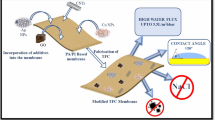
Abstract
Water desalination and recycling of wastewater is a key challenge to meet water shortage issues. Thin film composite polyamide membranes are widely used for desalination; however, their low permeability due to a poor hydrophilicity is a major drawback. Here, we designed novel thin film composite membranes having good hydrophilicity, permeability, and stability without compromising solute rejection. We improved the membrane hydrophilicity by incorporation of hydrophilic additives, such as glycine and l-glutamine, into the polyamide layer. Hence polyamide-based flat sheet membranes were fabricated via interfacial polymerization of m-phenylenediamine and trimesoyl chloride and then were coated over a polysulfone/sulfonated polyphenylsulfone (85:15) support. Polyamide membranes were then characterized and tested for desalination. Results show that the ridge and valley structure observed by scanning electron microscopy confirms the formation of the polyamide layer on membrane surface. The performance reached the highest pure water flux of 36.23 Lm−2 h−1 and flux recovery ratio of 89.18% for membranes with 2 wt% of l-glutamine. Incorporation of 2 wt% l-glutamine induced a high permeate flux and a maximum rejection of 87.87% for MgSO4, 83.50% for Na2SO4 and 60.77% for NaCl solutions. Overall, the polyamide nanofiltration membrane with hydrophilic groups displayed superior antifouling property and can be used as a potential candidate for desalination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid urbanization and industrialization have resulted in a severe scarcity of the clean potable water affecting the global economy, environment and normal health of the society. Providing an adequate supply of clean water to the society and industries at a reasonable cost is a major challenge to the municipal corporations. Recycling and reuse of the contaminated sewage/industrial water are one of the solutions for the increased water crises. However, in the long run, the effective and most economical source of water is seawater desalination. Different techniques are in practice across the globe for obtaining the potable water from different sources, namely adsorption, distillation, freezing, chemical treatment, electrodialysis and membrane technology (Adeniyi et al. 2016; Madhura et al. 2018). Among these, membrane technology has been proved as the most economical, eco-friendly and cost-effective as compared to any other techniques (Hebbar et al. 2017a, b; Crini and Lichtfouse 2018).
Desalination is the conventional process that removes salts from the saline water, which is a solution to the scarcity of potable water. Brackish and seawater desalination using thin film composite membranes is richly explored for the reverse osmosis and nanofiltration processes (Agboola et al. 2014; Zhao and Ho 2014). Polyamide membranes are generally fabricated by interfacial polymerization of an amine and acid chloride, for example, m-phenylenediamine and trimesoyl chloride (Hermans et al. 2014). However, the highly cross-linked network formed by m-phenylenediamine and trimesoyl chloride may lead to poor hydrophilicity that results in a significantly inferior water flux for nanofiltration membranes. Therefore, increasing the hydrophilicity of polyamide membranes has been a major concern in recent decades.
Many researchers have been attempted to modify polyamide layer or the porous substrate to produce thin film composite membrane with improved performance in terms of permeability, rejection and antifouling nature (An et al. 2013; Al Mayyahi and Deng 2018). Hence, tailoring of polyamide membranes based on the required properties for water purification can be brought about by changing the monomer or its concentration, usage of the hydrophilic substrate, by the functionalization of polyamide layer and by incorporation of hydrophilic additives. Various additives have been used in thin films and/or substrate to improve the membrane properties (Zhao et al. 2013; Nigiz 2018; Pandian et al. 2015).
In this work, organic molecules such as glycine and l-glutamine are used as new hydrophilic additives to improve the performance of thin film composite membranes. Glycine and l-glutamine are non-toxic amino acids with aromatic moiety and hydrophilic functional groups such as carboxylic acids and amides (Pandian et al. 2015). Introduction of hydrophilic and negatively charged carboxyl groups on the membrane surface by the incorporation amino acids as additives contributes to the improvement in membrane properties and performance. Moreover, the presence of the polar functional group aids its easy dissolution in aqueous m-phenylenediamine solution. The effect of these additives and its concentration on formation of polyamide membranes by the interfacial polymerization of m-phenylenediamine and trimesoyl chloride on polysulfone/sulfonated polyphenylsulfone substrate was studied. The membrane performance was analyzed by permeability, selectivity and fouling study.
Experimental
Materials
Polysulfone/sulfonated polyphenylsulfone membrane (85:15, water in coagulation bath) was fabricated as in the previously reported work and chosen as the substrate for the thin film composite membranes (Moideen et al. 2018). m-Phenylenediamine was procured from Sigma-Aldrich, India. N-methyl-2-pyrrolidone, H2SO4, glycine, NaCl, MgSO4 and Na2SO4 were procured from Merck India. Bovine serum albumin [Molecular weight (Mw) ~69 kDa], trimesoyl chloride and l-glutamine were purchased from Alfa Aesar (98% purity).
Preparation of thin film composite membranes
Polysulfone/sulfonated polyphenylsulfone substrate fixed on a glass plate was impregnated in an amine solution (Fig. 1a), and the solution was drained off after 15 min. The interfacial polymerization reaction was initiated by dipping impregnated membrane substrate in 0.1 wt% trimesoyl chloride in hexane for 1 min. The excess trimesoyl chloride solution was drained off and dried at room temperature. Subsequently, the membranes were cured for 10 min at 50 °C, washed and stored in water (Hermans et al. 2015). The procedure for membrane characterization by Fourier transform infrared spectroscopy, scanning electron microscope, atomic absorption spectroscopy, zeta potential analyzer, contact angle studies and water uptake capacity were followed according to the literature (Pereira et al. 2015; Hebbar et al. 2017a).
a The composition of amine solution used in preparation of thin film composite membrane series. b Plausible synthetic scheme for polyamide layer formation by reaction m-phenylenediamine and trimesoyl chloride (1) without any additives (2) on addition of glycine and (3) l-glutamine to the amine solution
Membrane performance study
The filtration and antifouling study of membranes were conducted using laboratory scale dead-end filtration setup at 0.8 MPa trans membrane pressure (Pereira et al. 2015). The flux (\(J_{\text{w}}\)) and rejection (R) of water, salt solution (1000 ppm) such as NaCl, Na2SO4 and MgSO4 were analyzed and calculated using the equations given below.
where ‘Q’ is the volume of permeate collected for a definite time interval ‘\(\Delta t\)’ through the effective area of membrane ‘A.’ ‘Cf’ and ‘Cp’ are the concentration of feed and permeate solution (gL−1), respectively.
The antifouling behavior of the membranes was carried out by passing pure water (Jw1) (Lm−2 h−1) for 80 min followed by 1000 ppm of bovine serum albumin solution (Jp). Later, the membranes were backwashed with clean water for 20 min, and then, the pure water flux (Jw2) of the cleaned membrane was measured. The extent of fouling by the membranes was estimated by calculating the flux recovery ratio, reversible fouling and irreversible fouling ratio using the following equation,
Results and discussion
Membrane characterization
Figure 1b demonstrates the reaction scheme for the interfacial polymerization of the amine (NH2) of m-phenylenediamine and acyl chloride (–COCl) of trimesoyl chloride through an amide linkage (CO–NH) with the elimination of HCl. The incorporation of hydrophilic additives such as glycine and l-glutamine into the aqueous phase containing m-phenylenediamine generates hydrophilic active sites on the polyamide layer formed by the reaction of NH2 group present in additive with –COCl of trimesoyl chloride.
The amide linkage formation was confirmed using Fourier transform infrared spectroscopy by the presence of a vibration peak around 3300 cm−1 and 1630–1660 cm−1 for –NH stretching and –C=O stretching, respectively (Fig. 2a). The generation of hydrophilic active sites on the incorporation of additives was detected by the vibrational peak around 1700 cm−1 for the carbonyl group of carboxylic acid present in the additives.
The zeta potential of the membrane surface was analyzed to determine the surface charge on the polyamide membrane. The prepared membranes showed isoelectric point within the pH range of 3.5–4.0. The zeta potential at pH 9 where complete deprotonation of the carboxylic acid group is likely to take place was found to be of − 38 mV, − 60 mV and − 62 mV for C1, C3 and C5, respectively. Hence, it confirms the presence of hydrophilic carboxylic acid groups on the polyamide layer. The increased number of carboxylic groups also facilitated the improvement of the surface charge, hydrophilicity, flux and rejection.
The roughness of the membrane surface was demonstrated by the presence of the peaks and valleys (Fig. 2b). The roughness parameters decreased on the incorporation of hydrophilic additives in the aqueous phase, as the incorporation of additives slows down the vigorous reaction between m-phenylenediamine and trimesoyl chloride (Zhao and Ho 2014). The average roughness reduced from 93.37 (C1) to 37.48 (C3) and 30.49 (C5) nm, whereas mean square roughness reduced from 113.60 (C1) to 49.68 (C3) and 39.05 (C5) nm on the addition of glycine and l-glutamine, respectively.
The scanning electron microscope images displayed the development of polyamide layer by the formation of a ‘ridge-and-valley’ morphology over the smooth surface of the substrate (Fig. 3). The ‘ridge-and-valley’ morphology of thin film composite membranes showed white areas depicting the ridges and the black areas for the valley, which became less pronounced as the additive concentration is increased, thereby resulting in a smoother surface. The defect-free polyamide layer formation can also be observed from the surface image. It is the presence of hydrophilic sulfonic group on the membrane substrate that triggers the uniform diffusion of m-phenylenediamine (Zhang et al. 2016).
Membrane hydrophilicity
Water contact angels and water uptake capacity of polyamide membranes demonstrated the membrane hydrophilicity (Fig. 4a). The decrease in contact angle and increase in water uptake capacity of the membrane on the incorporation of glycine and l-glutamine to amine solution showed an increase in the surface hydrophilicity. The carboxylic group present in the additives enhances the interaction of the polyamide layer and water by the formation of a hydration layer over the active layer of the membrane. Moreover, additional –NH2 groups present in the polyamide layer by incorporating l-glutamine resulted in higher hydrophilicity than the rest of the prepared membranes.
a Water contact angle and water uptake capacity, b permeate flux of water and salt solution, c rejection of MgSO4, Na2SO4 and NaCl during the desalination of feed solution studied separately and d fouling behavior and reusability of prepared membranes were studied by calculating the flux recovery ratio, reversible and irreversible fouling value at 0.8 MPa
Filtration study
The nanofiltration behavior of the thin film composite membranes was studied by measuring the permeate flux and salt rejection. The thin film composite membranes followed Donnan and size exclusion mechanism in its transport pathway, which makes them highly selective for the charge and size, respectively (Bera and Jewrajka 2016). There was a significant rise in pure water flux observed with the addition of hydrophilic additives in the aqueous amine solution. This is because of the improved surface hydrophilicity brought about by the functionalization of polyamide layer by the carboxylic group in hydrophilic additives. The permeate flux of salt solutions was recorded and a similar trend was observed (Fig. 4b). The permeate flux for salt solutions is in the order: NaCl > MgSO4 > Na2SO4, which is in accordance with the size of the salt ions. Therefore, the highest flux of 36.23, 14.47, 14.96 and 21.44 Lm−2 h−1 was shown by C5 membrane for pure water, Na2SO4, MgSO4 and NaCl solution, respectively.
Figure 4c shows the order of salt rejection as MgSO4 > Na2SO4 > NaCl. The rejection of salt by the thin film composite membrane is based on the Donnan exclusion principle, which functions on the basis of charge on the feed constituents and membranes. The figure indicates a higher rejection of MgSO4 and Na2SO4 than NaCl, as membrane surface showed strong electrostatic repulsion for divalent \({\text{SO}}_{4}^{2 - }\) than the monovalent \({\text{Cl}}^{ - }\) present in NaCl. The rejection of MgSO4 is observed to be highest, as the divalent Mg2+ showed stronger binding than monovalent Na+ due to their superior electrophilic nature than the monovalent cations (Remko et al. 2010). Salt rejection ability of thin film composite membrane improved on the incorporation of hydrophilic additives into the amine solution. This is because of net negative surface charge developed by functionalization of the polyamide layer with the carboxylic acid group present in glycine and l-glutamine. Thus, electrostatic interactions between the salt ions and negatively charged membranes caused the rejection of salt ions based on the Donnan exclusion principle. Moreover, the addition of l-glutamine showed higher rejection due to the collective effect of \(- {\text{COO}}^{ - }\) groups and an additional –NH2 group present in l-glutamine with the highest rejection of 87.87, 83.50 and 60.77% for MgSO4, Na2SO4 and NaCl, respectively, for C5 membrane. Hence, the results reveal that the polyamide membranes with hydrophilic additives displayed enhanced permeability without compromising the solute rejection, which is comparable with the results reported by Bera and Jewrajka (2016) and Tang et al. (2009).
Antifouling study
Thin film composite membranes showed good flux recovery ratio, which further increased with the incorporation of the hydrophilic additive into amine solution (Fig. 4d). The membrane with l-glutamine additive showed better antifouling than that with glycine as the additive, with the highest flux recovery ratio of 89.18%. The improved fouling resistance is attributed to the higher hydrophilicity of l-glutamine additive. Moreover, the irreversible fouling could be brought down from 23.33 to 10.81% by the incorporation of additives into the amine solution. This is because, the incorporation of hydrophilic additives functionalizes polyamide surface with –COOH, thereby elevating the negative surface charge. This, in turn, attributed to the strong electrostatic repulsion between the negatively charged bovine serum albumin molecules and membrane surface. The carboxyl group present on the membrane surface resulted in the formation of hydration layer that makes it difficult for the foulant to adhere on the membrane surface and aids easy removal of adsorbed foulant by simple hydraulic washing.
Conclusions
Thin film nanocomposite nanofiltration membranes were fabricated by the interfacial polymerization of m-phenylenediamine and trimesoyl chloride, on the polysulfone/sulfonated polyphenylsulfone membranes as substrate. The effects of glycine and l-glutamine as hydrophilic additives on membrane performance were studied. The additives functionalized the membrane surface with –COOH groups, which enhanced its hydrophilicity and electrostatic charge. The membrane performance improved with an increase in the additive concentration as the number of –COOH group over the surface also increased. The incorporation of hydrophilic additives resulted in membranes with the superior antifouling property, where irreversible fouling declined from 23.33 to 10.81%. Therefore, the membrane with 2 wt% of l-glutamine was found to be the best performing membrane among the series of thin film composite membranes, with a good salt rejection capability of 87.87, 83.50 and 60.77% for MgSO4, Na2SO4 and NaCl, respectively, and highest water flux of 36.23 Lm−2 h−1. Overall, the polyamide membranes with an active layer of hydrophilic groups on its surface displayed good permeability and can be used as a potential candidate for the desalination application.
References
Adeniyi A, Mbaya RKK, Onyango MS, Maree JP (2016) Efficient suspension freeze desalination of mine wastewaters to separate clean water and salts. Environ Chem Lett 14(4):449–454. https://doi.org/10.1007/s10311-016-0562-6
Agboola O, Maree J, Mbaya R (2014) Characterization and performance of nanofiltration membranes. Environ Chem Lett 12(2):241–255. https://doi.org/10.1007/s10311-014-0457-3
Al Mayyahi A, Deng B (2018) Efficient water desalination using photo-responsive ZnO polyamide thin film nanocomposite membrane. Environ Chem Lett. https://doi.org/10.1007/s10311-018-0758-z
An QF, Sun WD, Zhao Q, Ji YL, Gao CJ (2013) Study on a novel nanofiltration membrane prepared by interfacial polymerization with zwitterionic amine monomers. J Membr Sci 431:171–179. https://doi.org/10.1016/j.memsci.2012.12.043
Bera A, Jewrajka SK (2016) Tailoring polyamide thin film composite nanofiltration membranes by polyethyleneimine and its conjugates for the enhancement of selectivity and antifouling property. RSC Adv 6(6):4521–4530. https://doi.org/10.1039/C5RA21941H
Crini G, Lichtfouse E (2018) Advantages and disadvantages of techniques used for wastewater treatment. Environ Chem Lett. https://doi.org/10.1007/s10311-018-0785-9
Hebbar RS, Isloor AM, Ananda K, Abdullah MS, Ismail A (2017a) Fabrication of a novel hollow fiber membrane decorated with functionalized Fe2O3 nanoparticles: towards sustainable water treatment and biofouling control. New J Chem 41(10):4197–4211. https://doi.org/10.1039/C7NJ00221A
Hebbar RS, Isloor AM, Inamuddin, Asiri AM (2017b) Carbon nanotube-and graphene-based advanced membrane materials for desalination. Environ Chem Lett 15(4):643–671. https://doi.org/10.1007/s10311-017-0653-z
Hermans S, Mariën H, Dom E, Bernstein R, Vankelecom IF (2014) Simplified synthesis route for interfacially polymerized polyamide membranes. J Membr Sci 451:148–156. https://doi.org/10.1016/j.memsci.2013.10.005
Hermans S, Bernstein R, Volodin A, Vankelecom IF (2015) Study of synthesis parameters and active layer morphology of interfacially polymerized polyamide–polysulfone membranes. React Funct Polym 86:199–208. https://doi.org/10.1016/j.reactfunctpolym.2014.09.013
Madhura L, Kanchi S, Sabela MI, Singh S, Bisetty K (2018) Membrane technology for water purification. Environ Chem Lett 16(2):343–365. https://doi.org/10.1007/s10311-017-0699-y
Moideen IK, Isloor AM, Qaiser AA, Ismail AF, Abdullah MS (2018) Separation of heavy metal and protein from wastewater by sulfonated polyphenylsulfone ultrafiltration membrane process prepared by glycine betaine enriched coagulation bath. Korean J Chem Eng 35(6):1281–1289. https://doi.org/10.1007/s11814-018-0018-8
Nigiz FU (2018) Complete desalination of seawater using a novel polyvinylidene fluoride/zeolite membrane. Environ Chem Lett 16(2):553–559. https://doi.org/10.1007/s10311-017-0684-5
Pandian S, Katha AR, Moon JH, Kolake SM, Han S (2015) Exploring the effect of additives on polyamide membrane surface for seawater desalination using density functional tools. Desalination 367:28–36. https://doi.org/10.1016/j.desal.2015.03.034
Pereira VR, Isloor AM, Al Ahmed A, Ismail A (2015) Preparation, characterization and the effect of PANI coated TiO2 nanocomposites on the performance of polysulfone ultrafiltration membranes. New J Chem 39(1):703–712. https://doi.org/10.1039/C4NJ01594K
Remko M, Fitz D, Rode BM (2010) Effect of metal ions (Li+, Na+, K+, Mg2+, Ca2+, Ni2+, Cu2+ and Zn2+) and water coordination on the structure and properties of l-histidine and zwitterionic l-histidine. Amino Acids 39(5):1309–1319. https://doi.org/10.1007/s00726-010-0573-8
Tang CY, Kwon YN, Leckie JO (2009) Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes: II. Membrane physiochemical properties and their dependence on polyamide and coating layers. Desalination 242(1–3):168–182. https://doi.org/10.1016/j.desal.2008.04.003
Zhang X, Lv Y, Yang HC, Du Y, Xu ZK (2016) Polyphenol coating as an interlayer for thin-film composite membranes with enhanced nanofiltration performance. ACS Appl Mater Interfaces 8(47):32512–32519. https://doi.org/10.1021/acsami.6b10693
Zhao L, Ho WW (2014) Novel reverse osmosis membranes incorporated with a hydrophilic additive for seawater desalination. J Membr Sci 455:44–54. https://doi.org/10.1016/j.memsci.2013.12.066
Zhao L, Chang PCY, Ho WW (2013) High-flux reverse osmosis membranes incorporated with hydrophilic additives for brackish water desalination. Desalination 308:225–232. https://doi.org/10.1016/j.desal.2012.07.020
Acknowledgements
AMI is thankful to the Director, NITK Surathkal, India, for providing the research facilities. Authors also thank the head of Metallurgical and Materials Engineering and Chemical engineering department of NITK Surathkal, India, for the technical support. AMI also thank, Karnataka State Council for Science and Technology for the prestigious ‘Sir C.V. Raman’ young scientist award.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kolangare, I.M., Isloor, A.M., Inamuddin et al. Improved desalination by polyamide membranes containing hydrophilic glutamine and glycine. Environ Chem Lett 17, 1053–1059 (2019). https://doi.org/10.1007/s10311-018-00825-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-018-00825-1