce transplant shock. Then, the sugar beet plants were incubated in a glasshouse maintained at 25–27 °C with a 12-h photoperiod and watered whenever the surface soil appeared dry.
Sugar beet plants were harvested and assessed for disease incidence and disease index 6 weeks after inoculation. Severity on individual plants was rated from 0 to 4 based on the percentage of discoloration or necrosis of the vascular tissue (0 = no disease, 1 = less than 25% of vascular elements necrotic or localized lesions on a root, 2 = 26 to 50% vascular necrosis or less than 10% of taproot rotted, 3 = over 50% necrosis of vascular elements and 10 to 25% of taproot rotted, and 4 = more than 25% of taproot rotted) (Harveson and Rush 1998). The disease incidence and the disease index were calculated as follows: disease incidence = 100 × (n1 + n2 + n3 + n4)/N and disease index = 100 × (0n0 + 1n1 + 2n2 + 3n3 + 4n4)/4N, where n0 to n4 is the number of plants with each rating and N is the total number of inoculated plants. Dunnett’s one-tailed t test (Dunnett 1955) was used to compare disease index values of each isolate with the control, using a Dunnett- or Dunnett-Hsu-adjusted P value (Dunnett 1955, Hsu 1992), with P ≤ 0.05 considered the threshold for significance. Calculations were performed using the SPSS statistical program (version 20.0; IBM, Armonk, NY, USA). All the sugar beet roots were sampled to reisolate Fusarium and the reisolated Fusarium isolates were identified by morphological and molecular methods as previously described to fulfill Koch’s postulates. The pathogencity tests were conducted twice.
Results
Identification of Fusarium species and phylogenetic analysis
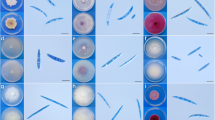
Based on their morphological characteristics, 307 isolates obtained from infected sugar beet root samples were identified as Fusarium species. The products obtained from the amplification of rDNA ITS and EF-1α were approximately 570 and 710 bp, respectively. Morphological characteristics, such as the appearance of the mycelia, growth rate, pigmentation, and sporodochia formation, varied among isolates within the same species, particularly for F. oxysporum, F. brachygibbosum, F. graminearum, F. tricinctum, and F. solani (Supplementary Figs. 1, 2). F. oxysporum strains varied the most in morphology when cultured on PDA. The combined morphological and rDNA ITS and EF-1α sequence analyses indicated that the 307 isolates belonged to 11 species of Fusarium: F. oxysporum, F. solani, F. equiseti, F. tricinctum, F. brachygibbosum, F. redolens, F. proliferatum, F. graminearum, F. verticillioides, F. nygamai, and F. culmorum.
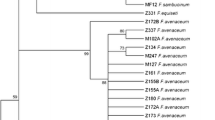
Sequence data obtained for rDNA ITS and EF-1α from 117 of the selected isolates, along with 11 reference sequences retrieved from GenBank were used to conduct a phylogenetic analysis. The phylogenetic analysis produced 11 well-defined clades in the maximum likelihood (ML) tree (Fig. 1). Each clade corresponded to a previously described species. F. culmorum and F. graminearum clustered in a single clade; F. nygamai and F. proliferatum clustered in another single clade. F. oxysporum clustered into two subgroups. F. oxysporum isolates from specific locations, such as NM1 and F138 isolates that were obtained from Inner Mongolia and Xinjiang Uygur, respectively, clustered in a single clade. In contrast, other F. oxysporum isolated clustered in different subclades, even though they were collected from the same region. For example, H57 and HL4 isolates from Heilongjiang Province clustered into two different subclades which was strongly supported in the ML tree (100%), indicating that there was no strong relationship between species composition and geographic origin (Fig. 1). Most of the F. solani isolates also clustered in a single subclade (Fig. 1). F. equiseti shared a high similarity to a Fusarium species that is a human pathogen (O’Donnell and Sutton 2009). This finding was based on the sequence alignment constructed from a BLAST query of the FUSARIUM-ID/FUNCBS database. A significant level of intraspecific diversity existed in the F. equiseti clade as evidenced by the low support of this clade in the analysis using the ML method (Fig. 1).
Phylogenetic tree based on combined ITS and EF-1α sequence data for 117 putative isolates of Fusarium using maximum likelihood method. Maximum parsimony bootstrap support values ≥ 70% based on 1,000 replications are shown at the nodes. The tree was rooted to Fusarium begoniae (U61673, AF160293) and 11 reference sequences. Sequences are labeled with a species designation, then the GenBank accession
Frequency of isolation of Fusarium species
The 307 isolates from the eight provinces or autonomous regions in northern China (Table 1) were identified as 11 different Fusarium species that represented the following percentages of the total number of isolates: Fusarium oxysporum (118 isolates, 38.4%), F. solani (64 isolates, 20.9%), F. equiseti (58 isolates, 18.9%), F. tricinctum (18 isolates, 5.9%), F. brachygibbosum (14 isolates, 4.6%), F. redolens (10 isolates, 3.3%), F. proliferatum (10 isolates, 3.3%), F. graminearum (7 isolates, 2.3%), F. verticillioides (5 isolates, 1.6%), F. nygamai (2 isolates, 0.7%), and F. culmorum (1 isolate, 0.3%) (Table 1). F. oxysporum was isolated at a much higher frequency than any of the other species, followed by F. solani and F. equiseti. The remaining Fusarium species were isolated less frequently (< 6%). F. culmorum was found in only one field within one region in Gansu Province. F. oxysporum, F. solani, and F. equiseti, the three most prevalent species, were also the most abundant species isolated in Heilongjiang, Xinjiang Uygur, Inner Mongolia, and Hebei, the main sugar beet producing provinces or autonomous regions in China (Table 1).
Pathogenicity tests
The identified Fusarium species all induced root rot symptoms, similar to natural infections, on sugar beet plants by 6 weeks after inoculation. Preliminary symptoms showed internal vascular discoloration on taproot. Then, the rot usually started from the root tip and developed as a black discoloration in infected parts. In some severely diseased roots, black rot was visible on the root surface, and the whole taproot was necrotic and visually destroyed (Fig. 2). In contrast, control plants that had been immersed in sterile SNB media remained healthy (Fig. 2). Fusarium isolates were consistently recovered from the diseased roots and identified as the species that had been used to inoculate the plants, thus fulfilling Koch’s postulates.
Symptoms of root rot in sugar beet caused by Fusarium isolates. Arrows indicate lesions on the taproots of sugar beet. a, f Healthy control plant, b H7, F. solani from Heilongjiang Province, collected in 2009, c S2, F. oxysporum from Shanxi Province, collected in 2010, d F51, F. oxysporum from Xinjiang Uygur Autonomous Region, collected in 2011, e NM1, F. oxysporum from Inner Mongolia Autonomous Region, collected in 2012; g XJ15, F. proliferatum from Xinjiang Uygur Autonomous Region, collected in 2012; h F43, F. brachygibbosum from Heilongjiang Province, collected in 2011; i H48, F. redolens from Heilongjiang Province, collected in 2009; j F69, F. solani from Heilongjiang Province, collected in 2011
The average disease incidence established by each of the 11 Fusarium species was above 80%, ranging from 84.2 to 100.0% (Table 2). No significant differences in disease incidence were observed between the different Fusarium species. The average disease index for the 11 Fusarium species ranged from 41.94 to 75.83 (Table 2). In pairwise comparisons of the disease index for the three most abundant species (F. oxysporum, F. solani, and F. equiseti), the disease index did not differ significantly betweeen F. oxysporum and F. solani, but it did differ significantly between F. equiseti, which had a much lower average disease index, and F. oxysporum and F. solani. The disease index for F. verticillioides and for F. brachygibbosum did not differ significantly compared to the other Fusarium species.
Diseased plants inoculated with the different Fusarium species developed root rot symptoms along with foliar yellowing. Entire plants wilted when disease was severe. The wilting, however, seemed like a secondary symptom, brought about by the loss of xylem function, rather than a primary symptom. In addition, the virulence of F. solani was equivalent to that of F. oxysporum; the resultant disease incidence or index did not differ between F. solani and F. oxysporum (Table 2). Given the proportion of F. solani among the isolates, its presence is regarded as a novel finding and an important aspect of the root rot disease of sugar beet in China. It was also interesting that the top three most virulent isolates were all collected from Heilongjiang Province, a traditional sugar beet production area that often suffers from outbreaks of root rot disease.
Discussion
Although root rot in sugar beets was first described by Martyn et al. (1989), the disease has not been widely studied and characterized in China. The present study is the first to identify Fusarium species associated with sugar beet root rot from among an extensive collection of samples obtained from the main sugar beet production areas in China. The results indicated a greater diversity of Fusarium species than previously recognized (Harveson and Rush 1998; Liu et al. 1997; Nitschke et al. 2009; Zhao et al. 2002). This detailed report is the first to demonstrate that F. tricinctum, F. brachygibbosum, F. redolens, F. proliferatum, F. nygamai, and F. culmorum cause root rot of sugar beet in China.
Two formae speciales of F. oxysporum on sugar beet have been described in the literature (Hanson et al. 2009; Harveson et al. 2009; Martyn et al. 1989); one is F. oxysporum f. sp. radicis-betae causing Fusarium root rot, and the other is F. oxysporum f. sp. betae causing Fusarium yellows. Since diseased plants with root rot symptoms were collected in the field to recover Fusarium isolates in this study, the formae specialis of F. oxysporum might be attributed to F. oxysporum f. sp. radicis-betae.
Fusarium species can infect several hosts in China, including wheat (Zhang et al. 2012), potato (Du et al. 2012), banana (Li et al. 2013), sugarcane (Lin et al. 2014), and linseed (Yuan et al. 2013), which are mainly infected by F. graminearum, F. sambucinum, F. oxysporum, F. verticillioides, and F. oxysporum, respectively. Wheat, potato, and linseed are the most common crops that are used as part of a rotation with sugar beets in the main sugar beet production areas in northern China. The Fusarium species isolated from sugar beet have also been reported to be pathogenic in wheat (Christ et al. 2011), dry edible bean, and onion (Webb et al. 2013). Results of a recent study also indicated that when sugar beets are planted before wheat, there may be an increased risk of Fusarium crown and foot rot in wheat (Tillmann et al. 2017). Therefore, cross-pathogenic tests between Fusarium species isolated from sugar beet and common rotation crops need to be conducted to identify crops that can be used as part of a crop rotation and serve as non-hosts or reduced risk crops.
F. oxysporum was reported to be more frequently isolated from soybean during the early stages of growth, while F. solani was more frequently isolated from reproductive stages than it was from vegetative stages (Farias et al. 1989; French and Kennedy 1963; Grant et al. 1981; Killebrew et al. 1993). Similarly, Fusarium can cause seedling damping off and root rot in sugar beet, where the main causal agents for damping off are F. lateritium, F. xylarioides, and F. camptocearas (Abo-Elnaga 2012), and the main causal agents for root rot are F. oxysporum, F. solani, and F. equiseti. The diversity, epidemiology, and impact of the pathogens that cause damping off and root rot in sugar beet need to be more thoroughly investigated in future studies.
The main focus in controlling root rot in sugar beet in China has been F. oxysporum. The diversity of Fusarium species associated with root rot in sugar beet in China has been significantly underestimated. Our study indicated that F. solani (20.9%) and F. equiseti (18.9%) were abundant enough to demand greater attention, especially for developing effective management strategies and screening relevant fungicides. Since different Fusarium species can also produce various mycotoxins, the toxicological risk in sugar beet residues, based on species diversity, should also be evaluated in future studies.
References
Abo-Elnaga HIG (2012) Biological control of damping off and root rot of wheat and sugar beet with Trichoderma harzianum. Plant Pathol J 11:25–31
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Arabiat S, Khan MFR, Chanda AK (2017) First report of Fusarium yellowing decline on sugar beet (Beta vulgaris) caused by Fusarium secorum in Montana, USA. Plant Dis 101:1328
Christ DS, Gödecke R, von Tiedemann A, Varrelmann M (2011) Pathogenicity, symptom development, and mycotoxin formation in wheat by Fusarium species frequently isolated from sugar beet. Phytopathology 101:1338–1345
Du M, Ren X, Sun Q, Wang Y, Zhang R (2012) Characterization of Fusarium spp. causing potato dry rot in China and susceptibility evaluation of Chinese potato germplasm to the pathogen. Potato Res 55:175–184
Dunnett CW (1955) A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc 50:1096–1121
FAOSTAT (FAO Statistical Division) (2016) Crops. http://www.fao.org/faostat/en/#data/QC. Accessed 28 Dec 2017
Farias GM, Griffin GJ (1989) Roles of Fusarium oxysporum and F. solani in Essex disease of soybean in Virginia. Plant Dis 73:38–42
Fong YK, Anuar S, Lim HP, Tham FY, Sanderson FR (2000) A modified filter paper technique for long-term preservation of some fungal cultures. Mycologist 14:127–130
Francis SA, Luttebacher MC (2003) Identification and exploitation of novel disease resistance genes in sugar beet. Pest Manag Sci 59:225–230
French ER, Kennedy BW (1963) The role of Fusarium in the root rot complex of soybean in Minnesota. Plant Dis Rept 47:672–676
Geiser DM, del M Jiménez-Gasco, Kang M, Makalowska S, Veeraraghavan I, Ward N, Zhang TJ, Kuldan N, O’donnell GAK (2004) FUSARIUM-ID v. 1.0: a DNA sequence database for identifying Fusarium. Eur J Plant Pathol 110:473–479
Grant CE, Phipps PM, Roane CW (1981) Etiology of a damping-off disease of soybeans in Virginia. (Abstract) Phytopathology 71:767
Hanson LE, Jacobsen BJ (2006) Beet root-rot inducing isolates of Fusarium oxysporum from Colorado and Montana. Plant Dis 90:247
Hanson LE, Lewellen RT (2007) Stalk rot of sugar beet caused by Fusarium solani on the Pacific coast. Plant Dis 91:1204
Hanson LE, Hill AL, Jacobsen BJ, Panella L (2009) Response of sugarbeet lines to isolates of Fusarium oxysporum f. sp. Betae from the United States. J Sugar Beet Res 46:11–26
Hanson L, Lucchi CD, Stevanato P, McGrath M, Panella L, Sella L, Biaggi MD, Concheri G (2018) Root rot symptoms in sugar beet lines caused by Fusarium oxysporum f. sp. betae. Eur J Plant Pathol 150:589–593
Harveson RM, Rush CM (1998) Characterization of Fusarium root rot isolates from sugar beet by growth and virulence at different temperatures and irrigation regimes. Plant Dis 82:1039–1042
Harveson RM, Hanson LE, Hein GL (eds) (2009) Compendium of beet diseases and pests, 2nd edn. American Phytopathological Society Press, St. Paul, MN, USA
Hill AL, Reeves PA, Larson RL, Fenwick AL, Hanson LE, Panella L (2011) Genetic variability among isolates of Fusarium oxysporum from sugar beet. Plant Pathol 60:496–505
Hsu JC (1992) The factor analytic approach to simultaneous inference in the general linear model. J Comput Gr Stat 1:151–168
Killebrew JF, Roy KW, Abney TS (1993) Fusaria and other fungi on soybean seedlings and roots of older plants and interrelationships among fungi, symptoms, and soil characteristics. Can J Plant Pathol 15:139–146
Leslie JF, Summerell BA (eds) (2006) The Fusarium laboratory manual. Blackwell Publishing, Ames, IA, USA
Li C, Zuo C, Deng G, Kuang R, Yang Q, Hu C, Sheng O, Zhang S, Ma L, Wei Y, Yang J, Liu S, Biswas MK, Viljoen A, Yi G (2013) Contamination of bananas with beauvericin and fusaric acid produced by Fusarium oxysporum f. sp. cubense. PLoS One 8:e70226
Lin Z, Xu S, Que Y, Wang J, Comstock JC, Wei J, McCord PH, Chen B, Chen R, Zhang M (2014) Species-specific detection and identification of Fusarium species complex, the causal agent of sugarcane pokkah boeng in China. PLoS One 9:e104195
Liu JX, Xian HQ, Liu GL (1997) Study on agricultural control of sugar beet root rot. Sugar Crop China 1:14–19 (in Chinese)
Martyn RD, Rush CM, Biles CL, Baker EH (1989) Etiology of a root rot disease of sugar beet in Texas. Plant Dis 73:879–884
Mulè G, Susca A, Stea G, Moretti A (2004) A species-specific PCR assay based on the calmodulin partial gene for identification of Fusarium verticillioides, F. proliferatum and F. subglutinans. Eur J Plant Pathol 110:495–502
Nelson PE, Toussoun TA, Marasas WFO (eds) (1983) Fusarium species: an illustrated manual for identification. Pennsylvania State University Press, University Park, PA, USA
Nitschke E, Nihlgard M, Varrelmann M (2009) Differentiation of eleven Fusarium spp. isolated from sugar beet, using restriction fragment analysis of a polymerase chain reaction-amplified translation elongation factor 1α gene fragment. Phytopathology 99:921–929
O’Donnell K, Cigelnik E (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenet Evol 7:103–116
O’Donnell K, Sutton DM (2009) Novel multilocus sequence typing scheme reveals high genetic diversity of human pathogenic members of the Fusarium incarnatum-F. equiseti and F. chlamydosporum species complexes within the United States. J Clin Microbiol 47:3851–3861
Ruppel EG (1991) Pathogenicity of Fusarium spp. from diseased sugar beets and variation among sugar beet isolates of F. oxysporum. Plant Dis 75:486–489
Secor GA, Rivera-Varas V, Christ DS, Mathew FM, Khan MFR, Varrelmann M, Bolton MD (2014) Characterization of Fusarium secorum, a new species causing Fusarium yellowing decline of sugar beet in North Central USA. Fungal Biol 118:764–775
Tillmann M, von Tiedemann A, Winter M (2017) Crop rotation effects on incidence and diversity of Fusarium species colonizing stem bases and grains of winter wheat. J Plant Dis Protect 124:121–130
Webb KM, Case AJ, Brick MA, Otto K, Schwartz HF (2013) Cross pathogenicity and vegetative compatibility of Fusarium oxysporum isolated from sugar beet. Plant Dis 97:1200–1206
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, NY, USA, pp 315–322
Yuan L, Mi N, Liu S, Zhang H, Li Z (2013) Genetic diversity and structure of the Fusarium oxysporum f. sp. lini populations on linseed (Linum usitatissimum) in China. Phytoparasitica 41:391–401
Zhang H, Van der Lee T, Waalwijk C, Chen W, Xu J, Xu J, Zhang Y, Feng J (2012) Population analysis of the Fusarium graminearum species complex from wheat in China show a shift to more aggressive isolates. PLoS One 7:e31722
Zhao SF, Li GY, Li H, Wang QY (2002) Identification of pathogen population of sugar beet root rot disease in Xinjiang Uygur autonomous region. Sugar Crop China 1:3–8 (in Chinese)
Acknowledgements
This work was supported by the earmarked fund for China Agriculture Research System (CARS-170304). Mention of trade names or commercial products in this report is solely for the purpose of providing specific information and does not imply recommendation or endorsement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10327_2018_792_MOESM2_ESM.tif
Morphological characteristics (colony, conidia, and sporulation) of Fusarium oxysporum, F. solani, F. equiseti, F. tricinctum, F. brachygibbosum, and F. redolens (TIF 9521 KB)
10327_2018_792_MOESM3_ESM.tif
Morphological characteristics (colony, conidia, and sporulation) of Fusarium proliferatum, F. graminearum, F. verticillioides, F. nygamai, and F. culmorum (TIF 9524 KB)
Rights and permissions
About this article
Cite this article
Cao, S., Yang, N., Zhao, C. et al. Diversity of Fusarium species associated with root rot of sugar beet in China. J Gen Plant Pathol 84, 321–329 (2018). https://doi.org/10.1007/s10327-018-0792-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-018-0792-5