Abstract
Purpose
To determine the effects of sex and age on cardiovascular autonomic parameters in healthy adults as assessed by Finapres (finger arterial pressure) method and prolonged head-up tilt (HUT).
Methods
We enrolled 81 healthy volunteers (41 females, 40 males, 18–74 years) for extensive cardiovascular autonomic function testing including blood pressure (BP) recordings, electrocardiography, and impedance cardiography at rest, under 60° HUT for 45 min, active standing for 5 min, Valsalva maneuver, and deep breathing (DB). Mean values and orthostatic changes, i.e., differences to baseline, of heart rate (HR), systolic and diastolic BP, stroke volume (SV), and total peripheral resistance (TPR), as well as DB ratio and Valsalva ratio were calculated. A generalized linear model (extended by generalized estimating equations) was used to assess sex- and age-related differences.
Results
Mean HR at rest was higher in women than in men (p = 0.035). In men, we observed significantly higher mean BP at rest (p < 0.001 systolic and p = 0.004 diastolic) and during HUT (p = 0.001 systolic and p < 0.001 diastolic), mean TPR at rest (p = 0.034), and mean SV during HUT (p < 0.001). We found no significant impact of sex on orthostatic changes of HR and BP. Mean TPR during HUT increased with age (p = 0.001), particularly in older women. Orthostatic changes of HR and diastolic BP, DB ratio, and Valsalva ratio became attenuated with age (p = 0.018, p = 0.006, p < 0.001, and p < 0.001, respectively).
Conclusions
Our study suggests that aging rather than sex needs to be taken into account when interpreting HR and BP changes during prolonged HUT performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Three main categories of autonomic disorders and their variants may be distinguished in a tilt-table laboratory: orthostatic hypotension (OH) including delayed OH, postural tachycardia syndrome (POTS), and neurally mediated syncope. Briefly, OH is defined as a fall in blood pressure (BP) of at least 20 mmHg (systolic) and/or at least 10 mmHg (diastolic) within 3 min of standing or passive head-up tilt (HUT) to at least 60° on a tilt table [1]. Delayed OH is defined as OH beyond 3 min of standing or passive HUT [1]. POTS is defined as development of orthostatic symptoms associated with a heart rate (HR) increase of ≥30 beats/min (bpm) with respect to supine values or HR above 120 bpm within 10 min of standing or HUT in absence of OH [1]. Syncope is defined as a transient loss of consciousness due to global cerebral hypoperfusion with rapid onset, short duration, and spontaneous recovery [2].
A previous report [3] suggests that prolonged (45 min) HUT should be applied to patients with recurrent syncopes of unknown etiology. A short (10 min) HUT test followed by an active standing test of 5 min appears to be sufficiently informative in patients with suspected OH or POTS. The minimum duration to detect delayed OH is still a subject of discussion [4, 5].
The gold standard in cardiovascular autonomic function testing is based on orthostatic changes of BP and HR. Different beat-to-beat BP monitors based on the Finapres (finger arterial pressure) method were developed for this purpose. The Task Force® Monitor (CNSystems, Graz, Austria) [6, 7] has been employed at our institution as a non-invasive tool for continuous monitoring of cardiovascular autonomic function parameters. To the best of our knowledge, few studies of cardiovascular autonomic parameters as assessed by Finapres methodology in well-defined healthy populations have been published to date, and none of these use the Task Force® Monitor system [8–14]. Two studies extensively assessed effects of sex and age on cardiovascular autonomic function using short tilting protocols (Braune et al. [8], 1 min tilting in 137 healthy volunteers and Low et al. [10], 5 min tilting in 270 normal subjects). Prolonged tilt-table testing up to 45 min is often required to diagnose orthostatic disorders [3, 15].
In the present study, we aimed at evaluating cardiovascular autonomic parameters as assessed by prolonged HUT under Task Force® Monitor in a population of well-defined healthy adults, with special emphasis on the effects of sex and age.
Methods
Subjects
One hundred healthy volunteers were initially screened over a period of 18 months (July 2012–December 2013) via local announcements in the hospital and local press in the region of Tyrol, Austria. A study flow chart is depicted in Fig. 1. Data from 81 subjects were entered for statistical analysis (41 females, 40 males; aged 18–74 years). More details on the baseline characteristics of the study population are provided in Table 1.
Study flowchart. We screened one hundred healthy volunteers. Subjects over 50 years underwent cardiological screening (F.H.) to exclude cardiovascular disease. The analyzed population consisted of 81 subjects. Asterisk for eight subjects, the prolonged (45 min) HUT protocol had to be shortened upon request. HUT head-up tilt
Exclusion criteria were history of neurologic, psychiatric, and cardiovascular disorders, including coronary heart disease or heart failure, known or suspected pregnancy, breastfeeding, and any drug dependence. All subjects were asked to avoid caffeine- or taurine-containing products on the day of the examination and to fast for 2 hours prior to the scheduled examination. Written informed consent was obtained from all subjects. The study was approved by the Ethical Committee of the Medical University Innsbruck and performed in accordance with the Declaration of Helsinki.
Study protocol
For all subjects, hemodynamic parameters were continuously monitored by means of continuous non-invasive beat-to-beat BP recording, oscillometric BP recording, a 6-lead electrocardiogram, and impedance cardiography using the Task Force® Monitor.
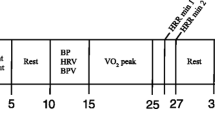
In a quiet setting, with mean room temperature of 22 ± 1 °C, the test protocol was applied as shown in Fig. 2. The arm with the finger cuff was fixed at heart level during measurements. Details on the tools used for autonomic testing are provided elsewhere [15, 16]. Testing took place between 8:30 and 11:00 am for 42 subjects and between 11:00 am and 4:00 pm for 39 subjects. All tests were conducted by a neurologist well experienced in cardiovascular autonomic testing (A.F.).
Data processing
For each subject, beat-to-beat values of HR, systolic BP, diastolic BP, SV, and TPR in supine and upright positions were calculated by means of Task Force® Monitor software (version 2.2.22.2; methods and formula used for calculating hemodynamic parameters are provided elsewhere [6]).
Data were exported in an Excel file (Microsoft Excel®, Microsoft, Redmond, US) and further processed offline. Mean values (average of 15 beat-to-beat values) of HR, systolic BP, diastolic BP, SV, and TPR at various time points (supine and after 1, 3, 5, 10, 20, 30, and 45 min of HUT; supine and after 1, 3, and 5 min of standing) were collected. Orthostatic changes, i.e., differences between orthostatic values and values in the preceding supine position, were calculated. Deep breathing (DB) ratio was calculated as mean value of 6 ratios obtained by calculating the longest R–R interval of ECG recording during expiration versus the shortest interval during inspiration of the DB test. Valsalva ratio was obtained by choosing the best performed trial of Valsalva maneuver (VM) and by dividing the highest HR in phase II by the lowest HR in phase IV. A VM was excluded from the analysis, if artifacts or extra-systole occurred during task execution, or if the subject was not able to reach the expiratory pressure of 40 mmHg.
Statistical analysis
Data are presented as mean (95 % confidence interval), if not otherwise stated. To assess age-related differences, subjects were divided into three age groups (<30, 30–50, and >50). Sex and age effects were assessed using a generalized linear model (GLM) and univariate analysis of variance (ANOVA) or Kruskal–Wallis (KW) test in case variances were not equal. GLM was extended by generalized estimating equations (GEE) [17] to account for within-subject variation on time. The within-subject correlation matrix was autoregressive of first order [AR (1)]. Parameters entered in the model were sex, age (categorized as mentioned above), time (subdivided in various points of time of the orthostatic stress as described in the methods section), time of day (testing before and after 11:00 pm), and body mass index (BMI). ANOVA was adjusted for sex, age, time of day, and BMI. LSD (least significant difference) correction was used for multiple comparisons. LOESS (locally weighted scatterplot smoothing) method (75 % of points to fit; Epanechnikov kernel) was used to visualize sex and age group differences. Statistical analysis was conducted with IBM® SPSS® Statistics, version 22. Results with p < 0.05 were considered as statistically significant.
Results
Subjects
Baseline characteristics of the study population are presented in Table 1. Women and men had similar age characteristics. Body height was greater in men than in women (p < 0.001, ANOVA); BMI was lower in women than in men (p = 0.003, KW).
Significant effects of time of day (time schedule for tilting) were observed by both indexes of cardiovagal function; values of Valsalva ratio and DB ratio were smaller (p < 0.001 and p = 0.048, respectively, GEE) in the morning than in the afternoon.
Passive HUT
Mean HR at rest was significantly higher in women than in men (p = 0.035, ANOVA), but not age-related (p = 0.298, ANOVA). During HUT, we found significantly different mean HR in age categories (p = 0.001, GEE). Mean HR was greater in younger subjects as compared with older subjects. More details are provided in online Table 1. Mean change of HR was 10.3 (8.4–12.3) bpm after 1 min and increased significantly (p < 0.001, GEE) to 19.1 (17.1–21.0) bpm after 45 min HUT. Further details, depicted in age groups, are shown in Table 2. Mean HR changes were significantly different in age groups (p = 0.018, GEE), being greater in younger subjects as compared with older subjects. We did not observe any sex effect on HR changes during passive HUT (p = 0.882, GEE) (see also online Figure 1).
Systolic and diastolic BP were significantly higher in men than in women both at rest (p < 0.001 and p = 0.004 respectively, ANOVA) and during passive HUT (p = 0.001 and p < 0.001, respectively, GEE). Differences in BP between age groups were neither observed at rest nor during HUT. More details are provided in online Table 1. After 1 min, systolic BP increased on average by 10.9 (8.7–13.0) mmHg and mean change of diastolic BP was 15.9 (14.0–17.8) mmHg. Further details are shown in Table 2. Changes of systolic BP were neither sex- nor age-related. By contrast, we observed significantly smaller mean diastolic BP increase in subjects older than 50 years (p = 0.006, GEE). Details are shown in Table 2 (see also online Figures 2 and 3).
Mean TPR at rest was significantly higher in men than in women (p = 0.034, KW). During passive HUT, mean TPR was age-related (p = 0.001, GEE), being higher in subjects older than 50 years as compared with subjects in the two remaining groups (more details are provided in online Table 2). In addition, mean change of TPR during passive HUT was sex-related (p = 0.011, GEE). Age and female gender were associated with greater orthostatic increments in TPR (Fig. 3a).
Mean SV at rest was not statistically different between sex and age groups (p = 0.078 and p = 0.137 respectively, ANOVA). Sex and age effects on mean SV were highly significant (p < 0.001 for sex and p = 0.005 for age) during passive HUT. Subjects younger than 30 years irrespective of gender as well as men irrespective of age had higher mean values of SV during passive HUT. Mean SV changes showed no sex or age difference (p = 0.863; p = 0.556 respectively, GEE). More details are provided in online Table 3.
Active standing
After 1 min, mean HR change was 17.5 (15.5–19.5) bpm. We observed a significant age difference in changes of HR (p = 0.008, GEE). Latter were 20.5 (15.8–25.2) bpm in subjects younger than 30 years, 15.6 (12.8–18.3) bpm in subjects between 30 and 50 years, and 17.3 (13.9–20.7) bpm in subjects older than 50 years after 1 min. We did not observe any sex effect on HR changes during active standing (p = 0.945, GEE, Table 3).
After 1 min, mean changes of BP were 11.7 (9.5–14.0) mmHg (systolic) and 17.9 (15.8–19.9) mmHg (diastolic). No effects of sex and age on changes of BP were observed during active standing (Table 3).
DB and VM
No association between DB ratio and sex was observed. DB ratio decreased significantly with age (p < 0.001, ANOVA, Table 4).
We did not observe any effect of sex on mean Valsalva ratio. However, mean Valsalva ratio decreased highly significantly with age (p < 0.001, ANOVA).
Discussion
This is the first study using a prolonged HUT protocol to evaluate sex and age effects on cardiovascular autonomic function in a well-defined healthy population. The major findings are summarized as follows: women had higher mean HR at rest. In men, we observed higher mean BP at rest and during HUT, higher mean TPR at rest and higher mean SV during HUT. We observed no significant impact of sex on changes of HR and BP during HUT. Orthostatic changes of HR and diastolic BP became attenuated with age. Mean TPR during HUT increased with age, particularly in older women. Mean SV during HUT was lower in older subjects. DB ratio and Valsalva ratio decreased with age.
Sex effects on cardiovascular autonomic function during passive HUT
Our findings are consistent with previously reported data in healthy subjects undergoing shorter HUT protocols. Mean HR at rest was significantly higher in women than in men [8, 10]. Systolic and diastolic BP during HUT [10, 18] were higher in men than in women, especially younger women. The lower BP values observed in women may reflect the greater vasodilatory response to vascular β2 adrenoreceptors stimulation occurring in women, especially in premenopausal age [19]. In men, higher TPR at rest and higher SV during orthostatic challenges may also partially explain higher BP values observed at rest and during HUT. An inverse relationship between muscle sympathetic activity and cardiac output contributes to normal BP in young men [20]. Hart et al. [21] found in their study no relationship between cardiac output and muscle sympathetic nerve activity in young women and concluded that sexes may rely on different mechanisms to maintain a normal BP, female reproductive hormones possibly accounting for these differences. Sex differences in BP could also be related to body height. London et al. [22] assessed the influence of body size on arterial function and found a greater amplification of systolic BP from central to peripheral arteries in men, due to their greater body height. In the present study, men had significantly greater body heights in all age groups (p < 0.001, Table 1). This could provide a further explanation for higher BP values found in our study in men. Despite the aforementioned sex differences in regulation of BP, we observed no significant sex effect on changes in HR and BP during HUT. These findings were consistent with the results of Low et al. [10]. Braune et al. [8] also reported no significant impact of sex on changes in HR, yet a significant influence of sex on changes in BP during passive HUT. Latter were greater in women than in men.
Age effects on cardiovascular autonomic function during passive HUT
During passive HUT, mean HR and changes of HR became attenuated with increasing age. This might be due to attenuated cardiovagal inhibition [23] and decreased ß-adrenergic cardiac sensitivity [24] of the heart with age. Changes of HR were greater in younger subjects than in older subjects. HR changes increased progressively with time and exceeded in some cases 30 bpm, the cut-off value for diagnosing POTS, but rather beyond 10 min of tilting. We found no significant difference in changes of systolic BP between younger and older subjects, yet a significant reduction in diastolic BP with age during HUT (Table 2 and online Figure 3), a finding consistent with a previous report by Low et al. [10]. Changes of BP were the lower, the longer the tilting time, especially in older healthy subjects (online Figures 2d–f and 3d–f). Age-related diminished orthostatic tolerance during prolonged tilt-table testing was also observed in a recent study [11].Use of prolonged tilt-table testing may be well-thought-out especially in older patients.
Aging is accompanied by increased arterial stiffening [12, 14, 25], which likely explains the age-related increase of TPR during orthostatic challenge in our study population. Barnes et al. [26] and Narkiewicz et al. [12] reported greater sympathetic traffic in older women. This may explain the greater changes of TPR observed in older women in our study (Fig. 3a). Older subjects had lower mean SV during tilting, likely associated with age-related left ventricular stiffening [27]. Similar findings were reported by Baldi et al. [28].
Active standing versus passive HUT
Active standing and head-up tilt are associated with different physiological processes upon orthostatic change [29]. However, the effects of sex and age observed during active standing were generally similar to those observed during passive HUT (Tables 2, 3). Low et al. [10] compared active standing up and passive HUT in patients with OH. The orthostatic BP fall was greater under HUT than under active standing and was smaller than 5 mmHg. In the present study with healthy subjects, we observed an orthostatic BP and HR increase, which was numerically greater during active standing. Calculating the mean values of 1, 3, and 5 min of HUT and standing, the difference in BP was on average 2.2 (systolic) and 4.1 mmHg (diastolic) and the difference in HR was on average 6.9 bpm. We agree with a recent report [4] arguing that both challenge tests are not interchangeable but complementary in the assessment of orthostatic syndromes.
DB and VM
DB ratio and Valsalva Ratio, both indexes of cardiovagal function, decreased significantly with age. This is in agreement with previous findings [8, 10], which emphasized an age-related decrease of cardiac parasympathetic control and possibly explains the higher incidence of neurally mediated, especially vasovagal, syncopes among healthy subjects with respect to older ones.
Circadian variation
Endogenous and exogenous factors could affect the circadian rhythm on responses of cardiovascular autonomic tests. Sympathetic activity is basically increased during daytime, while parasympathetic activity rises during the night or after meals. Hu et al. [30] assessed in a recent study the effect of circadian system on responses to HUT and found significant circadian rhythms in hemodynamic parameters. Our study showed no significant contribution of time of day on hemodynamic parameters and their changes during HUT. Significant effects of time of day were observed only by both indexes of cardiovagal function. Latter was higher in the afternoon group than in the morning group. This could be explained with a post-prandial vagal activation, despite asking the subjects to fast for 2 hours prior to the scheduled examination.
Limitations
Our study has some limitations. First, both physical activity and inactivity can alter vascular function. We did not assess deconditioning in our study. However, all recruited subjects reported a regular active lifestyle in accordance with local recreational activities (hiking, skiing). Second, 51.9 % of the subjects in this study were tilted between 8:30 and 11:00 am and the rest (49.1 %) after 11:00 am. The circadian system could have affected responses to orthostatic stress. Our statistical model was therefore adjusted for “time of day” in order to control for possible circadian influences in our data.
Conclusions
To our knowledge, this is the first study evaluating sex and age effects using a prolonged HUT protocol and the Task Force® Monitor in a well-defined healthy population. Our study suggests that aging rather than sex needs to be taken into account when interpreting HR and BP changes during prolonged HUT testing.
References
Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG (2011) Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 21:69–72
Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB, Deharo JC, Gajek J, Gjesdal K, Krahn A, Massin M, Pepi M, Pezawas T, Ruiz Granell R, Sarasin F, Ungar A, van Dijk JG, Walma EP, Wieling W (2009) Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J 30:2631–2671
Fitzpatrick AP, Theodorakis G, Vardas P, Sutton R (1991) Methodology of head-up tilt testing in patients with unexplained syncope. J Am Coll Cardiol 17:125–130
Gibbons CH, Freeman R (2006) Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology 67:28–32
Gurevich T, Machmid H, Klepikov D, Ezra A, Giladi N, Peretz C (2014) Head-up tilt testing for detecting orthostatic hypotension: how long do we need to wait? Neuroepidemiology 43:239–243
Fortin J, Habenbacher W, Heller A, Hacker A, Grullenberger R, Innerhofer J, Passath H, Wagner C, Haitchi G, Flotzinger D, Pacher R, Wach P (2006) Non-invasive beat-to-beat cardiac output monitoring by an improved method of transthoracic bioimpedance measurement. Comput Biol Med 36:1185–1203
Fortin J, Marte W, Grullenberger R, Hacker A, Habenbacher W, Heller A, Wagner C, Wach P, Skrabal F (2006) Continuous non-invasive blood pressure monitoring using concentrically interlocking control loops. Comput Biol Med 36:941–957
Braune S, Auer A, Schulte-Monting J, Schwerbrock S, Lucking CH (1996) Cardiovascular parameters: sensitivity to detect autonomic dysfunction and influence of age and sex in normal subjects. Clin Auton Res 6:3–15
Laitinen T, Niskanen L, Geelen G, Lansimies E, Hartikainen J (2004) Age dependency of cardiovascular autonomic responses to head-up tilt in healthy subjects. J Appl physiol (Bethesda, Md: 1985) 96:2333–2340
Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O’Brien PC, Slezak JM (1997) Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve 20:1561–1568
Mellingsaeter MR, Wyller VB, Wyller TB, Ranhoff AH (2013) Gender differences in orthostatic tolerance in the elderly. Aging Clin Exp Res 25:659–665
Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK (2005) Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45:522–525
Petersen ME, Williams TR, Gordon C, Chamberlain-Webber R, Sutton R (2000) The normal response to prolonged passive head up tilt testing. Heart (British Cardiac Society) 84:509–514
Tahvanainen A, Leskinen M, Koskela J, Ilveskoski E, Nordhausen K, Oja H, Kahonen M, Koobi T, Mustonen J, Porsti I (2009) Ageing and cardiovascular responses to head-up tilt in healthy subjects. Atherosclerosis 207:445–451
Freeman R (2006) Assessment of cardiovascular autonomic function. Clin Neurophysiol 117:716–730
Mathias CJ, Low DA, Iodice V, Bannister R (2013) Investigation of autonomic disorders. In: Mathias CJ, Bannister R (eds) Autonomic failure. A textbook of clinical disorders of the autonomic nervous system. University Press, Oxford, pp 259–289
Zeger SL, Liang KY (1986) Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42:121–130
Barnett SR, Morin RJ, Kiely DK, Gagnon M, Azhar G, Knight EL, Nelson JC, Lipsitz LA (1999) Effects of age and gender on autonomic control of blood pressure dynamics. Hypertension 33:1195–1200
Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM (2000) Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36:1233–1238
Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG (2005) Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol 568:315–321
Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ (2009) Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension 53:571–576
London GM, Guerin AP, Pannier B, Marchais SJ, Stimpel M (1995) Influence of sex on arterial hemodynamics and blood pressure. Role of body height. Hypertension 26:514–519
Jones PP, Shapiro LF, Keisling GA, Jordan J, Shannon JR, Quaife RA, Seals DR (2001) Altered autonomic support of arterial blood pressure with age in healthy men. Circulation 104:2424–2429
Brodde OE, Leineweber K (2004) Autonomic receptor systems in the failing and aging human heart: similarities and differences. Eur J Pharmacol 500:167–176
Smulyan H, Asmar RG, Rudnicki A, London GM, Safar ME (2001) Comparative effects of aging in men and women on the properties of the arterial tree. J Am Coll Cardiol 37:1374–1380
Barnes JN, Hart EC, Curry TB, Nicholson WT, Eisenach JH, Wallin BG, Charkoudian N, Joyner MJ (2014) Aging enhances autonomic support of blood pressure in women. Hypertension 63:303–308
Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA (2005) Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation 112:2254–2262
Baldi JC, Lalande S, Carrick-Ranson G, Johnson BD (2007) Postural differences in hemodynamics and diastolic function in healthy older men. Eur J Appl Physiol 99:651–657
Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME (2007) Initial orthostatic hypotension: review of a forgotten condition. Clin Sci (London, England: 1979) 112:157–165
Hu K, Scheer FA, Laker M, Smales C, Shea SA (2011) Endogenous circadian rhythm in vasovagal response to head-up tilt. Circulation 123:961–970
Acknowledgments
We thank all subjects who took part in the present study, for their patience and stamina. This study was supported by grants of the Austrian Science Fund (FWF): F04404-B19 and KLI380. The funder had neither a role in study design, data collection, and analysis, nor in the decision to publish or in the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ndayisaba, JP., Fanciulli, A., Granata, R. et al. Sex and age effects on cardiovascular autonomic function in healthy adults. Clin Auton Res 25, 317–326 (2015). https://doi.org/10.1007/s10286-015-0310-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-015-0310-1