Abstract
(3R)-Acetoin and (2R,3R)-2,3-butanediol are important pharmaceutical intermediates. However, until now, the quantity of natural microorganisms with the ability to produce single configuration of optically pure (3R)-acetoin and (2R,3R)-2,3-butanediol is rare. In this study, a meso-2,3-butanediol dehydrogenase encoded by the slaC gene from Serratia marcescens MG1 was identified for meso-2,3-butanediol and (2S,3S)-2,3-butanediol biosynthesis. Inactivation of the slaC gene could significantly decrease meso-2,3-butanediol and (2S,3S)-2,3-butanediol and result in a large quantity of (3R)-acetoin accumulation. Furthermore, a (2R,3R)-2,3-butanediol dehydrogenase encoded by the bdhA gene from Bacillus subtilis 168 was introduced into the slaC mutant strain of Serratia marcescens MG1. Excess (2R,3R)-2,3-butanediol dehydrogenase could accelerate the reaction from (3R)-acetoin to (2R,3R)-2,3-butanediol and lead to (2R,3R)-2,3-butanediol accumulation. In fed-batch fermentation, the excess (2R,3R)-2,3-butanediol dehydrogenase expression strain could produce 89.81 g/l (2R,3R)-2,3-butanediol with a productivity of 1.91 g/l/h at 48 h. These results provided potential applications for (3R)-acetoin and (2R,3R)-2,3-butanediol production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As one of the promising bulk chemicals, 2,3-butanediol (2,3-BD) has attracted increasing attention due to its potential industrial application in butadiene and liquid fuel [2, 6, 26]. 2,3-BD contains two stereo centers and has three stereo isomers: (2R,3R)-2,3-BD, meso-2,3-BD and (2S,3S)-2,3-BD [21, 25]. The optically active isomers of 2,3-BD can be used as building blocks for asymmetric synthesis of highly valuable chiral compounds [7]. Due to its low freezing point of −60 °C, optically pure 2,3-BD is also used as an antifreeze agent [21]. Acetoin (AC), the precursor of 2,3-BD, exists in two stereoisomeric forms, (3R)-AC and (3S)-AC, which are widely used to synthesize novel optically active α-hydroxyketone derivatives and liquid crystal composites [17, 18]. In addition, all of the isomers of 2,3-BD and AC are important potential pharmaceutical intermediates [8, 21]. Therefore, production of 2,3-BD and AC with high optical purities is desirable.
AC and 2,3-BD isomers can be produced via the mixed acid fermentation pathway by different native strains such as Klebsiella pneumoniae [1], Klebsiella oxytoca [4], Paenibacillus polymyxa [3], Bacillus amyloliquefaciens [20] and Bacillus licheniformis [15]. Of these, Paenibacillus polymyxa can produce 111 g/l (2R,3R)-2,3-BD with a purity up to 98 % [3]. At the present, the highest production of 150 g/l and 152 g/l 2,3-BD was, respectively, with S. marcescens H30 [24] and K. pneumoniae SDM [9] without considering its isomers. So S. marcescens and K. pneumonia were potential strains to produce optically pure (2R,3R)-2,3-BD with high concentration after the purity was taken into consideration.
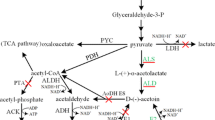
In previous studies, several heterologous hosts such as E. coli [5, 19] and Synechocystis [12] were also metabolically engineered to produce optically pure AC or 2,3-BD. α-Acetolactate, diacetyl and AC are the three main intermediate compounds of 2,3-BD fermentation in bacteria [25]. In general, 2,3-BD can be produced by natural microorgansims as a mixture of 2,3-BD isomers, and the stereoisomeric composition of 2,3-BD formed by bacteria differs among strains, which is related to the existence of various 2,3-butanediol dehydrogenases (BDHs) differing in their stereospecificities [14, 25]. Several BDHs with different stereospecificities have been identified and characterized in previous studies. K. pneumoniae mainly produced meso-2,3-BD with a small amount of (2S,3S)-2,3-BD from (3R)-AC and (3S)-AC due to the existence of meso-BDH [8, 23]. Brevibacterium saccharolyticum could express (2S,3S)-BDH which led to (2S,3S)-2,3-BD production from (3S)-AC [13, 14], whereas (2R,3R)-BDH from Bacillus subtilis and Paenibacillus polymyxa could catalyze (3R)-AC and (3S)-AC into (2R,3R)-2,3-BD and meso-2,3-BD, respectively [10, 22]. So some gene modifications of native strains are necessary for single configuration production of optically pure AC and 2,3-BD.
Previously, we reported an S. marcescens MG1 strain, which mainly produces (3R)-AC and meso-2,3-BD with a small amount of (2S,3S)-2,3-BD and (2R,3R)-2,3-BD from sucrose and demonstrated the potential for industrial-scale (3R)-AC and meso-2,3-BD production [11]. In this present study, we identified a meso-BDH enzyme encoded by the slaC gene in S. marcescens MG1, which was responsible for meso-2,3-BD and (2S,3S)-2,3-BD formation from (3R)-AC and (3S)-AC. Further, inactivation of the slaC gene from S. marcescens MG1 could lead to a large quantity of optically pure (3R)-AC accumulation. The expression of (2R,3R)-BDH from B. subtilis 168 in the slaC mutant strain could accelerate (3R)-AC into (2R,3R)-2,3-BD and result in a high purity of (2R,3R)-2,3-BD production. These results would provide further useful hints for single configuration production of AC and 2,3-BD.
Materials and methods
Enzymes, chemicals and primers
Restriction enzymes, T4 DNA ligase and Taq DNA polymerase were from Takara Biotech (Dalian, China). Bacterial DNA kit, plasmid mini kit, cycle-pure kit and gel extraction kit were purchased from Omega Biotech (Norcross, America). Oligonucleotide primers were synthesized in SBSbio (Shanghai, China). Diacetyl, (3S/3R)-AC, (2S,3S)-2,3-BD (97.0 %), (2R,3R)-2,3-BD (97.0 %) and meso-2,3-BD (99.0 %) were obtained from Sigma–Aldrich (Shanghai, China).
Bacterial strains, plasmids and growth conditions
The strains, plasmids and primers used in this study are listed in Table 1. Luria–Bertani (LB) broth was used for culturing E. coli, S. marcescens MG1 and its derivatives. S. marcescens MG1 was grown at 30 °C, and E. coli was grown at 37 °C. Plasmid pET-28a (+) (Novagen) was used for the meso-BDH expression. Antibiotics were added in the following amounts (per ml) if necessary: 50 μg kanamycin or 100 μg ampicillin.
For fermentation experiments, a full loop of S. marcescens MG1 strains or its derivatives from the slants were inoculated into 250-ml shake flasks containing 30 ml of fresh seed medium and cultivated for 12 h at 200 rpm and 30 °C. Seed culture (5 %, v/v) was then inoculated into 250-ml shake flasks containing 50 ml of fresh fermentation medium, followed by 30 h of incubation at 30 °C on a rotary shaker (200 rpm). The seed medium was composed of (g/l): glucose 10, peptone 2, yeast extract 1, (NH4)2SO4 6, KH2PO4 10, NaCl 0.5 and MgSO4 0.5 at pH 7.2. The fermentation medium was composed of (g/l): sucrose 90, yeast extract 33.36, sodium citrate 10, sodium acetate 4, NH4H2PO4 1, MgSO4 0.3 and MnSO4 0.1 [24].
For fed-batch fermentations, the seed culture was inoculated (5 %, v/v) into the above fermentation medium in a 7-l jar (ALF, Bioengineering, Switzerland) with an initial broth volume of 3 l. The cultivation was carried out at 30 °C with an aeration rate of 1.0 vvm and agitation speed of 500 rpm during the first 15 h of strain growth. After 15 h, the aeration rate and agitation speed were decreased to 0.5 vvm and 300 rpm, respectively. The feeding substrate was pumped into the bioreactor using a computer-coupled peristaltic pump. Sucrose solution (80 %, w/v) was added to 40 g/l into the bioreactor at different time points when the residual sucrose concentration was below 5 g/l. When the pH decreased to 6.0, it was maintained at 6.0 by automatic addition of 4 M KOH using a computer-coupled peristaltic pump.
Construction of the slaC recombinant strain
The ORF of the slaC gene was amplified by PCR with the genomic DNA of S. marcescens MG1 as template using the primer pair slaC-1 and slaC-2, which contain the EcoRI and HindIII restriction sites, respectively. The amplified product was digested and ligated into the vector pET-28a (+) at EcoRI and HindIII sites to generate pET28a-slaC. The recombinant pET28a-slaC vector was transformed into E. coli BL21(DE3) for protein expression.
Enzyme preparation and enzymatic reactions
The recombinant E. coli BL21(DE3)/pET28a-slaC strain was cultivated at 37 °C in a 250-ml flask containing 30 ml LB medium (pH 7.0) with kanamycin (50 μg/ml). The cells were induced at about 0.6 OD600 with 1 mM IPTG and harvested by centrifugation after 5 h. The meso-BDH was purified by Ni-affinity chromatography using a His-trap column according to the purification protocol (GE healthcare, USA).
The enzymatic reactions were carried out following a previously described method [25]. Briefly, for the oxidation reactions, a mixture containing 100 mM substrates (meso-2,3-BD, (2S,3S)-2,3-BD and (2R,3R)-2,3-BD), 4 mM NAD+, 50 mM of potassium phosphate buffer (pH 8.0) and 10 μl of purified enzyme in a final volume of 1 ml was incubated at 40 °C for 2 h. The reduction reactions was performed in a 1-ml mixture containing 100 mM substrates (diacetyl and (3S/3R)-AC), 0.2 mM NADH, 50 mM of sodium acetate buffer (pH 5.0) and 10 μl of purified enzyme at 40 °C for 2 h. The products in these reaction systems were analyzed by gas chromatography.
Construction of S. marcescens-ΔslaC and S. marcescens-ΔslaC-bdhA
Two DNA fragments from upstream sequence (about 800 bp) and downstream sequence (about 1,000 bp) of the slaC gene with overlapping ends were amplified from S. marcescens MG1 genomic DNA using the primers listed in Table 1. The two fragments were then fused by overlapping PCR, generating an in-frame deletion construct of the slaC gene. The overlapping PCR fragment was cloned into the suicide vector pUTkm1 to produce pUT-slaC. The pUT-slaC vector was transformed into E. coli S17-1 λpir for conjugation with S. marcescens MG1. The single-crossover strains were selected on LB medium agar plate containing 50 μg/ml kanamycin. Then, a single-corssover colony was grown in LB broth overnight and plated onto LB agar, which was incubated overnight at 30 °C. Colonies were screened through the double-crossover resistance phenotype. The kanamycin-sensitive colonies were verified by PCR using the primers UpslaC-1/DnslaC-2. The slaC disruption mutant was designated as S. marcescens-ΔslaC and stored in a glycerol suspension at −80 °C.
To develop a constitutive expression vector for expression of (2R,3R)-BDH in S. marcescens, the slaC promoter (P slaC ) sequence and bdhA gene [coding for (2R,3R)-BDH] were amplified from the genomic DNA of S. marcescens MG1 and B. subtilis 168 using the primers of pslaC-1/pslaC-2 and bdhA-1/bdhA-2. Two amplified products were combined by overlapping PCR resulting in the merged fragment, designated as p-bdhA. The p-bdhA fragment was digested and ligated into the vector pUC19 at BamHI and HindIII sites to produce the recombinant pUC-pbdhA vector. The pUC-pbdhA vector was then transformed into the slaC mutant strain by electroporation, generating S. marcescens-ΔslaC-bdhA strain.
The stability of pUC-pbdhA plasmid in S. marcescens-ΔslaC
The stability of pUC-pbdhA plasmid in S. marcescens-ΔslaC was investigated using the following experimental procedure. Appropriately diluted fermentation samples were spread on selective (100 μg/ml ampicillin) and nonselective LB agar plates. The plates were incubated at 37 °C for 24 h. Plasmid stability was estimated as the ratio of number of colonies on the selective agar plates to the number on the nonselective plates.
Analytical methods
The sucrose concentration of the samples was measured by reagent kit (Jiemen Bio-Tech Co. China) of glucose after centrifugation and sucrose hydrolysis.
The biomass concentration was determined from the optical density (OD) at 600 nm using a spectrophotometer (UV-2008 h, Unic) and correlated with dry cell weight (DCW).
AC and 2,3-BD isomers in the samples were analyzed and quantified by GC (Agilent GC9860) equipped with a chiral column (FID-detector, Supelco β-DEX™ 120, 30 m length, 0.25 mm inner diameter). The operation conditions were as follows: N 2 was used as the carrier gas at a flow rate of 1.2 ml/min; the injector temperature and the detector temperature were 215 and 245 °C, respectively; the oven temperature was maintained at 50 °C for 1.5 min, then raised to 180 °C at a rate of 8 °C/min.
Results and discussion
Expression, purification and stereospecific characteristics of meso-BDH
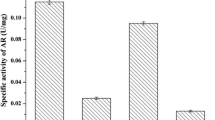
The slaC gene from S. marcescens MG1 was obtained by PCR amplification using the primers (slaC-1/slaC-2) designed according to our previous submitted sequence (Genbank accession number JF519738). The PCR-amplified products were ligated into the expression vector pET-28a (+) and transformed into E. coli BL21(DE3) for heterologous expression. As shown in Fig. 1, SDS-PAGE analysis showed that the meso-BDH was successfully expressed in E. coli. The subunit of meso-BDH was approximately 26 kDa which was consistent with the predicted size. The stereospecific characteristics of the meso-BDH were studied by catalysis reactions using 2,3-BD, diacetyl and AC as substrates. The results are given in Table 2. In oxidation reactions, meso-BDH could convert meso-2,3-BD, and (2S,3S)-2,3-BD to (3R)-AC and (3S)-AC, respectively, while it could not convert (2R,3R)-2,3-BD to any form of AC. In reduction reactions, the meso-BDH could convert diacetyl to (3S)-AC and could not further convert (3S)-AC into any form of 2,3-BD at pH 5.0. As reported by Zhang et al. [25], the (3S)-AC product from diacetyl could be further transformed into (2S,3S)-2,3-BD at pH 9.0. When racemic acetoin was used as the substrate, only meso-2,3-BD was observed. Considering (2S,3S)-2,3-BD from 3S-acetoin, therefore, meso-2,3-BD was formed from 3R-acetoin as substrate.
Effects of slaC inactivation on cell growth and metabolic profiles
Figure 2 demonstrated the effects of slaC inactivation on cell growth and metabolic profiles in batch culture. The maximum cell concentration (6.67 g/l) of the slaC mutant strain was lower than that of the wild-type strain MG1 (7.47 g/l), which indicated that the cell growth was somewhat impaired due to slaC inactivation. Similar to cell growth, the sucrose consumption rate for S. marcescens-ΔslaC appeared significantly lower when compared with that of the wild-type strain MG1. The sucrose was completely exhausted by the wild-type strain MG1 at 21 h, whereas for S. marcescens-ΔslaC the residual sucrose of 2.56 g/l remained in the broth after 30 h. This might be because excess NADH produced by S. marcescens-ΔslaC led to the decrease of bacterial glycolysis rate. As described in previous studies, 2,3-BD production played a major role in oxidizing NADH and regulated the intracellular redox balance during sugar metabolism [18]. Inactivation of the slaC gene in S. marcescens MG1 blocked the 2,3-BD biosynthesis pathway and resulted in NADH accumulation, thus inhibiting the glycolysis pathway of S. marcescens. For AC and 2,3-BD production, inactivation of the slaC gene could result in a large quantity of AC accumulation (21.78 g/l), implying that the slaC gene played a major role in 2,3-BD biosynthesis in S. marcescens MG1. However, S. marcescens-ΔslaC still produced a small amount of 2,3-BD (6.07 g/l), which suggested that another enzyme involved in 2,3-BD formation might exist in S. marcescens MG1. According to our previous study [24] and another study [16], glycerol dehydrogenase was proved to be involved in 2,3-BD formation. Therefore, it is possible that glycerol dehydrogenase was responsible for the formation of 2,3-BD in the ΔslaC mutant. The production of meso-2,3-BD and (2R,3R)-2,3-BD in the ΔslaC mutant also support the hypothesis. As shown in Fig. 3 and Table 3, 21.78 g/l of (3R)-AC with 1.45 g/l meso-2,3-BD and 4.62 g/l (2R,3R)-2,3-BD was produced by S. marcescens-ΔslaC at 30 h. (2S,3S)-2,3-BD and (3S)-AC could not be detected in the broth of S. marcescens-ΔslaC. In the broth of the wild-type strain MG1, 30.58 g/l of meso-2,3-BD with a small amount of (2R,3R)-2,3-BD (0.67 g/l), (2S,3S)-2,3-BD (0.92 g/l) and (3R)-AC (1.39 g/l) could be observed at 30 h. These results indicated that optically pure 3R-acetoin production could be achieved by S. marcescens-ΔslaC.
Effects of slaC inactivation and expressed bdhA on cell growth and metabolic profiles
The above results showed that meso-BDH encoded by the slaC gene from S. marcescnes MG1 were responsible for meso-2,3-BD and (2S,3S)-2,3-BD biosynthesis and slaC inactivation could lead to optically pure (3R)-AC accumulation. Here, we attempted to introduce a (2R,3R)-BDH enzyme encoded by the bdhA gene from B. subtilis 168 into S. marcescens-ΔslaC for (2R,3R)-2,3-BD production, since the (2R,3R)-BDH enzyme showed the ability of the conversion from (3R)-AC to (2R,3R)-2,3-BD and diacetyl to (2R,3R)-2,3-BD via (3R)-AC with concomitant oxidation of NADH to NAD+. The expression vector pUC-pbdhA was constructed and transformed into S. marcescens-ΔslaC as described in “Materials and methods”. The obtained recombinant strain designated as S. marcescens-ΔslaC-bdhA was cultured in the fermentation medium to analyze plasmid stability, expression of the bdhA gene and its metabolic profiles. As shown in Fig. 4, the fraction of plasmid-containing cells remained about 93 % at 30 h, suggesting that the vector pUC-pbdhA was stably expressed in S. marcescens-ΔslaC. SDS-PAGE analysis showed that (2R,3R)-BDH was successfully expressed in the S. marcescens-ΔslaC (Fig. 4).
The effects of bdhA gene expression in S. marcescens-ΔslaC on cell growth, sucrose consumption, AC and 2,3-BD production were investigated in 250-ml flask containing 50 ml of fresh fermentation medium. As shown in Fig. 2, the maximum cell concentration (4.87 g/l) of S. marcescens-ΔslaC-bdhA was obviously lower than that of the wild-type strain MG1 due to slaC inactivation and bdhA gene expression. Similar to S. marcescens-ΔslaC, the sucrose consumption rate of S. marcescens-ΔslaC-bdhA was lower than that of the wild-type strain MG1, even though BDH was working to consume NADH for BDO biosynthesis. For AC and 2,3-BD production, excess (2R,3R)-BDH in S. marcescens-ΔslaC could increase 2,3-BD yield and decrease AC accumulation. Ultimately, 28.31 g/l of 2,3-BD with a small amount of 1.53 g/l AC could be produced by S. marcescens-ΔslaC-bdhA at 30 h, which indicated that slaC inactivation could be relieved by bdhA gene expression. Configuration analysis of the products by S. marcescens-ΔslaC-bdhA was also determined by GC with a chiral column. As shown in Fig. 3 and Table 3, 27.56 g/l of (2R,3R)-2,3-BD occupying a weight fraction of 97.4 % could be produced by S. marcescens-ΔslaC-bdhA at 30 h, showing that most of (3R)-AC was converted into (2R,3R)-2,3-BD by (2R,3R)-BDH. Additionally, a small amount of meso-2,3-BD (0.75 g/l) was observed in the broth. The reason might be related to another enzyme existing in S. marcescens MG1. Further identification of another enzyme would be helpful for optically pure (2R,3R)-2,3-BD production.
Fed-batch fermentation with S. marcescens-ΔslaC-bdhA strain
To improve the final concentration of (2R,3R)-2,3-BD, the strain S. marcescens-ΔslaC-bdhA was used to perform fed-batch culture in a 7-l bioreactor with an initial broth volume of 3.0 l. As shown in Fig. 5 and Table 3, the maximum cell concentration was 12.56 g/l, which was more than twice that of shake flask culture, indicating that the engineered strain could also grow to a higher density in a more suitable environment. When the residual sucrose decreased below 5 g/l, a fed-batch culture was performed to increase the residual sucrose concentration to about 40 g/l. Finally, 255.05 g/l sucrose was consumed and 89.81 g/l of (2R,3R)-2,3-BD with 2.11 g/l of meso-2,3-BD was produced by S. marcescens-ΔslaC-bdhA at 48 h. The productivity and yield of (2R,3R)-2,3-BD were 1.91 g/l/h and 0.35 g (2R,3R)-2,3-BD/g sucrose, respectively. These results suggested that the S. marcescens-ΔslaC-bdhA strain had potential to improve its (2R,3R)-2,3-BD production and might be a candidate strain for large-scale production of (2R,3R)-2,3-BD with high optical purity. In addition, the AC concentration was just 1.98 g/l, showing again that the exogenous (2R,3R)-BDH was very efficient in converting AC to 2,3-BD.
Conclusion
In this study, a meso-BDH enzyme encoded by the slaC gene from S. marcescens MG1 was identified and responsible for meso-2,3-BD and (2S,3S)-2,3-BD formation. Inactivation of the slaC gene could lead to a large quantity of optically pure (3R)-AC accumulation, while (2R,3R)-BDH from B. subtilis 168 introduced in the slaC mutant strain could result in (2R,3R)-2,3-BD accumulation which occupied with a weight fraction of 97.4 %. Therefore, the two recombinant strains might be potential alternatives for optically pure (3R)-AC and (2R,3R)-2,3-BD production.
References
Borim K, Lee S, Park J, Lu M, Oh M, Kim Y, Lee J (2012) Enhanced 2,3-butanediol production in recombinant Klebsiella pneumoniae via overexpression of synthesis-related genes. J Microbiol Biotechnol 22:1258–1263
Celińska E, Grajek W (2009) Biotechnological production of 2,3-butanediol-current state and prospects. Biotechnol Adv 27:715–725
Häßler T, Schieder D, Pfaller R, Faulstich M, Sieber V (2012) Enhanced fed-batch fermentation of 2,3-butanediol by Paenibacillus polymyxa DSM 365. Bioresour Technol 124:237–244
Han S, Lee J, Park K, Park Y (2013) Production of 2,3-butanediol by a low-acid producing Klebsiella oxytoca NBRF4. New Biotechnol 30:166–172
Ji X, Huang H, Ouyang P (2011) Microbial 2,3-butanediol production: a state-of-the-art review. Biotechnol Adv 29:351–364
Lee S, Kim B, Park K, Um Y, Lee J (2012) Synthesis of pure meso-2,3-butanediol from crude glycerol using an engineered metabolic pathway in Escherichia coli. Appl Biochem Biotechnol 166:1801–1813
Li L, Yu W, Zhang L, Ma C, Wang A, Tao F, Xu P (2012) Biocatalytic production of (2S,3S)-2,3-butanediol from diacetyl using whole cells of engineered Escherichia coli. Bioresour Technol 115:111–116
Liu Z, Qin J, Gao C, Hua D, Ma C, Li L, Wang Y, Xu P (2011) Production of (2S,3S)-2,3-butanediol and (3S)-acetoin from glucose using resting cells of Klebsiella pneumonia and Bacillus subtilis. Bioresour Technol 102:10741–10744
Ma C, Wang A, Qin J, Li L, Ai X, Jiang T, Tang H, Xu P (2009) Enhanced 2,3-butanediol production by Klebsiella pneumoniae SDM. Appl Microbiol Biotechnol 82:49–57
Nicholson WL (2008) The Bacillus subtilis ydjL (bdhA) gene encodes acetoin reductase/2,3-butanediol dehydrogenase. Appl Environ Microbiol 74:6832–6838
Rao B, Zhang L, Sun J, Su G, Wei D, Chu J, Zhu J, Shen Y (2012) Characterization and regulation of the 2,3-butanediol pathway in Serratia marcescens. Appl Microbiol Biotechnol 93:2147–2159
Savakis P, Angermayr S, Hellingwerf K (2013) Synthesis of 2,3-butanediol by Synechocystis sp. PCC6803 via heterologous expression of a catabolic pathway from lactic acid- and enterobacteria. Metab Eng 20:121–130
Takusagawa Y, Otagiri M, Ui S, Ohtsuki T, Mimura A, Ohkuma M, Kudo T (2001) Purification and characterization of L-2,3-butanediol dehydrogenase of Brevibacterium saccharolyticum C-1012 expressed in Escherichia coli. Biosci Biotechnol Biochem 65(8):1876–1878
Ui S, Otagiri M, Mimura A, Dohmae N, Takio K, Ohkuma M, Kudo T (1998) Cloning, expression and nucleotide sequence of the L-2,3-butanediol dehydrogenase gene from Brevibacterium saccharolyticum C-1012. J Ferment Bioeng 86:290–295
Wang Q, Chen T (2012) Metabolic engineering of thermophilic Bacillus licheniformis for chiral pure D-2,3-butanediol production. Biotechnol Bioeng 109:1610–1621
Wang Y, Tao F, Xu P (2014) Glycerol dehydrogenase plays a dual role in glycerol metabolism and 2,3-butanediol formation in Klebsiella pneumonia. J Biol Chem 289:6080–6090
Xiao Z, Lv C, Gao C, Qin J, Ma C, Liu Z, Liu P, Li L, Xu P (2010) A novel whole-cell biocatalyst with NAD+ regeneration for production of chiral chemicals. PLoS One 5:e8860
Xu Q, Xie L, Li Y, Lin H, Sun S, Guan X, Hu K, Shen Y, Zhang L (2014) Metabolic engineering of Escherichia coli for efficient production of (3R)-acetoin. J Chem Technol Biotechnol. doi:10.1002/jctb.4293
Xu Y, Chu H, Gao C, Tao F, Zhou Z, Li K, Li L, Ma C, Xu P (2014) Systematic metabolic engineering of Escherichia coli for high-yield production of fuel bio-chemical 2,3-butanediol. Metab Eng 23:22–33
Yang T, Zhang X, Rao Z, Gu S, Xia H, Xu Z (2012) Optimization and scale-up of 2,3-butanediol production by Bacillus amyloliquefaciens B10-127. World J Microbiol Biotechnol 28:1563–1574
Yan Y, Lee C, Liao J (2009) Enantioselective synthesis of pure (R, R)-2,3-butanediol in Escherichia coli with stereospecific secondary alcohol dehydrogenases. Org Biomol Chem 7:3914–3917
Yu B, Sun J, Rajesh R, Song L, Zeng A (2011) Novel (2R,3R)-2,3-Butanediol dehydrogenase from potential industrial strain Paenibacillus polymyxa ATCC 12321. App Environ Microbiol 77:4230–4233
Zhang G, Wang C, Li C (2012) Cloning, expression and characterization of meso-2,3-butanediol dehydrogenase from Klebsiella pneumoniae. Biotechnol Lett 34:1519–1523
Zhang L, Sun J, Hao Y, Zhu J, Chu J, Wei D, Shen Y (2010) Microbial production of 2,3-butanediol by a surfactant (serrawettin)-deficient mutant of Serratia marcescens H30. J Ind Microbiol Biotechnol 37:857–862
Zhang L, Xu Q, Zhan S, Li Y, Lin H, Sun S, Sha L, Hu K, Guan X, Shen Y (2014) A new NAD(H)-dependent meso-2,3-butanediol dehydrogenase from an industrially potential strain Serratia marcescens H30. Appl Microbiol Biotechnol 98:1175–1184
Zhang L, Yang Y, Sun J, Shen Y, Wei D, Zhu J, Chu J (2010) Microbial production of 2,3-butanediol by a mutagenized strain of Serratia marcescens H30. Bioresour Technol 101:1961–1967
Acknowledgments
This work was supported by Shanghai Leading Academic Discipline Project (project B505) and National Special Fund for State Key Laboratory of Bioreactor Engineering (no. 2060204).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Bai, F., Dai, L., Fan, J. et al. Engineered Serratia marcescens for efficient (3R)-acetoin and (2R,3R)-2,3-butanediol production. J Ind Microbiol Biotechnol 42, 779–786 (2015). https://doi.org/10.1007/s10295-015-1598-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-015-1598-5