Abstract
Purpose
Compensatory hyperhidrosis (CH) is the most common adverse complication of sympathectomy. It often has a major negative impact on life quality. No efficient treatment of CH is available. We report nine cases of CH after sympathectomy, which were treated with botulinum toxin A/B (BTX) and anticholinergics.
Methods
The patients responded to a dermatology life quality index (DLQI) questionnaire before injections with BTX and 3 weeks after treatment. At the follow-up visit, the participants also ranked the effect of the treatment on a five-grade scale. Three patients had residual sweating after BTX treatment, and received additional anticholinergics at the follow-up visit. Those subjects eventually had a third evaluation with the DLQI.
Results
The DLQI score was, on average, 16.4 before treatment and decreased to 4.8 after BTX injections. Eight out of nine patients were satisfied with the treatment. The average DLQI score decreased to 2.2 when the patients with residual sweating (n = 3) received additional anticholinergics. Adverse events from BTX were mild and temporary, but dry mouth was substantial in one patient using anticholinergics.
Conclusions
A combination of BTX A/B and anticholinergics alleviated the hyperhidrosis with minor side-effects. We consider this treatment safe, effective, and well tolerated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Hyperhidrosis is characterized as excessive sweating beyond physiologic need. It can be defined as primary (idiopathic or essential) or secondary to various medical disorders [28, 30]. Primary focal hyperhidrosis includes excessive sweating from palms, feet, axillae, groin, and craniofacial area [28–30]. Craniofacial hyperhidrosis can be an isolated complaint or can be seen as a part of primary generalized hyperhidrosis, which also involves the abdomen and extremities [28]. The prevalence of hyperhidrosis is 2.8 % among the population of the Western world [27].
Treatment options include aluminum salts, iontophoresis, anticholinergics, and botulinum toxin (BTX) [22]. Severe cases of primary palmar hyperhidrosis and facial blushing are treated surgically by endoscopic thoracic sympathectomy (ETS), which involves electrocautery or clamping of the sympathetic chain at levels Th2–Th4, depending on the indication [5, 9, 15].
The techniques of open surgery that have been used since the 1920s have almost been abandoned in favor of ETS [7]. However, the prevalence of long-term side-effects seems to be independent of whether the open or endoscopic surgical approach is utilized [9].
The most common side-effects of sympathectomy are compensatory hyperhidrosis (CH), followed by phantom sweating, gustatory sweating, neuralgia, and Horner’s syndrome [9]. The prevalence of CH is reported in 30–100 % of patients who have gone through ETS [30]. Most often, 1–6 months postoperatively, excessive sweating appears after little or no effort below the denervation zone, especially on the abdomen [9]. One in four patients who developed late sequelae after sympathectomy considered it disabling and worse than the condition (palmar hyperhidrosis) before surgery [9].
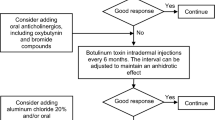
A major problem in the treatment of CH is that patients typically have large areas with excessive sweating which limit the use of BTX type A. However, BTX type B has a greater affinity for sudomotor fibers and alleviates excessive sweating from large areas in relatively small doses [1, 6, 23, 24]. Both BTX type A and B block the presynaptic release of acetylcholine and cotransmitters by destroying different key enzymes in the nerve end-terminals [10]. Another option in the treatment of hyperhidrosis over large areas is the use of anticholinergics, which block the nerve impulse post-synapse at the muscarinic receptor on the sweat gland [13].
The aim of this open prospective study was to investigate life quality before and after injections with BTX alone or in combination with anticholinergics.
Patients and methods
Patients
Nine consecutively included patients, aged 36–69 years, 6 male and 3 female, with CH after sympathectomy, being followed at the Sweat Clinic of Sophiahemmet, Stockholm, between the 1st of September to the 31st of October 2009, participated in the study. Exclusion criteria were neuromuscular diseases, pregnancy, lactation, and previous treatment of CH with BTX and/or anticholinergics. However, none of the patients with CH after sympathectomy were excluded.
The patients had, on average, suffered 16 (12–21) years from CH post-sympathectomy. The indications of sympathectomy were facial blushing (n = 1), facial blushing and palmar hyperhidrosis (n = 1), palmar hyperhidrosis (n = 4), palmar and axillary hyperhidrosis (n = 1), and axillary hyperhidrosis (n = 2).
Eight out of nine patients had suffered from primary hyperhidrosis or facial blushing since childhood. Four of eight patients with hyperhidrosis had a family history.
Before sympathectomy, all patients with primary hyperhidrosis from the axillae and palms had tried aluminum chloride; two had tried iontophoresis; and one had tried anticholinergics. However, none had tried BTX. One patient (no. 6) had, in another clinic, been treated with Dysport® (Ipsen Limited, Slough, UK) for axillary hyperhidrosis (primary focal) after the sympathectomy.
The most common triggering factors for CH were stress, physical activity, and heat. Other contributing factors were certain foods and beverages, and pressure from weight bearing areas.
As shown in Table 1, hyperhidrosis at different locations in combination was common. The most common complaint was excessive sweating from the trunk (see Fig. 1).
The study was approved by the Swedish regional ethics committee in Stockholm and informed consent was collected from all patients.
Methods
The primary objective of this study was to evaluate the clinical effect (change in life quality) of BTX in CH approximately 3 weeks post-treatment. The secondary objectives were to investigate the clinical effect of additional anticholinergics in patients with insufficient response to BTX alone, as well as the safety of these treatments. The primary end-point was the difference in total dermatology life quality index (DLQI) score before and approximately 3 weeks after treatment [8, 11]. The secondary end-points were the score of the global assessment of therapy [18] and the frequency of adverse events.
The patients answered the Swedish translation of the DLQI questionnaire before receiving injections with Xeomin® (Merz Pharma GmbH & Co. KGaA, Frankfurt/Main, Germany) and/or NeuroBloc®/Myobloc® (Eisai Limited, Hatfield, United Kingdom/Solstice Neurosciences, LLC, Louisville, USA) and at the follow-up visit approximately 3 weeks after the treatment. At the follow-up visit all of the patients also ranked the effect on the five-grade scale global assessment of therapy: 1 = no effect at all; 2 = slight, but insufficient reduction of sweating; 3 = moderate, but insufficient reduction of sweating; 4 = marked reduction, residual sweating acceptable; and 5 = sweating disappeared completely.
Patients with insufficient treatment response to BTX received additional treatment with Oxybutynin (Mylan Inc. Morgantown, USA) 5 mg taken 1–3 times per day. These patients answered the DLQI questionnaire a third time, 2 weeks after the addition of Oxybutynin and 5 weeks after the initial injections of BTX (when the maximum effect of the BTX treatment was still expected to be sustained).
At each follow-up, any adverse events were recorded.
Three years after the first treatment, a review of the participants medical records was performed, as well as a telephone interview to evaluate the long-term results.
Injection procedure
Before the injections, the patients had to rank the hyperhidrotic areas from most to least troublesome, in order to prioritize local treatment of the worst areas, when the total affected area was too large to be completely treated with BTX. Large areas, such as the trunk, were injected with NeuroBloc/Myobloc, while small areas, such as feet and palms, were injected with Xeomin; total doses and treated locations are described in Table 1. NeuroBloc/Myobloc 250 U/ml, was given every 15 mm with 7.5 U at each spot and Xeomin 20 U/ml, was given every 15 mm with 1.5 U per injection. To avoid muscle weakness from the palms, NeuroBloc/Myobloc 250 U/ml was injected every 15 mm over the thenar eminence with 7.5 U per injection. All injections were given intradermally with a 0.3-ml syringe and a 0.33 × 12-ml needle (Omnican, Braun, Belgium).
Anesthesia
Bilateral intravenous regional anesthesia with low tourniquet at the wrists or ankles with prilocaine (Citanest®, 5 mg/ml, AstraZeneca), 0.4 mg per kg, was used to treat palmoplantar hyperhidrosis [2]. One patient initially preferred nerve block of the ulnar and median nerves at the wrists with mepivacaine (Carbocaine® 10 mg/ml, AstraZeneca), 5 ml at each nerve. The axillae were injected without anesthesia. The trunk was injected after anesthesia with alfentanil (Rapifen®, 0.5 mg/ml, Janssen) 1–4 ml intravenously.
Statistical analyses
Descriptive statistics were used and calculations were performed in Microsoft Excel.
Results
The DLQI score was, collectively, 16.4 (8–23) before treatment and 4.8 (0–15) at the follow-up, on average 21 days after the injections. However, three patients still had high DLQI scores: patient number 4 (score = 11) , patient number 8 (score = 15) , and patient number 9 (score = 8). Therefore, they received additional treatment with Oxybutynin. The three patients who answered the DLQI questionnaire a third time (2 weeks after the addition of the anticholinergic agent) and had the following score series: patient number 4 scores = 23-11-1; patient number 8 scores = 14-15-10; and patient number 9 scores = 19-8-0. The average DLQI score for the whole group decreased to 2.2 (0–10) when patients with residual sweating received additional anticholinergics (see Fig. 2).
Change in dermatology life quality index (DLQI) score during the study. About 3 weeks after their first botulinum toxin (BTX) injection, the DLQI was normal in six out of nine patients. Patients no. 4, 8, and 9 received additional Oxybutynin at the follow-up visit, which normalized the values of the DLQI in patients no. 4 and 9. The effect on quality of life from BTX alone or in combination with Oxybutynin was remarkably positive in eight out of nine patients
With respect to the global assessment of therapy, eight of nine patients were satisfied, ranked as a 4 or 5, after the first treatment with BTX. Four patients assessed the effect as “sweating disappeared completely” while four other patients assessed the effect as “marked reduction, residual sweating acceptable”. One patient assessed the effect as “moderate, but insufficient reduction of sweating”.
Reported adverse events at the follow-up visits included the following: moderate dry mouth (n = 3); sensation of warmth without fever (n = 1); fatigue, dizziness, and metal taste in the mouth during the 2 weeks after treatment (n = 1); dry feet (n = 1); and compensatory sweating (n = 1). Dry mouth was a more prominent adverse event using oxbutynin, which led patient number 4 to change to another anticholinergic agent, tolterodin, which was accepted with a satisfying effect.
Table 2 shows the adverse events reported during the study visits in detail and also the long-term results captured from the medical records and the telephone interviews. No tachyphylaxis was observed after repeated treatments with BTX. The effect duration was, on average, 3.6 (1–6) months based on the long-term data.
Discussion
CH is a life-long problem in most patients who have gone through sympathectomy, independent of open or endoscopic approach [9], and nearly 14 % regret the surgery [25]. Our study confirms that CH is a major handicap with high pretreatment scores of DLQI. Furthermore, our study clearly shows that CH can effectively be treated with BTX alone or in combination with Oxybutynin with high positive impact on patient’s life quality, reducing high scores of DLQI to normal in 8 of 9 patients. Adverse events were moderate and temporary.
The patients reported CH from the trunk, below the denervation zone, and most prominently under the breast and along the spine of the back. These areas of the trunk are the most important to treat with local BTX injections. An iodine–starch test can be performed prior to injections, but is impractical and unnecessary, because the area of hyperhidrosis is clearly visible. However, before administering injections it is advisable to encourage the patient to do light exercise, such as walking up or down stairs, in order to elicit the area of hyperhidrosis. Other important areas of CH are the groin, buttocks, and feet. These areas are typically affected by primary focal hyperhidrosis, however, sweating from these locations becomes more severe after surgery, which was shown in the study population. In general, the hyperhidrotic areas in CH follow the lines of primary hyperhidrosis, but the margins are widened. When injecting BTX into feet, it is important to treat both the plantar as well as the dorsal areas because the pattern of hyperhidrosis changes after surgery (see Fig. 3).
Illustrative pictures of the difference between primary focal and secondary compensatory hyperhidrosis of the feet. In primary hyperhidrosis of the feet the typical pattern of the affected area is in the shape of a ballerina shoe. Sympathectomy often worsens an already existing primary hyperhidrosis, and widens the area, which results in a pattern of socks
Most studies on BTX are performed on muscles and α-motor neurons with divergent effects from different brands of BTX. When treating muscular disorders, 1 U of Xeomin or Botox® (Allergan Inc, Irvine, CA, USA) correspond to 50–100 U of Neurobloc/Myobloc [3, 4, 12, 17]. However, recent studies on sympathetic sudomotor neurons show that BTX A and BTX B have a similar anhidrotic effect with ratios of 1:1–2 when similar concentrations are used [23, 24]. It therefore seems that BTX B has a weaker effect on α-motor neurons and muscles compared to autonomic sympathetic sudomotor neurons and sweat glands. This discrepancy of effect on cholinergic, somatic, and autonomic fibers can conveniently be used when treating multiple areas of hyperhidrosis, for example CH. In general, BTX A seems to have a longer anhidrotic effect than BTX B at the group level [1, 14, 23], but not necessarily at the individual level. To achieve as long of an effect as possible without systemic side-effects, BTX A was used in small areas such as the feet, and BTX B was used in large areas such as the trunk in the study population. Oxybutynin has more systemic side-effects than local BTX injections, and therefore, we chose BTX for the most bothersome areas of hyperhidrosis. For six out of nine patients this treatment regimen normalized the DLQI scores. When also adding Oxybutynin to the treatment of three patients still having high DLQI scores after BTX injections, eight out of nine were satisfied, with normalized scores.
BTX acts presynaptically and Oxybutynin acts postsynaptically at the sudomotor synapse—the addition of Oxybutynin seems to strengthen and prolong the effect of BTX, while also working at non-injected areas. Oxybutynin is a modern anticholinergic agent that acts on muscarinic receptors type 1 and 3 [20], both present in the sweat glands [16]. The drug has shown good anhidrotic effect in focal hyperhidrosis [31], but seems to be more suitable in general hyperhidrosis and as a complement to potentiate and prolong the effect of BTX.
The patients in the study were intolerant to stress and even more so to warmth, and they suffered from excessive sweating during everyday activities such as walking, dealing with a stressful situation, or being in a very warm room. Their clothes, underwear, and shoes became wet and uncomfortable, leading to a social stigma. The pretreatment DLQI value of 16.4 in our study indicates a severely negatively affected life quality [11] and is similar to DLQI scores in patients with severe psoriasis using biologic drugs [26].
The profession has already abandoned ETS for axillary hyperhidrosis due to its poor effect [21]. For palmar and craniofacial hyperhidrosis, we recommend all other anhidrotic treatments, including BTX and anticholinergics, before ETS. This is in congruence with the guidelines from The National Board of Health and Welfare [19].
Conclusions
CH postsympathectomy is a severe physical and social problem that interferes with daily life, which can be alleviated with BTX alone or in combination with Oxybutynin.
References
Birklein F, Eisenbarth G, Erbguth F, Winterholler M (2003) Botulinum toxin type B blocks sudomotor function effectively: a 6 month follow up. J Invest Dermatol 121:1312–1316
Bosdotter Enroth S, Rystedt A, Covaciu L, Hymnelius K, Rystedt E, Nyberg R, Naver H, Swartling C (2010) Bilateral forearm intravenous regional anesthesia with prilocaine for botulinum toxin treatment of palmar hyperhidrosis. J Am Acad Dermatol 63:466–474
Brashear A, Lew MF, Dykstra DD, Comella CL, Factor SA, Rodnitzky RL, Trosch R, Singer C, Brin MF, Murray JJ, Wallace JD, Willmer-Hulme A, Koller M (1999) Safety and efficacy of NeuroBloc (botulinum toxin type B) in type A-responsive cervical dystonia. Neurology 53:1439–1446
Brin MF, Lew MF, Adler CH, Comella CL, Factor SA, Jankovic J, O’Brien C, Murray JJ, Wallace JD, Willmer-Hulme A, Koller M (1999) Safety and efficacy of NeuroBloc (botulinum toxin type B) in type A-resistant cervical dystonia. Neurology 53:1431–1438
Cramer MN, Jay O (2012) Compensatory hyperhidrosis following thoracic sympathectomy: a biophysical rationale. Am J Physiol Regul Integr Comp Physiol 302:R352–R356
Dressler D, Adib Saberi F, Benecke R (2002) Botulinum toxin type B for treatment of axillar hyperhidrosis. J Neurol 249:1729–1732
Drott C, Claes G, Rex L, Dalman P, Gothberg G, Fahlen T (2001) Long-term effects after surgery for hand sweating and facial blushing. Patients are satisfied in spite of troublesome side-effects. Lakartidningen 98:1766–1772
Finlay AY, Khan GK (1994) Dermatology life quality index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol 19:210–216
Furlan AD, Mailis A, Papagapiou M (2000) Are we paying a high price for surgical sympathectomy? A systematic literature review of late complications. J Pain Off J Am Pain Soc 1:245–257
Grumelli C, Verderio C, Pozzi D, Rossetto O, Montecucco C, Matteoli M (2005) Internalization and mechanism of action of clostridial toxins in neurons. Neurotoxicology 26:761–767
Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY (2005) Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol 125:659–664
Jost WH, Blumel J, Grafe S (2007) Botulinum neurotoxin type A free of complexing proteins (XEOMIN) in focal dystonia. Drugs 67:669–683
Kim WO, Kil HK, Yoon KB, Yoo JH (2010) Treatment of generalized hyperhidrosis with Oxybutynin in post-menopausal patients. Acta Dermato Venereologica 90:291–293
Kranz G, Paul A, Voller B, Posch M, Windischberger C, Auff E, Sycha T (2011) Long-term efficacy and respective potencies of botulinum toxin A and B: a randomized, double-blind study. Br J Dermatol 164:176–181
Krasna MJ (2008) Thoracoscopic sympathectomy: a standardized approach to therapy for hyperhidrosis. Ann Thorac Surg 85:S764–S767
Kurzen H, Berger H, Jager C, Hartschuh W, Naher H, Gratchev A, Goerdt S, Deichmann M (2004) Phenotypical and molecular profiling of the extraneuronal cholinergic system of the skin. J Invest Dermatol 123:937–949
Matarasso SL (2003) Comparison of botulinum toxin types A and B: a bilateral and double-blind randomized evaluation in the treatment of canthal rhytides. Dermatol Surg 29:7–13; discussion 13
Naver H, Swartling C, Aquilonius SM (2000) Palmar and axillary hyperhidrosis treated with botulinum toxin: 1-year clinical follow-up. Eur J Neurol 7:55–62
Raf L (2001) Postoperative complications are frequent after surgery for palmar sweating and facial redness. Effects of the treatment must be considered with regard to the risk of side-effects. Lakartidningen 98:1764–1765
Reitz AB, Gupta SK, Huang Y, Parker MH, Ryan RR (2007) The preparation and human muscarinic receptor profiling of Oxybutynin and N-desethyloxybutynin enantiomers. Med Chem 3:543–545
Rex LO, Drott C, Claes G, Gothberg G, Dalman P (1998) The Boras experience of endoscopic thoracic sympathicotomy for palmar, axillary, facial hyperhidrosis and facial blushing. Eur J Surg 164(S1):23–26
Rodriguez PM, Freixinet JL, Hussein M, Valencia JM, Gil RM, Herrero J, Caballero-Hidalgo A (2008) Side effects, complications and outcome of thoracoscopic sympathectomy for palmar and axillary hyperhidrosis in 406 patients. Eur J Cardio Thorac Surg Off J Eur Assoc Cardio Thorac Surg 34:514–519
Rystedt A, Karlqvist M, Bertilsson M, Naver H, Swartling C (2013) Effect of botulinum toxin concentration on reduction in sweating: a randomized, double-blind study. Acta Dermato Venereologica 93:674–678
Rystedt A, Swartling C, Naver H (2008) Anhidrotic effect of intradermal injections of botulinum toxin: a comparison of different products and concentrations. Acta Dermato Venereologica 88:229–233
Smidfelt K, Drott C (2011) Late results of endoscopic thoracic sympathectomy for hyperhidrosis and facial blushing. Br J Surg 98:1719–1724
Smith CH, Anstey AV, Barker JN, Burden AD, Chalmers RJ, Chandler DA, Finlay AY, Griffiths CE, Jackson K, McHugh NJ, McKenna KE, Reynolds NJ, Ormerod AD (2009) British association of dermatologists guidelines for biologic interventions for psoriasis 2009. Br J Dermatol 161:987–1019
Strutton DR, Kowalski JW, Glaser DA, Stang PE (2004) US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol 51:241–248
Swartling C, Brismar K, Aquilonius SM, Naver H, Rystedt A, Rosell K (2011) Hyperhidrosis—the silent handicap. Lakartidningen 108:2428–2432
Walling HW (2011) Clinical differentiation of primary from secondary hyperhidrosis. J Am Acad Dermatol 64:690–695
Weksler B, Blaine G, Souza ZB, Gavina R (2009) Transection of more than one sympathetic chain ganglion for hyperhidrosis increases the severity of compensatory hyperhidrosis and decreases patient satisfaction. J Surg Res 156:110–115
Wolosker N, de Campos JR, Kauffman P, Puech-Leao P (2012) A randomized placebo-controlled trial of Oxybutynin for the initial treatment of palmar and axillary hyperhidrosis. J Vasc Surg 55:1696–1700
Conflict of interest
Carl Swartling is a shareholder in Hidroskliniken i Sverige AB. Alma Rystedt has received travel cost support in relation to conferences from Merz.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karlsson-Groth, A., Rystedt, A. & Swartling, C. Treatment of compensatory hyperhidrosis after sympathectomy with botulinum toxin and anticholinergics. Clin Auton Res 25, 161–167 (2015). https://doi.org/10.1007/s10286-015-0278-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-015-0278-x