Abstract
An alkaline protease secreting Haloalkaliphilic bacterium (Gene bank accession number EU118361) was isolated from the Saurashtra Coast in Western India. The alkaline protease was purified by a single step chromatography on phenyl sepharose 6 FF with 28% yield. The molecular mass was 40 kDa as judged by SDS-PAGE. The enzyme displayed catalysis and stability over pH 8–13, optimally at 9–11. It was stable with 0–4 M NaCl and required 150 mM NaCl for optimum catalysis at 37 °C; however, the salt requirement for optimal catalysis increased with temperature. While crude enzyme was active at 25–80 °C (optimum at 50 °C), the purified enzyme had temperature optimum at 37 °C, which shifted to 80 °C in the presence of 2 M NaCl. The NaCl not only shifted the temperature profile but also enhanced the substrate affinity of the enzyme as reflected by the increase in the catalytic constant (K cat). The enzyme was also calcium dependent and with 2 mM Ca+2, the activity reached to maximum at 50 °C. The crude enzyme was highly thermostable (37–90 °C); however, the purified enzyme lost its stability above 50 °C and its half life was enhanced by 30 and sevenfold at 60 °C with 1 M NaCl and 50 mM Ca+2, respectively. The activity of the enzyme was inhibited by PMSF, indicating its serine type. While the activity was slightly enhanced by Tween-80 (0.2%) and Triton X-100 (0.05%), it marginally decreased with SDS. In addition, the enzyme was highly stable with oxidizing-reducing agents and commercial detergents and was affected by metal ions to varying extent. The study assumes significance due to the enzyme stability under the dual extremities of pH and salt coupled with moderate thermal tolerance. Besides, the facts emerged on the enzyme stability would add to the limited information on this enzyme from Haloalkaliphilic bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Haloalkaliphiles are the group of extremophiles which grow at dual extremities of high salt and alkaline pH and therefore, their extracellular enzymes might be active and stable under these conditions. The first Haloalkaliphilic bacterium was isolated by Tindall in 1984 [54] and since then many representatives of this group have been described [8, 11, 15, 24, 38, 47, 48, 53]. While majority of the studies are focused on molecular phylogeny and diversity, exploration of their enzymatic potential is still in fancy [12, 17, 22, 28, 41, 51].
Proteases have focused attention with respect to their significance in cellular and commercial context. The proteases produced worldwide on commercial scale are greater than any other biotechnologically used enzymes [37]. Among the industrial enzymes, 75% are hydrolytic in which 60% is contributed by proteases [42]. Proteases have diverse industrial applications in peptide synthesis, protein processing [36], bioprocessing of used X-ray films [29], leather [25], dairy and detergent industries [29, 50]. Among these applications, majority of the sales is for detergent industries [20]. Typically, a detergent protease needs to be active and stable in alkaline environment (pH 9–11) at 30–60 °C prevailing under harsh washing conditions that also include high salt and surfactants. Proteases in general are not stable under these conditions and therefore, the emphasis has been on newer sources. Besides, production of recombinant enzymes from the extremophiles would be another strategy for producing large quantities of suitable enzymes. While proteolytic enzymes are reported from halo-neutrophilic bacteria, alkaline proteases from haloalkaliphiles have rarely been investigated [12, 17, 22, 41, 51]. We have been involved with the screening and characterization of alkaline proteases from haloalkaliphiles [9, 19, 27, 39, 40]. Enzymes derived from these bacteria are potential biocatalysts due to their optimal activities under the conditions of high salt, surfactants, high temperature and alkaline pH [34], whereas the mesophilic counter parts fail to act under similar conditions [55].
Purification and characterization of proteins from halophilic organisms has been less frequently investigated due to the difficulties associated with the protein stability in the absence of salt. Enzyme characterization from haloalkaliphiles in general has been further restricted due to the coupled extremities of pH and salt [33]. This enzyme has been studied from only few Haloalkaliphilic bacteria. Extracellular serine protease from a haloalkaliphilic strain Natronobacterium, was partially purified and characterized by Yu [56] and a serine protease from Natrialba magadii has also been purified and characterized [17]. Preliminary studies of a serine protease from Natronococcus occultus were carried out by Studdert and his co-workers [52], followed by the characterization at biochemical level [13, 51]. Recently, the serine alkaline proteases from 18AG haloalkaliphilic species belonging to the Salinivibrio genus [31] have been purified and characterized. Therefore, in view of the limited information on this enzyme from haloalkaliphiles, it was quite interesting to purify and characterize it from a newly isolated bacterium.
We isolated a Haloalkaliphilic bacterium Strain AH-6 from coastal Gujarat (India). The bacterium secreted a novel serine protease active at wide range of alkaline pH, high salt and high temperatures; besides being able to catalyze in the presence of surfactants. In the present work, we also describe a one-step purification protocol based on hydrophobic interaction chromatography for efficient purification of protease from a newly isolated Haloalkaliphilic sp. AH-6.
Materials and methods
Materials
Phenyl sepharose 6 FF was purchased from Sigma (St. Louis, MO, USA). Casein was from Sisco Research Laboratories (Mumbai, India) and other media components were purchased from Hi Media Laboratories (Mumbai, India). All other chemicals were of analytical grade.
Isolation and screening of bacterial strain
Haloalkaliphilic sp. AH-6 was isolated from saline soil collected from the seashore near Jamnagar (Latitude 22.27 N, Longitude 70.07 E) in Gujarat (Western India). One g sample was suspended in sterile distilled water and 2 ml of the resulting suspension was transferred to 500 ml Erlenmeyer flask containing 100 ml of the enrichment medium which contained Solution A (g/l); NaCl, 200 and Na2CO3.10H2O, 50 and Solution B (g/l); Yeast extract, 10 g; Casamino acids, 7.5 g; Tri-sodium citrate, 3 g; KCl, 2 g; MgSO4.H2O, 1 g; FeSO4, 50 mg; MnCl2.4H2O, 0.36 mg. The solutions A and B were mixed in equal volume after autoclaving and the pH was adjusted, after autoclaving, to 10 with previously autoclaved Na2CO3 (20%, w/v). The cultures were incubated at 37 °C at 100 rpm for 72 h and then were spread on enrichment agar plate. The pure isolates were obtained by repeated streaking. The protease producers were screened by plating on gelatin agar medium (pH 9.0), which contained (g/l): gelatin, 30; peptone, 10 and NaCl, 100. The AH-6 strain showing maximum ration of clear zone vs. colony diameter on gelatin agar plate was selected as potent producer of the enzyme and was characterized on the basis of 16S rRNA gene sequencing, biochemical properties and antibiotic profile.
The genomic DNA was subjected to 16S rRNA amplification (1.5 kb rDNA fragment) using consensus primers. The PCR product was bi-directionally sequenced using the forward, reverse and an internal primer. Sequence data was aligned and analyzed for finding the closest homologs for the microbe. The biochemical tests for catalase, oxidase, urease, indole production, H2S production, ammonia production, nitrate reduction, triple sugar iron reaction and sugar fermentation test were carried out by inoculating the respective media. The sensitivity and resistances against antibiotics was carried out employing 5 different types of Octadisc from Hi Media.
Enzyme production
For protease production, the AH-6 was grown at 37 °C in CMB medium (pH 9.0) consisting of (g/l): glucose, 10.0; KH2PO4, 10.0; yeast extract, 5.0; peptone, 5.0; casein acid hydrolysate, 5.0 and NaCl, 100.0. The 24 h grown mother culture (A 660; 1.0, 3 ml) was inoculated to 100 ml of production medium, which contained (g/l): gelatin, 10.0; casein acid hydrolysate, 10.0 and NaCl, 100; pH 9. The culture was incubated at 37 °C at 100 rpm and 96 h grown cells were harvested by centrifugation at 5,500 g for 10 min. The supernatant was used as crude enzyme preparation. The crude enzyme preparation was stored at 4 °C until further use.
Enzyme purification by hydrophobic interaction chromatography
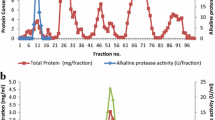
The crude enzyme was concentrated by ammonium sulphate (75% saturation, w/v) and precipitates were suspended in a minimum volume of 20 mM Borax-NaOH buffer (pH 10). Purification was achieved by a single step purification method using hydrophobic interaction chromatography as described earlier [19]. Hydrophobic interaction chromatography on a phenyl sepharose 6 fast flow column (1 cm × 6.5 cm), equilibrated with 0.1 M sodium phosphate buffer (pH 8.0) containing 1 M ammonium sulfate, was performed. The crude protease preparation (20.0 ml in 1 M ammonium sulfate) was loaded onto this column. The bound enzyme was eluted by 0.1 M sodium phosphate buffer, pH 8.0 containing a decreasing step gradient of ammonium sulfate (1.0–0.1 M). Fractions at a flow rate of 0.7 ml min−1 were collected by BIO-RAD fraction collector (BIO-RAD, California, USA) and analyzed for protease activity. The active fractions were pooled and used for further characterization.
Enzyme assay and estimation of protein
The protease activity was measured by Anson–Hagihara’s method [21–31] as described earlier [40]. Enzyme solution (0.5 ml) was added to 3.0 ml of substrate solution (0.6% casein in 20 mM borax–NaOH buffer, pH 10.0) and the mixture was incubated at 37 °C for 20 min. The reaction was stopped by addition of 3.2 ml of TCA mixture (containing 0.11 M trichloroacetic acid, 0.22 M sodium acetate and 0.33 M acetic acid) and kept at room temperature for 20 min followed by filtration through Whatman filter paper No. 1. The absorbance of the filtrate was measured at 280 nm. In control, the enzyme was added after adding TCA mixture. One unit of alkaline protease activity was defined as the amount of enzyme liberating 1 μg of tyrosine per min under standard assay conditions. Enzyme units were measured using tyrosine (0–100 μg) as standard. Protein was estimated by dye-binding method [6], using bovine serum albumin as standard protein.
SDS-Polyacrylamide gel electrophoresis
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out according to the method of Laemmli [30] using 12% crosslinked polyacrylamide gel. The protein bands were visualized on the gel by Coomassie blue staining.
Zymography
The zymogram staining for the detection of proteolytic activity was performed according to Heussen and Dowdel [23] with slight modifications. Casein (0.1%) was co-polymerized with 12% polyacrylamide gel. The reducing agent β–mercaptoethanol was excluded from the sample buffer. After electrophoresis the gel was rinsed in 2.5% Triton X-100 for 1 h at 4 °C to remove SDS followed by the incubation in 20 mM Borax-NaOH buffer for 3 h. The gels were stained with Coomassie brilliant blue R-250 for 1 h before destaining for over night. A clear zone against blue background on the gel revealed the protease activity.
Determination of K m and V max
K m and V max values of the pure enzyme were determined by measuring the activity with various concentrations of casein substrate (0.025–1 g/100 ml). Kinetic constants were calculated from Lineweaver-Burke plot.
Influence of pH on activity and stability of AH-6 protease
The optimum pH was determined by enzyme assay at different pH at 37 °C using purified enzyme. The % maximal activity was calculated by considering the maximum activity under the given conditions as 100%. The buffers (20 mM) were: Sodium Phosphate (pH 5.5–8), Tris-HCl (pH 8–9.5), NaOH-Borax (pH 9.5–10), Glycine-NaOH (pH 8.5–12) and KCl-NaOH (pH 12–13). To determine the stability at various pH, the enzyme was incubated with respective buffers (listed above) and after an incubation of 1 h and 24 h, the residual activities were measured by considering the initial activity as 100%.
Effect of NaCl on protease activity and stability
To determine the effect of NaCl on enzyme activity, the assay was carried out at 37 °C with purified enzyme in the presence of varying salt concentrations; 0–500 mM. For stability, the enzyme was incubated with NaCl (w/v) in the range of 0–4 M and the aliquots were withdrawn at regular time intervals. The % maximal activity and residual activities were calculated as described above.
Effect of NaCl and CaCl2 on the temperature optimum
The temperature optimum was assessed by carrying out the enzyme assay, using purified enzyme, at different temperatures in the range of 30–90 °C. Effect of NaCl and CaCl2 on the temperature profile was monitored; where at each temperature (30–90 °C) assay, the substrate (casein) was supplemented with varying concentrations of NaCl (0–3 M NaCl, w/v) and CaCl2 (0–10 mM). The % maximal activity and the catalytic constant K cat were calculated.
Determination of thermal stability and half-life of the enzyme
The thermal stability of protease was studied by incubating the enzyme (crude and purified) at different temperatures (37–90 °C). The aliquots were withdrawn at different time intervals till the enzyme got completely denatured. The half-life of the enzyme was calculated by considering the time at which the enzyme retained 50% of the maximal activity.
In order to determine the effect of NaCl, 1 M NaCl (w/v) was added to the purified enzyme, whereas for the effect of CaCl2, the enzyme was supplemented with 50 mM CaCl2. The enzymes along with the effectors were then incubated at different temperatures (37–80 °C). The aliquots were withdrawn at different time intervals and the half-life and % residual activity were calculated.
Effect of inhibitors and surfactants on enzyme activity
The influence of various effectors on enzyme activity was studied under standard assay conditions where the assay cocktail was supplemented with 5 and 10 mM of inhibitors; p-chloromercuribenzoate, phenylmethanesulfonyl fluoride (PMSF), ethylene diamine tetra acetate (EDTA), diopropyl flurophosphate (DFP), phenethroline and thiourea. The surfactants used were (0–0.2%, v/v); SDS, Triton X-100 and Tween-80 (0–2%, v/v). The effect was assessed by considering the control (without inhibitors and surfactants) as 100%.
Effect of surfactants, oxidizing and reducing agents and commercial detergents on protease stability
To investigate the effect on the protease stability the enzyme was incubated with various effectors; hydrogen peroxide (oxidizing agent) and β-mercaptoethanol (reducing agent) (5 and 10 mM); SDS, Triton X-100 and Tween-80 (0.5 and 1.0%); various commercial detergents (1%, w/v); Aerial (Procter & Gamble), Surf Excel (Hindustan Lever), Tide (Proctor & Gamble), Rin Supreme (Hindustan Lever), Nirma (Nirma Industries, India), Wheel (Hindustan Lever) and Flash (Local Brand). The enzyme aliquots were then withdrawn at definite time interval and the residual activities were determined.
Results and discussion
The present study describes the isolation of a new Haloalkaliphilic bacterial strain and the purification and characterization of the extracellular alkaline protease. The report assumes significance in the light of the fact that alkaline proteases have been characterized only from few haloalkaliphiles [17, 22, 51, 52, 56]. Another important feature of the present study relates to the habitat from where the organism has been isolated. While majority of the halophiles and haloalkaliphiles have been reported from Soda Lakes or Dead Sea, the present report highlights the occurrence of these bacteria from a natural saline habitat in western coast of India.
A total of eight haloalkaliphilic strains were isolated from the saline soil collected from Jamnagar in Gujarat (India). The isolates were screened for the secretion of extracellular proteases and among them, strain AH-6 produced maximum alkaline protease and used for further studies. The cells were cocci arranged in tetrad and clusters displaying gram-positive character. Based on 16S rRNA gene homology, the organism (Gene bank accession number EU118361) displayed nearest relatedness with a Halophilic bacterium MBIC3303. The next closest homolog was Bacillus marismortui sp. (GenBank entry: AJ009793|g4826393).
Enzyme purification by hydrophobic interaction chromatography
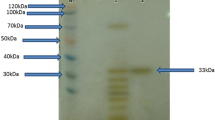
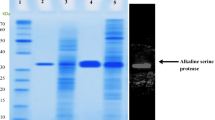
The purification results are summarized in Table 1. A 23-fold purification of AH-6 protease was achieved by a single step purification method with phenyl sepharose 6 FF [19] with a specific activity of 7,596 U/mg and 28% yield. Hydrophobic interaction chromatography has been a successful technique for the purification of many alkaline proteases. However, on previous occasions, it has been used in combination with other chromatographic techniques [25, 45]. The AH-6 protease was a monomeric protein with a molecular mass of 40 kDa as revealed by native and SDS-PAGE (Fig. 1). This value was lower than other halophilic and alkaline proteases where the molecular weight ranged from 40–130 kDa [17, 31, 35, 51]. The zymogram revealed the high level of protease activity on the gel, which corresponded to a single band, obtained in SDS-PAGE (Fig. 1). Earlier, some alkaline protease activities were also demonstrated by zymography [7, 43, 52]. The purified AH-6 enzyme was characterized for substrate kinetics towards casein, where K m and V max were 2.5 mg/ml and 625 U/min, respectively. A Km value of 2.5 mg/ml was quite comparable with 2 mg/ml (for casein) obtained for the alkaline protease from a haloalkaliphilic Bacillus sp. [19].
SDS-PAGE pattern of purified AH-6 protease. Lane 1 molecular mass marker proteins, Lane 2 purified protease, Lane 3 casein zymogram of the purified protease as described in “Materials and Methods” section
Influence of pH on activity and stability of AH-6 protease
The AH-6 protease remained active in the range of pH 7–13 (Fig. 2). The activity of the enzyme was almost negligible at pH 7 and 13. The enzyme was optimally active in a broad range of pH; 9.5–11. The decrease in enzyme activity was rather sharp at pH above 11 when compared with the decrease at acidic pH. However, the enzyme retained 50% of the maximal activity at pH 8 and 11.5. The solubility of casein was poor at pH below 7; therefore, lower pH range could not be attempted with casein (Fig. 2). Alkaline proteases from some other organisms have also displayed the similar pH range; 9–11 [2, 9, 16, 40]. However, the studies on pH stability of AH-6 protease demonstrated a relatively different pattern than the activity profile. The enzyme was almost completely denatured at pH 5 and 6 after 24 h incubation; however, it retained 40–50% of the activity in the pH range of 7–13 (Fig. 3).
Effect of pH on purified AH-6 protease stability. The pH stability was determined by incubating the enzyme in different buffers of varying pH values at 37 °C.The buffers used were sodium phosphate (filled diamond), Tris-HCl (filled circle), NaOH-Borax (filled square) and Glycine-NaOH (filled triangle). Where the open symbols represent incubation of 1 h and closed symbols represent incubation after 24 h with the respective buffers
Effect of NaCl on protease activity and stability
The findings have suggested that the halophilic enzymes generally require 1–2 M salt for their optimal activity and the catalytic activity was lost irreversibly when exposed to lower salt concentrations [1]. An extracellular protease from Natrialba magadii was optimally active with 1–1.5 M NaCl/KCl [17] and extracellular protease from Halobacterium halobium required 3 M NaCl for optimal activity [26]. Alkaline protease from AH-6 did not depend on high salt concentrations for their optimum catalysis as it was optimally active with 150–200 mM NaCl; however, it also retained 70% of the maximal activity in the absence of salt, indicating the salt tolerant nature of the enzyme (Fig. 4). The enzyme was quite stable up to 4 M NaCl and retained nearly 75% of the original activity after 4 h incubation, which remained stable even up to 48 h (Fig. 5). The stability and activity of the enzyme in the presence and absence of salt make it quite interesting for wider applications in biotechnological processes. Similar results were also reported for CP1 protease from a moderately halophilic bacterium Pseudoalteromonas sp., where the enzyme was active in the range of 0–4 M salt [43].
Effect of NaCl on stability of purified AH-6 protease. Enzyme was incubated with NaCl (w/v) in the range of 0–4 M and the aliquots were withdrawn and assayed at regular time intervals. The concentrations were 1 M (filled circle), 2 M (filled triangle), 4 M (×) and control, without NaCl (filled diamond)
Determination of temperature optimum
The crude AH-6 protease was active at temperatures; 37–80 °C, the optimum being at 50 °C and retained 65 and 30% of the maximal activity at 60 and 37 °C, respectively (Fig. 6). At temperatures above 60 °C, however, the enzyme activity rapidly decreased. The purified protease, on the other hand, was active in a relatively lower temperature range; 20–60 °C, the optimum being at 30–37 °C. The enzyme retained 55% of the maximal activity at 50 °C, above which the activity sharply decreased (Fig. 6).
Effect of NaCl and CaCl2 on the temperature optimum
The temperature optimum of the crude protease was higher than purified enzyme, a feature apparently linked with the presence of NaCl in the crude extract. To investigate this phenomenon further, the temperature profile of the purified enzyme under the influence of NaCl (0.2–2 M) and Ca+2 (0–10 mM) was carried out. An interesting feature that emerged was the NaCl and Ca+2 dependence of the enzyme for its optimal activity at higher temperatures. The optimum activity in the absence of salt shifted from 37 to 60 °C upon the addition of 500 mM NaCl; the shift with 1 M NaCl, however, was from 37 to 70 °C. Increase in salt concentrations above 1 M did not shift the temperature profile any further. The NaCl has not only shifted the temperature optima towards higher temperatures but also increased the substrate affinity of the enzyme as reflected by the increase in K cat values of the enzyme (Table 2). The K cat was maximum at 70 °C with 1 M NaCl indicating the highest substrate affinity of the enzyme under these conditions. In the absence of salt at 37 °C; however, K cat was only 6.18 indicating the role of salt on substrate affinity at high temperatures (Table 2). A similar trend was also observed for a serine protease from haloalkaliphilic archaea, Natronococcus occultus where the enzyme was optimally active at 60 °C in the presence of 2 M NaCl [52]. However, the temperature optimum was lower as compared to AH-6 protease under the similar salt concentrations. The above relationship could also be related to the fact that at higher temperatures, the salt requirement for optimum activity was also increased. At 37 °C, the enzyme was optimally active with 150 mM NaCl whereas at 70 °C, it required 2 M salt for optimal activity. To the best of our efforts, we have not come across with any report from the literature signifying the importance of salt on the temperature profile of alkaline protease from Haloalkaliphilic bacteria. However, it has been shown that compatible solutes (glycine, betaine, hydroxyectoine) shifted the activity profile towards higher temperatures [18]. The reasons for this shift may relate to the ionic strength of the enzymatic solutions, which might be one of the most important factors affecting the catalysis at higher temperatures. The high salt in the reaction mixture would increase the ionic strength and thus enhancing the electrostatic coupling of the salts and the proteins, thereby forming more stable salt/enzyme aggregates. Apparently, with salts, the degree of stabilization strongly depends on the protein:salt ratio [32].
A universal feature among the subtilisin type serine proteases is the presence of calcium-binding sites, which largely contributes to their stability against thermal denaturation and autolytic digestion [3, 16, 46]. As shown in Table 3, there was marked increase in the K cat (sec−1) of the purified AH-6 protease with the increasing concentrations of Ca+2. The maximum value of K cat (10.02 sec−1) was obtained with 2 mM Ca+2 at 50 °C, a value nearly twofold greater than the K cat at 37 °C (4.86 sec−1) in the absence of Ca+2. These results clearly indicated the calcium dependent protection against thermal denaturation of AH-6 protease. However, further increase in the Ca+2 did not affect the temperature optima of the AH-6 protease. Similar trend was also observed for an alkaline protease from B. pseudofirmus, where the enzyme was optimally active in the presence of 5 mM Ca+2 and retained only 50% of the maximal activity in its absence [16].
Determination of thermal stability and half-life of the enzyme
The thermostability of crude and purified enzyme was investigated in the range of 37–90 °C. Table 4 clearly indicated the resistant nature of the crude enzyme against thermal denaturation as it was highly stable at temperatures; 37–80 °C, whereas the purified enzyme was not stable above 60 °C, as also reflected by the half life of both enzyme preparations. In order to confirm the enzyme stability at higher temperatures, effect of NaCl (1 M) and calcium (50 mM) on the thermostability was investigated with purified enzyme. The half-life of the purified enzyme was only 30 and 2 min at 50 and 60 °C, respectively, which was correspondingly increased by 4 and 30-fold in the presence of 1 M NaCl (Table 4). The enzyme was completely denatured within 5 min of incubation at 60 °C in the absence of NaCl; however, the enzyme retained 65 and 60% of the original activity in the presence of 1 M NaCl even after 30 min at 60 and 70 °C, respectively (Table 4). The possible mechanism of salt-based thermal protection could be due to increase in water activity at the surface of proteins leading to enhanced core hydrophobicity of the proteins. This will lead to the improved core packing and enhanced rigidity of the proteins, the major factors providing stability to the proteins at higher temperatures [32].
The thermostability of the AH-6 protease was also increased in the presence of 50 mM Ca+2. However, the extent of protection was lower as compared to NaCl (Table 4). Calcium binding is an excellent strategy to allow survival of the enzymes in the extracellular environment but with a limitation during the use of the enzymes in detergents that contain sequestering agents to complex calcium and magnesium [44]. There are some reports on calcium dependent alkaline serine proteases where the enzyme requires 5 mM of the calcium for optimum activity [2].
Effect of cations on protease activity
In general, cations protect the enzyme against thermal denaturation and play a vital role in maintaining the active conformation of the enzyme at high temperatures [10, 49]. The AH-6 protease was exceptionally stable in the presence of heavy metal, HgCl2 where the enzyme retained 100 and 50% of the original activity at 10 and 100 mM HgCl2, respectively. Our results contradict a report on a thermostable alkaline protease from Bacillus brevis where the enzyme lost its activity completely at even 5 mM HgCl2 [4]. Generally, Mg+2 stimulate the protease activity [2] but with our protease, the activity decreased with increasing concentrations of Mg+2. However, the enzyme was highly stable and active with KCl in the range of 0–100 mM. The enzyme retained nearly 50% activity with 50 mM CaCl2. At higher concentrations, the enzyme was further inactivated and reached to 15% with 100 mM CaCl2 (Fig. 7). Similar observation of inhibitory effect of Ca+2 was also reported for alkaline protease from Pseudomonas aeruginosa MN1 [5].
Effect of inhibitors on protease activity
The purified enzyme was completely inhibited by phenylmethanesulfonyl fluoride, indicating its serine type. The enzyme was partially inhibited by pCMB, EDTA, dithiothritol, thiourea and phenethroline (Table 5). Literatures revealed that majority of the alkaline proteases from halophilic and haloalkaliphilic organisms were of serine type [19, 31, 51, 52].
Effect of oxidizing-reducing agents, surfactants and commercial detergents on the protease stability
Alkaline proteases have been of interest to detergent industries for their ability to remove proteinaceous stains and to deliver unique benefits that cannot otherwise be obtained with conventional detergent technologies. For an enzyme to be used as detergent additives, it should be active in the presence of surfactants, bleaching and oxidizing agents including fabric softeners. Some of the chemical environment necessary for better detergent action could be provided by the genetically modified recombinant enzymes with enhanced stability under varying redox conditions [14]. However, AH-6 protease was highly stable in the presence of hydrogen peroxide and retained 77% of the original activity with 10 mM H2O2 after 2 h of incubation. Similar kind of resistance to oxidation has also been exhibited by an alkaline protease from Nesterenkonia sp. [3]. The enzyme had high stability in the presence of SDS, Tween-80; however, it was not much stable with Triton X-100. Tween-80 within limited concentrations enhanced the AH-6 protease activity (Tables 5, 6). The enzyme retained stability and catalyzed the reaction with various commercial detergents even after 24 h, except Surf, where it decreased to 55%. However, with the rest of the detergents, the enzyme retained 70–80% of the activity after 24 h (Table 7). These characteristics of the AH-6 protease qualify it for the list of enzymes that can be used as a detergent additive.
Conclusion
The stability of AH-6 protease at three extremities of salt, pH and temperature displayed unique features for biotechnological applications along with the suitability of this enzyme for detergent additives. The results are particularly important in view of the fact that only few enzymes have been purified and biochemically characterized from Haloalkaliphilic bacteria. Characterization of more enzymes in this category would facilitate the understanding on the biochemical and structural basis of the molecular stability under extreme conditions. In additions, the findings have also highlighted the presence of a novel Haloalkaliphilic bacterium from the unexplored saline habitats in western coast of India.
References
Adams RJ, Bygraves M, Kogut, Russell NJ (1987) The role of osmotic effects in haloadaptation of Vibrio costicola. J Gen Microbiol 133:1861–1870
Adinarayana K, Ellaiah P, Prasad DS (2003) Purification and partial characterization of thermostable serine alkaline protease from a newly isolated Bacillus subtilis PE-11. AAPS PharmScitech 4:56
Bakhtiar S, Andersson M, Gessesse A, Mattiasson B, Kaul R (2002) Stability characteristics of a calcium-independent alkaline protease from Nesterenkonia sp. Enz Microbial Technol 32:525–531
Banerjee UC, Sani RK, Azmi W, Soni R (1999) Thermostable alkaline protease from Bacillus brevis and its characterization as a laundry detergent additive. Process Biochem 35:213–219
Bayoudh A, Gharsallah N, Chamkha M, Dhouib A, Ammar S, Nasri M (2000) Purification and characterization of an alkaline protease from Pseudomonas aeruginosa MN1. J Ind Microbiol Biotechnol 24:291–295
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Catara G, Ruggiero G, La Cara F, Digilio FA, Capasso A, Rossi MA (2003) Novel extracellular subtilisin-like protease from the hyperthermophile Aeropyrum pernix K1: biochemical properties, cloning, and expression. Extremophiles 7:391–399
David R, Arahal M, Marquez C, Volcani BE, Schleifer KH, Ventosa A (1999) Bacillus marismortui sp. nov., a new moderately halophilic species from the Dead Sea. Int J Syst Evol Microbiol 49:521–530
Dodia MS, Joshi RH, Patel RK, Singh SP (2006) Characterization and stability of extracellular alkaline proteases from moderately halophilic and alkaliphilic Bacteria isolated from saline habitat of coastal Gujarat, India. Braz J Microbiol 37:276–282
Donaghy JA, McKay AM (1993) Production and properties of an alkaline protease by Aureobasidium pullulans. J Appl Bacteriol 74:662–666
Doronina N, Darmaeva T, Trotsenko Y (2003) Methylophaga natronica sp. nov., a new alkaliphilic and moderately halophilic, restricted-facultatively methylotrophic bacterium from soda lake of the Southern Transbaikal region. Syst Appl Microbiol 26:382–389
Eddy ML, Jablonski PE (2000) Purification and characterization of a membrane-associated ATPase from Natronococcus occultus, a haloalkaliphilic archaeon. FEMS Microbiol Lett 189:211–214
Elsztein C, Herrera SMK, Sanchez JJ, de Castro RE (2001) Autoproteolytic activation of the haloalkaliphilic archaeon Natronococcus occultus extracellular serine protease. J Basic Microbiol 41:319–27
Estell DA, Graycar TP, Wells JA (1985) Engineering an enzyme by site-directed mutagenesis to be resistant to chemical oxidation. J Biol Chem 260:6518–6521
Foti M, Ma S, Sorokin DY, Rademaker JL, Kuenen JG, Muyzer G (2006) Genetic diversity and biogeography of haloalkaliphilic sulphur-oxidizing bacteria belonging to the genus Thioalkalivibrio. FEMS Microbiol Ecol 56:95–101
Gessesse A, Kaul RH, Gashe BA, Mattiasson B (2003) Novel alkaline proteases from alkaliphilic bacteria grown on chicken feather. Enz Microbial Technol 32:519–524
Gimenez MI, Studdert CA, Sanchez J, De Castro RE (2000) Extracellular protease of Natrialba magadii: purification and biochemical characterization. Extremophiles 4:181–188
Goller K, Galinski EA (1999) Protection of a model enzyme (lactate dehydrogenase) against heat, urea and freeze-thaw treatment by compatible solute additives. J Mol Catl B Enzym 7:37–45
Gupta A, Roy I, Patel RK, Singh SP, Khare SK, Gupta MN (2005) A One-step purification and characterization of an alkaline protease from haloalkaliphilic Bacillus sp. Journal of Chromatogr A 1075:103–108
Gupta R, Beg QK, Lorenz P (2002) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59:15–32
Hagihara B (1958) The Enzymes, vol 4. Academic press Inc, New York
Herrera SK, Studdert C, Sanchez J, De Castro R (1997) Intracellular proteolytic activity of the haloalkaliphilic archaeon Natronococcus occultus. Effect of starvation. J Basic Microbiol 7:313–322
Heussen C, Dowdle EB (1980) Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulphate and copolymerized substrates. Anal Biochem 102:196–202
Hoover RB, Pikuta EV, Bej AK, Marsic D, Whitman WB, Tang J, Krader P (2003) Spirochaeta americana sp. nov., a new haloalkaliphilic, obligately anaerobic spirochaete isolated from soda Mono Lake in California. Int J Syst Evol Microbiol 53:815–821
Huang Q, Peng Y, Li X, Wang Y, Zhang Y (2003) Purification and characterization of an extracellular alkaline serine protease with dehairing function from Bacillus pumilus. Curr Microbiol 46:169
Izotova LS, Strongin AY, Chekulaeva LN, Sterkin VE, Ostoslavskaya VI, Lyublinskaya LA, Timokhina EA, Stepanov VM (1983) Purification and properties of serine protease from Halobacterium halobium. J Bacteriol 155:826–830
Jogi C, Joshi RH, Dodia MS, Singh SP (2005) Extracellular alkaline protease from haloalkaliphilic bacteria isolated from sea water along coastal Gujarat. J Cell Tissue Res 5:439–444
Kobayashi T, Kanai H, Hayashi T, Akiba T, Akaboshi R, Horikoshi K (1992) Haloalkaliphilic maltotriose-forming a-amylase from the archaebacterium Natronococcus sp. Strain Ah-36. J Bacteriol 174:3439–3444
Kumar CG, Takagi H (1999) Microbial alkaline proteases: from bioindustrial viewpoint. Biotechnol Adv 17:561–594
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lama L, Romano I, Calandrelli V, Nicolaus B, Gambacorta A (2005) Purification and characterization of a protease produced by an aerobic haloalkaliphilic species belonging to the Salinivibrio genus. Res Microbiol 156:478–84
Lippert K, Galinski E (1992) Enzyme stabilization by ectoine-type compatible solutes: protection against heating, freezing adn drying. Appl Microbial Biotechnol 37:61–65
Madern D, Camacho M, Rodriguez-Arnedo A, Bonete MJ, Zaccai G (2004) Salt-dependent studies of NADP-dependent isocitrate dehydrogense from the halophilic archaeon Haloferax volcanii. Extremophiles 8:377–384
Margesin R, Schinner F (2001) Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles 5:73–83
Miyaji T, Otta Y, Nakagawa T, Watanabe T, Niimura Y, Tomizuka N (2006) Purification and molecular characterization of subtilisin-like alkaline protease BPP-A from Bacillus pumilus strain MS-1. Lett Appl Microbiol 42:242–247
Neklyudov AD, Ivankin AN, Berdutina AV (2000) Properties and uses of protein hydrolysates (review). Appl Biochem Microbiol 36:452–459
Niehaus F, Bertoldo C, Kahler M, Antranikian G (1999) Extremophiles as a source of novel enzymes for industrial application. Appl Microbiol Biotechnol 51:711–729
Nowlan B, Dodia MS, Singh SP, Patel BKC (2006) Bacillus okhensis nov. sp., a halotolerant alkaliphile from an Indian salt pan. Int J Syst Evol Microbiol 56:1073–1077
Patel RK, Dodia MS, Joshi RH, Singh SP (2006) Production of extracellular halo-alkaline protease from a newly isolated Haloalkaliphilic Bacillus sp. isolated from seawater in Western India. World J Microb Biot 24:375–382
Patel RK, Dodia MS, Singh SP (2005) Extracellular alkaline protease from a newly isolated haloalkaliphilic Bacillus sp.: production and optimization. Process Biochem 40:3569–3575
Polosina YY, Zamyatkin DF, Kostyukova AS, Filimonov V, Fedorov OV (2002) Stability of Natrialba magadii NDP kinase: comparisons with other halophilic proteins. Extremophiles 6:135–142
Rao MB, Tanksale AM, Ghatge MS, Deshpande VV (1998) Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 62:597–635
Sanchez-Porro C., Mellado E., Bertoldo C., Antranikian G., Ventosa A (2003) Screening and characterization of the protease CP1 produced by the moderately halophilic bacterium Pseudoalteromonas sp. strain CP76. Extremophiles 7:221–228
Sato M., Yoshikawa K, Minagawa M (1990) The effect of builders on the activity of protease enzymes. J Am Oil Chem Soc 67:711–716
Smacchi E, Fox PF, Gobbetti M (1999) Purification and characterization of two extracellular proteinases from Arthrobacter nicotianae 9458. FEMS Microbiol Lett 170:327–333
Smith CA, Toogood HS, Baker HM, Daniel RM, Baker EN (1999) Calcium-mediated thermostability in Subtilisin superfamily: the crystal structure of Bacillus AK.1 protease at 1.8 Å resolution. J Mol Biol 294:1027–1040
Sorokin DY, Kuenen JG (2005) Chemolithotrophic haloalkaliphiles from soda lakes. FEMS Microbiol Ecol 52:287–95
Sorokin DY, Kuenen JG (2005) Haloalkaliphilic sulfur-oxidizing bacteria in soda lakes. FEMS Microbiol Rev 29:685–702
Steele DB, Fiske MJ, Steele BP, Kelley VC (1992) Production of a low molecular weight, alkaline active, thermostable protease by a novel spiral-shaped bacterium, Kurthia spiroforme sp. nov. Enz Microbiol Technol 14:358–360
Stoner MR, Dale DA, Gualfetti PJ, Becker T, Manning MC, Carpenter JF, Randolph TW (2004) Protease autolysis in heavy-duty liquid detergent formulations: effects of thermodynamic stabilizers and protease inhibitors. Enz Microbiol Technol 34:114–125
Studdert CA, Seitz MKH, Gilv MIP, Sanchez JJ, De Castro RE (2001) Purification and biochemical characterization of the haloalkaliphilic archaeon Natronococcus occultus extracellular serine protease. J Basic Microbiol 41:375–383
Studdert CA, De Castro RE, Herrera SK, Sanchez JJ (1997) Detection and preliminary characterization of extracellular proteolytic activities of the haloalkaliphilic archaeon Natronococcus occultus. Arc Microbiol 168:532–535
Tikhonova TV, Slutsky A, Antipov AN, Boyko KM, Polyakov KM, Sorokin DY, Zvyagilskaya RA, Popov VO (2006) Molecular and catalytic properties of a novel cytochrome c nitrite reductase from nitrate-reducing haloalkaliphilic sulfur-oxidizing bacterium Thioalkalivibrio nitratireducens. Biochem Biophys Acta 1764(4):715–723
Tindall BJ, Ross HNM, Grant WD (1984) Natronobacterium gen. nov. and Natronococcus gen. nov., to new genera of haloalkalophilic archaebacteria. Syst Appl Microbiol 5:41–57
Ventosa A, Nieto JJ (1995) Biotechnological applications and potentialities of halophilic microorganisms. World J Microbiol Biotechnol 11:85–94
Yu TX (1991) Protease of haloalkaliphiles. In: Horikoshi K, Grant WD (eds) Super bugs. Springer, New York, pp 76–83
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dodia, M.S., Rawal, C.M., Bhimani, H.G. et al. Purification and stability characteristics of an alkaline serine protease from a newly isolated Haloalkaliphilic bacterium sp. AH-6. J Ind Microbiol Biotechnol 35, 121–131 (2008). https://doi.org/10.1007/s10295-007-0273-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-007-0273-x