Abstract
The First International Symposium on Cardiac Sympathetic Neuroimaging brought together for the first time clinical and preclinical researchers evaluating autonomic and neurocardiologic disorders by this modality. The invited lectures and posters presented some uses of cardiac sympathetic neuroimaging for diagnosis, prognosis, and monitoring treatments. The Symposium also included a discussion about whether and how to expand the availability of cardiac sympathetic neuroimaging at medical centers in the United States. Here, we review the background for the Symposium, provide an annotated summary of the lectures and posters, discuss some of the take-home points from the roundtable discussion, and propose a plan of action for the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Clinical investigators at a variety of medical centers, including Vanderbilt University Medical Center, the University of Texas, the University of Michigan, the Mayo Clinic, the Cleveland Clinic, Mt. Sinai Medical Center, New York University, and The Johns Hopkins Hospital, have noted that their centers offer 123I-metaiodobenzylguanidine (123I-MIBG) scanning for evaluation of possible pheochromocytoma but not for cardiac sympathetic neuroimaging. Cardiac sympathetic neuroimaging by 123I-MIBG scanning has been used extensively (almost 1,000 published studies culled on “MIBG” and “heart”) in Japan and to a lesser extent in Europe—but hardly at all in the United States.
This discrepancy may be the result of a vicious cycle of lack of availability because of lack of insurance coverage because of lack of clinical trials because of lack of availability. Recognition in the autonomic and more generally in the neurological communities that cardiac sympathetic neuroimaging can be a valuable part of testing of patients with suspected disorders of the autonomic nervous system may help break this cycle. Such recognition requires greater understanding of the clinical settings in which cardiac sympathetic neuroimaging is indicated and agreement about the strengths and weaknesses of the different modalities of testing, the types of information the testing provides, cost factors, and standardization.
The First International Symposium on Cardiac Sympathetic Neuroimaging had the general goal of introducing cardiac sympathetic neuroimaging as an approach for autonomic function testing in research and clinical practice, on a footing equal to physiological, pharmacologic challenge, and neurochemical tests, by convening for the first time researchers who are experts in the field, update the science, educate the membership of the American Autonomic Society (AAS), delineate problems and opportunities, and develop an action plan to increase the use of this modality in the United States.
The Symposium provided an international forum on strengths and weaknesses of cardiac versus brain neuroimaging in the differential diagnosis of Lewy body diseases, assignment of prognosis in common multisystem disorders such as congestive heart failure, and monitoring treatment responses in clinical trials. Importantly, the Symposium included a roundtable to discuss issues such as relative advantages and disadvantages of different imaging techniques and agents and about how to expand the availability of cardiac sympathetic neuroimaging as a research and clinical tool at medical centers in the United States. Having the Symposium take place as part of the AAS annual meeting, the largest annual conference about disorders of the autonomic nervous system, was important for achieving this goal.
Members of the AAS are less familiar with cardiac sympathetic neuroimaging than one might expect. Research and clinical practice in this area consist mainly of medical history and physical examination, physiological tests such as hemodynamic responses to tilt table testing and sweat testing, and occasionally measurements of catecholamine levels or pharmacologic challenge tests. The First International Symposium on cardiac sympathetic neuroimaging familiarized the membership better with this new testing method.
Synopses of invited lectures
Cardiac and extra-cardiac noradrenergic denervation in Parkinson disease (D.S. Goldstein, NINDS, NIH, Bethesda, MD USA)
This presentation provided an update about the status of cardiac and extra-cardiac noradrenergic innervation in Parkinson disease (PD) and related disorders, based on sympathetic neuroimaging using 6-[18F]fluorodopamine positron-emission tomographic (PET) scanning.
Neuroimaging results were analyzed from patients with PD and neurogenic orthostatic hypotension (PD + NOH), PD without NOH, multiple system atrophy (MSA), and pure autonomic failure (PAF). Nigrostriatal 6-[18F]fluorodopa PET scanning was also performed. Skeletal muscle microdialysate levels of dihydroxyphenylglycol (DHPG) provided a neurochemical index of extra-cardiac noradrenergic innervation in patients off levodopa. Relationships were assessed of University of Pennsylvania Smell Identification Test (UPSIT) scores with interventricular septal myocardial 6-[18F]fluorodopamine-derived radioactivity and with the putamen:occipital cortex ratio (PUT:OCC) of 6-[18F]fluorodopa-derived radioactivity.
Virtually all patients with PD + NOH had interventricular septal myocardial 6-[18F]fluorodopamine-derived radioactivity more than two standard deviations below the normal mean. PD + NOH was also characterized by decreased radioactivity in the renal cortex, whereas PD without NOH was not. PD + NOH patients had lower microdialysate DHPG levels than did PD patients without NOH [17].
Among PD patients without NOH, a substantial minority had decreased radioactivity confined to the apex or free wall. In this group, septal 6-[18F]fluorodopamine-derived radioactivity decreased progressively over years, with about a 10% annual decline [3]. In one well-documented case, cardiac sympathetic denervation preceded the onset of the movement disorder by several years [5].
The putamen:occipital cortex (PUT:OCC) ratio of 6-[18F]fluorodopa-derived radioactivity was unrelated to septal 6-[18F]fluorodopamine-derived radioactivity. UPSIT scores were positively correlated with septal 6-[18F]fluorodopamine-derived radioactivity [3] but not with the PUT:OCC ratio of 6-[18F]fluorodopa-derived radioactivity (Fig. 1).
Positron emission tomographic scans of the left ventricular myocardium after i.v. injection of the sympathetic neuroimaging agent, 6-[18F]fluorodopamine. Left panel shows a normal scan, middle panel shows a scan from a patient with Parkinson disease (PD) without neurogenic orthostatic hypotension (NOH), and right panel shows a scan from a patient with PD + NOH. Note neuroimaging evidence for partial loss of sympathetic nerves in the left ventricular free wall in the patient with PD and no NOH and diffusely decreased 6-[18F]fluorodopamine-derived radioactivity in the patient with PD + NOH
Pure autonomic failure patients had normal PUT:OCC ratios but decreased substantia nigra (SN):OCC ratios that resembled those in PD. Post-mortem analyses of tissues from a PAF patient and a PD patient showed similarly low SN tissue concentrations of dopamine and tyrosine hydroxylase activity, but the PD patient had 10-fold lower PUT dopamine and the PAF patient 15-fold lower myocardial norepinephrine concentrations [4].
Conclusions
Virtually all patients with PD + NOH have cardiac sympathetic denervation, as assessed by cardiac 6-[18F]fluorodopamine PET scanning. About half of patients with PD and no NOH also have cardiac sympathetic denervation. Loss of cardiac sympathetic innervation progresses over years in patients with PD and no NOH. Cardiac sympathetic denervation can precede the onset of the movement disorder in PD. PD + NOH, but not PD without NOH, is associated with noradrenergic denervation in the renal cortex, thyroid, skeletal muscle, and the body as a whole. Whereas both PD + NOH and PAF entail cardiac and extra-cardiac noradrenergic denervation, striatal dopaminergic denervation distinguishes PD from PAF.
Implications
PD + NOH seems to be between PAF and PD without NOH. Because PD + NOH and PAF entail both cardiac and extra-cardiac noradrenergic denervation, biopsy of accessible tissues in these disorders may yield valuable information about pathogenesis of Lewy body diseases. In alpha-synucleinopathies, the severity of striatal dopaminergic denervation in individual patients is independent of the severity of cardiac noradrenergic denervation, whereas olfactory dysfunction is related to cardiac noradrenergic denervation in these disorders.
Cardiac noradrenergic denervation in Lewy body diseases (LBD): clinical-pathologic correlations (S. Orimo, Kanto Central Hospital, Tokyo, Japan)
This presentation focused on clinical-pathologic correlations of cardiac noradrenergic denervation in Lewy body diseases (LBDs) such as PD and dementia with Lewy bodies (DLB).
Degeneration of the cardiac sympathetic nerves occurs in both PD and DLB and begins early in the disease progression of PD, accounting for reduced cardiac uptake of 123I-metaiodobenzylguanidine (123I-MIBG) or 6-[18F]fluorodopamine even in early stages of these forms of LBD. We previously reported that degeneration of the distal cardiac sympathetic axons precedes loss of the neuronal cell bodies in the paravertebral ganglia, suggesting distal-dominant degeneration and a “dying-back” centripetal pathogenetic process [13].
Alpha-synuclein is a key molecule in the pathogenesis of PD. This protein is abundant in Lewy bodies and Lewy neurites, and mutations or multiplications of the gene encoding alpha-synuclein cause rare inherited forms of PD. We therefore investigated whether and how alpha-synuclein aggregates are involved in the distal-dominant degeneration of cardiac sympathetic nerves.
Some people who die without clinical parkinsonism have Lewy bodies detected pathologically. Accumulating evidence indicates that incidental Lewy body disease (ILBD) constitutes early, pre-symptomatic PD [2]. Cardiac tissues and paravertebral sympathetic ganglia were therefore obtained for comparison from patients with ILBD. Post-mortem tissues were also obtained from patients with MSA or control subjects.
Immunohistochemical analyses were performed using antibodies against tyrosine hydroxylase (TH), the rate-limiting enzyme in norepinephrine biosynthesis and therefore a marker of noradrenergic nerves, phosphorylated neurofilament as a marker of axons, and phosphorylated alpha-synuclein as a marker of abnormal alpha-synuclein deposits.
Key findings from this study were the following [15]:
-
1.
Alpha-synuclein aggregates in distal epicardial nerve fascicles were more abundant in ILBD with preserved TH-immunoreactive axons than in ILBD with decreased TH-immunoreactivity (Fig. 2).
Fig. 2 Alpha-synuclein and tyrosine hydroxylase (TH) immunoreactivity in epicardial nerve fascicles of patients with incidental Lewy body disease (ILBD), Parkinson disease (PD), multiple system atrophy (MSA), or control subjects. In controls (a, f, k), TH-immunoreactive axons were abundant in epicardial nerves (a), and there were no alpha-synuclein aggregates in the axons (f) or myocardium (k). In ILBD with preserved TH axons (b), alpha-synuclein aggregates were more abundant (g, l) than in ILBD with decreased TH-immunoreactivity (c, h, m). In PD, TH-immunoreactive axons were nearly absent (d), and alpha-synuclein aggregates were sparse (I, n). In the MSA patient, TH-immunoreactive axons were abundant (e), and there were no alpha-synuclein aggregates in the epicardial nerve (j) or myocardium (o). Figure adapted from Orimo et al [15], with permission
-
2.
Alpha-synuclein aggregates in the epicardial nerve fibers were closely related to disappearance of TH-immunoreactive axons.
-
3.
In ILBD with preserved TH-immunoreactive axons, alpha-synuclein aggregates were consistently more abundant in the epicardial nerves than in the paravertebral sympathetic ganglia.
-
4.
Distal-dominant accumulation of alpha-synuclein aggregates was reversed in ILBD with decreased TH-immunoreactive axons and in PD, because both conditions involved fewer alpha-synuclein aggregates in axons and more abundant aggregates in the paravertebral sympathetic ganglia.
-
5.
MSA differed markedly from both ILBD and from PD, because in MSA, TH-immunoreactive axons were basically well preserved, and alpha-synuclein aggregates were rarely observed in both epicardial nerves and paravertebral sympathetic ganglia. Unusual patients with MSA and decreased epicardial TH-immunoreactive axons also had evidence of Lewy bodies in sympathetic ganglia [14].
Conclusions
Accumulation of alpha-synuclein aggregates in distal cardiac sympathetic axons precedes aggregation in neuronal somata or ganglionic neurites, heralding centripetal degeneration of cardiac sympathetic nerves in PD, which sharply contrasts with slight changes in most patients with MSA. This chronological and dynamic relationship between alpha-synuclein aggregation and distal-dominant degeneration of cardiac noradrenergic nerves may represent the pathological mechanism underlying a common degenerative process in PD.
Implications
Cardiac noradrenergic denervation in LBDs, even in early stages, accounts for reduced cardiac uptake of 123I-MIBG and 6-[18F]fluorodopamine in PD. Alpha-synuclein aggregation seems intimately involved in the cardiac noradrenergic denervation that attends LBDs.
Takotsubo cardiomyopathy: a form of severe, reversible heart failure evoked by emotional distress in postmenopausal women (T. Ueyama, Wakayama Medical University, Wakayama, Japan)
Several relatively recent case reports and series have described a condition featuring symptoms and signs of acute myocardial infarction, without demonstrable coronary artery stenosis or spasm, in which the heart takes on the appearance of a Japanese octopus fishing pot, called a takotsubo. In takotsubo cardiomyopathy (also called transient apical ballooning and stress cardiomyopathy), left ventricular dysfunction, which can be remarkably depressed, recovers within a few weeks.
The most frequent symptoms of takotsubo cardiomyopathy on admission are chest pain and dyspnea, resembling acute myocardial infarction. Electrocardiographic findings on admission often include ST elevation in precordial leads.
Takotsubo cardiomyopathy occurs predominantly in postmenopausal women, soon after exposure to sudden, unexpected emotional or physical stress.
The myocardial histological changes in takotsubo cardiomyopathy resemble strikingly those seen in catecholamine cardiotoxicity. These changes, which differ from those in ischemic cardiac necrosis, include contraction band necrosis, neutrophil infiltration, and fibrosis. It has been proposed that ionized calcium overload in myocardial cells produces the ventricular dysfunction in catecholamine cardiotoxicity. Although diffuse heart failure can produce high circulating catecholamine concentrations, the attained levels are not nearly as high as in takotsubo cardiomyopathy and would not explain the takotsubo pattern.
The development of appropriate animal models has begun to address how psychological stress triggers the condition, whether regional heterogeneity of adrenoceptor numbers sufficient to explain apical ballooning, and why elderly women are particularly susceptible to develop takotsubo cardiomyopathy. In rats, simple immobilization (IMMO) evokes profound sympathoadrenal activation [10]. During IMMO, the increment in plasma EPI is derived wholly from the adrenal medulla, whereas most of the increment in plasma NE is derived from sympathetic nerves. Patients with takotsubo cardiomyopathy have increased plasma EPI and NE levels comparable to those seen during IMMO in rats [21].
In response to IMMO, pathologically high EPI and NE levels are associated with characteristic electrocardiographic changes including ST segment elevation and, importantly, reversible left ventricular apical ballooning, reproducing strikingly the abnormalities seen in takotsubo cardiomyopathy.
Estrogen receptors (ERα and ERβ) are expressed widely in the cardiovascular and central nervous systems. We hypothesized that reduced estrogen levels following menopause explains the predisposition of elderly women to develop takotsubo cardiomyopathy. Estrogen supplementation attenuated the IMMO-induced cardiac dysfunction and increased heart rate and blood pressure [18]. Reduced estrogen levels induced vulnerability to stress, whereas estrogen supplementation attenuated the exaggerated responses, including the sympathoadrenal activation and vagal inhibition, which is observed in takotsubo cardiomyopathy.
Emotional stress causes rapid, transient expression of immediate early genes in the brain. Treatment with estrogen attenuated IMMO-induced increase of c-Fos immunoreactivity or c-fos mRNA expression in the lateral septum, medial amygdaloid nucleus, paraventricular hypothalamic nucleus, dorsomedial hypothalamic nucleus, laterodorsal tegmental nucleus, and locus ceruleus, regions that are parts of the central autonomic network and possess immunoreactive estrogen receptors. Estrogen attenuated IMMO-induced c-fos mRNA expression in the adrenal gland and the heart, suggesting protective effects at the level of the target organs [19]. Estrogen treatment also increased levels of possibly cardioprotective substances such as atrial natriuretic peptide and heat shock protein-70 in the heart. Conversely, bilateral ovariectomy (OVX) increased the sensitivity of cardiac myofilaments to ionized calcium, and estrogen replacement abolished this change. The density and protein content of β1-adrenergic receptors were upregulated in OVX, and estrogen/progesterone supplementation reversed these changes.
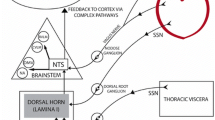
Taken together, these data suggest that reduced estrogen levels, by actions in both the nervous system and the heart, following menopause might constitute the basis of susceptibility of elderly women to takotsubo cardiomyopathy. Based on the above clinical literature and findings from the rat IMMO model, we propose a possible mechanism for the pathogenesis of takotsubo cardiomyopathy [1]. In response to sudden, unexpected, severe emotional distress, neurons of the central autonomic network expressing estrogen receptors are activated, followed by marked increases in sympathetic neuronal and adrenomedullary hormonal outflows (Fig. 3). EPI released from the adrenal medulla and NE from cardiac and extra-cardiac sympathetic nerves reach adrenoceptors in the blood vessels and heart. Contraction of the resistance vessels rapidly increases the systemic blood pressure and cardiac afterload. Meanwhile, within the heart, high circulating levels of NE and EPI, along with increased release and decreased reuptake by sympathetic nerves, induce catecholamine toxicity in the cardiomyocytes via occupation of adrenoceptors. Hypercontraction and possibly functional basal obstruction of left ventricular outflow result in increased mechanical wall stress in the left ventricular apex, in conjunction with high BNP levels and increased end-diastolic pressure. Contraction bands and rupture of myocardial cells occur in a regionally heterogeneous manner related to greater apical expression of adrenoceptors. Disturbance of the coronary microcirculation, as suggested by diffuse expression of immediate early genes in coronary arteries, and increases of oxygen demand in the cardiomyocytes alter the redox state and trigger oxidative stress, which precipitates a variety of positive feedback loops that leads to stunning of the apical myocardium. In postmenopausal women, the loss of estrogen effects exaggerates the responses of central neurons and cardiac cells and possibly attenuates production of cardioprotective substances.
Concept diagram for the degenerative process of the cardiac sympathetic nervous system. In ILBD, alpha-synuclein aggregates are abundant in distal axons, in contrast to sparse aggregates in paravertebral sympathetic ganglia. In ILBD, the number of alpha-synuclein aggregates in distal axons decreases, while the number increases in the ganglia. In PD, alpha-synuclein aggregates in the distal axons disappear with regression of TH-immunoreactive axons, while alpha-synuclein aggregates accumulate abundantly in the ganglia. In most patients with MSA and in controls, alpha-synuclein aggregates are not seen. Black areas indicate alpha-synuclein aggregates. Dashed lines indicate degeneration of TH-immunoreactive axons. ILBD(a) refers to Idiopathic Lewy body disease with preserved TH-immunoreactive neurons, and ILBD(b) to ILBD with decreased TH-immunoreactive neurons
Brain and cardiac neuroimaging to distinguish Parkinson disease from multiple system atrophy (G. Wenning, University of Innsbruck, Austria)
Early, severe autonomic involvement has been thought to be exclusionary for Parkinson disease (PD) [16]; however, in patients with parkinsonism and autonomic failure, PD and the parkinsonian form of multiple system atrophy (MSA-P) overlap in terms of autonomic symptom scores and physiological measures such as beat-to-beat blood pressure responses to the Valsalva maneuver, thermoregulatory sweat testing, and the sympathetic skin response.
Many studies using radioiodinated metabiodobenzylguanidine (MIBG) have noted a decrease in the ratio of radioactivity in the heart to that in the mediastinum (H:M). Here, we report the relative diagnostic efficiency of MIBG scanning and diffusion-weighted magnetic resonance imaging (DWI) in separating PD from MSA.
Subjects received 185 MBq of 131I- or 123I-MIBG. For 123I-MIBG scintigraphy, planar images of the thorax in anterior view were acquired 15 minutes (early images) and 4 hours (late images) after intravenous injection of a single dose of 185 MBq 123I-MIBG using a double-headed gamma camera (ADAC Vertex Epic, Phillips) equipped with VXHR-collimators (optimized collimator for MIBG). 123I-MIBG uptake was quantified by comparing regions of interest (ROI) over heart and mediastinum. An irregular region was drawn enclosing the heart. A rectangular region outside the lungs and below the thyroid was used to quantify uptake of the upper and anterior mediastinum. Based on the ROI, cardiac 123I-MIBG uptake was expressed as heart to mediastinum ratio (H:M ratio). The ratio was calculated by dividing the counts per pixel in the region enclosing the heart by the counts per pixel in the mediastinal area.
Diffusion-weighted magnetic resonance imaging sequences were performed using a 1.5 T whole body MR scanner (Magnetom Vision, Siemens, Erlangen, Germany) and a circular polarized head coil. DWI scans were acquired using an echoplanar imaging sequence with diffusion-sensitizing gradients switched in three orthogonal directions and three different b values (0, 500, and 1,000 seconds/mm2). Sequential sampling of k-space was used with an effective TE of 110 ms, a bandwidth of 833 Hz/pixel, and an acquisition matrix of 128 × 128 interpolated to 256 × 256 during image calculation. Apparent diffusion coefficient (ADC) maps were calculated by fitting the logarithm of the signal intensity as a function of the gradient factor b over three different b values for each pixel. After calculation of ADC maps, regional ADC values were determined by segmentation of the striatum using the echoplanar images with the lowest b value. These images provide a good contrast between gray and white matter and permit a sufficient differentiation of different brain structures. The segmented regions of interests (ROIs) were copied on the ADC maps to obtain mean regional (r)ADC values. Additionally, we placed ROIs in the cerebrospinal fluid space at mid-ventricular level of each patient. To avoid CSF contamination and partial volume effects, we excluded all ADC pixel values that were higher than the mean CSF rADC minus 2 SD.
In the MIBG study, there were 11 PD, 9 MSA-P patients, and 9 controls. Five of the 9 MSA patients and 4 of the 11 PD patients had orthostatic hypotension, defined by a fall in systolic blood pressure of more than 20 mm Hg or in diastolic pressure of more than 10 mm Hg. At both 15 minutes and 4 hours after 131I-MIBG injection, PD patients had lower mean H:M ratios of 131I-MIBG-derived radioactivity than did MSA patients or control subjects, whereas the MSA and control groups did not differ. Calculations of regional ADCs after DWI scanning showed increased mean values in MSA-P with respect to PD and controls, whereas the PD and control groups did not differ [9]. Overall, MIBG scanning had a sensitivity of 72.7% and specificity of 66.7%, and DWI had a sensitivity of 100% and specificity of 100%.
Conclusions
Both cardiac MIBG scanning and brain DWI can distinguish MSA from PD. H:M ratios of MIBG-derived radioactivity are low in PD but not in MSA. ADC scores are high in MSA but not in PD. DWI seems more sensitive and specific.
Implications
Either MIBG scanning or DWI can separate MSA from PD; however, DWI seems more sensitive and specific.
Relationships among nigrostriatal dopamine deficiency, cardiac norepinephrine deficiency, and olfactory dysfunction in Parkinson disease (P.H. Lee, Ajou University School of Medicine, Suwon, Korea)
Patients with Parkinson disease (PD) have markedly impaired olfactory function. Olfactory impairment can be an early manifestation of PD and can precede development of the movement disorder. Moreover, pathological studies have recognized deposition of alpha-synuclein in the olfactory bulb as one of the earliest findings in PD.
Cardiac sympathetic denervation is also common in PD, can be found even in patients with de novo disease, and can precede the movement disorder. Recent pathological studies have supported the view that autonomic involvement also occurs early in the pathogenesis of PD.
Olfaction and cardiac sympathetic innervation therefore represent two non-motor systems that, in addition to the nigrostriatal dopaminergic system, seem to be part of the widespread pathological manifestations of PD. To evaluate the correlation between these two systems, we investigated whether there is an association between olfaction function and cardiac 123I-MIBG uptake in patients with PD.
We prospectively enrolled 26 patients with PD, and 15 healthy controls. PD was diagnosed according to the United Kingdom PD Society Brain Bank Clinical Diagnosis Criteria. For assessing olfactory function and the cardiac uptake experiments, the Cross-Cultural Smell Identification (CCSI) test and the ratio of 123I-MIBG uptake in the heart to that in the mediastinum were used. The severity of parkinsonism was assessed according to the classification of Hoehn and Yahr (H&Y).
The mean CCSI score in patients with PD was 4.4 ± 2.2, significantly lower than that in controls (7.3 ± 2.6). There was a significant positive correlation of cardiac 123I-MIBG uptake and the CCSI score in patients with PD (r = 0.56, P = 0.003), and the correlation remained significant after adjusting for age (r = 0.47, P = 0.014). The CCSI score and cardiac 123I-MIBG showed a significant inverse correlation with age (r = 0.39, P = 0.048 and r = 0.62, P = 0.001); however, neither the CCSI score nor cardiac 123I-MIBG uptake was significantly correlated with disease duration or the H&Y stage [11].
Conclusions
Our data suggest that in PD, functional losses of the olfactory and cardiac sympathetic systems are closely coupled and are independent of the clinical rating of motor status.
Implications
Because the olfactory bulb receives scant dopaminergic innervation but substantial noradrenergic innervation derived from the locus ceruleus, the association between olfactory function and cardiac sympathetic innervation, despite no relationship with H&Y scores, suggests that the pathogenetic process leading to loss of noradrenergic neurons may differ in some respects from the process leading to loss of nigrostriatal neurons and the movement disorder.
Cardiac sympathetic innervation and function in patients with heart failure (S. Kasama, Gunma University School of Medicine, Gunma, Japan)
In chronic heart failure (CHF), abnormalities of cardiac uptake or retention of 123I-MIBG-derived radioactivity are associated with adverse prognosis, and treatments that improve cardiac function or symptoms also improve abnormalities of cardiac 123I-MIBG-derived radioactivity [7, 8, 12, 20].
Approximately 50% of patients with CHF show preservation of the left ventricular ejection fraction (LVEF). It has been reported that angiotensin-receptor blocker (ARB) therapy improves cardiac sympathetic nerve activity in CHF patients who have reduced LVEF. The effect of ARB therapy on cardiac sympathetic nerve activity evaluated by 123I-MIBG scintigraphy has not been determined in CHF patients with preserved LVEF. Accordingly, this study was performed to determine whether candesartan could improve cardiac sympathetic nerve activity in patients with CHF and an LVEF above 40%.
We selected 50 patients with non-ischemic CHF and an LVEF greater than 40% who were treated with standard therapy. Twenty-five patients were randomized to receive candesartan additionally, while the remaining 25 patients received a placebo additionally. The delayed heart:mediastinum (H:M) ratio, delayed total defect score (TDS), and washout rate (WR) were determined by 123I-MIBG scintigraphy before and after 6 months of therapy. The left ventricular end-diastolic volume (LVEDV) and LVEF were determined by echocardiography, and the plasma brain natriuretic peptide (BNP) concentration was also measured.
In patients receiving candesartan, TDS decreased consistently, from 28 ± 8 to 23 ± 8 (P < 0.0005), the H:M ratio increased from 1.87 ± 0.24 to 2.00 ± 0.22 (P < 0.005), and WR decreased from 37 ± 11 to 32 ± 8% (P < 0.005). LVEDV decreased from 114 ± 38 to 90 ± 27 ml (P < 0.05), and LVEF increased from 54 ± 7% to 58 ± 10% (P < 0.05). In contrast, there were no significant changes in these parameters in patients receiving placebo. There was a significant correlation between the changes in 123I-MIBG scintigraphic findings and the percent change in BNP from baseline to 6 months in patients receiving candesartan (TDS, r = 0.587, P < 0.005; H:M ratio, r = −0.509, P < 0.01; and WR, r = 0.602, P < 0.005) [6].
Conclusions
The TDS, H:M, and WR determined by 123I-MIBG scintigraphy all improve after 6 months of candesartan treatment. The left ventricular volume and cardiac functions also improve. Furthermore, the plasma BNP concentration decreases. There is a significant correlation between changes in 123I-MIBG scintigraphic findings and percent change in BNP from baseline to 6 months in patients receiving candesartan. These findings suggest that adding candesartan to standard therapy can improve cardiac sympathetic and left ventricular performance in CHF patients with preserved LVEF.
Implications
Treatments such as angiotensin II receptor blockade that improve clinical and cardiac function status in CHF also improve cardiac sympathetic function, as indicated by 123I-MIBG scanning.
Poster highlights
Marumoto et al. carried out 123I-MIBG scintigraphy in groups of patients with PD, dementia with Lewy bodies (DLB), progressive supranuclear palsy (PSP), the parkinsonian form of multiple system atrophy (MSA-P), the cerebellar form of MSA (MSA-C), vascular parkinsonism (VP), drug-induced parkinsonism (DIP), juvenile parkinsonism (JP), and controls. Mean H:M ratios of 123I-MIBG-derived radioactivity were subnormal in PD and DLB but not in MSA, VP, DIP, or JP. In some MSA-P patients, however, 123I-MIBG-derived radioactivity was low, and some PD patients had normal radioactivity.
Takeda et al. examined 123I-MIBG scintigraphy in patients with early parkinsonism and assessed its usefulness for differential diagnosis. Seventeen patients with more than one parkinsonian symptom (resting tremor, akinesia, rigidity, retropulsion, masked face, or parkinsonian gait) within 2 years from their onset were enrolled. The uptake ratio of heart to mediastinum (H:M) and washout ratio (WR) were calculated. All 17 patients were reassessed after more than a year, when a final clinical diagnosis was made. The diagnoses were as follows: PD, 10 cases; DLB, 1 case; essential tremor, 3 cases; MSA, 1 case; multiple cerebral infarctions, 1 case; and idiopathic normal pressure hydrocephalus, 1 case. In the groups with PD or DLB, the mean H:M was decreased. There were statistically significant differences in both H:M (P = 0.002) and WR (P < 0.001) between the PD/DLB group and the non-PD/DLB group. Evaluation of H:M and WR, therefore, is useful to differentiate PD and DLB from other related disorders in the early phase of neurodegenerative disorders.
Uchiyama et al. assessed whether increased 123I-MIBG washout has an independent differential diagnostic value in patients with PD and to clarify the clinical significance of the washout rate (WR). After excluding patients with diabetes mellitus and obvious cardiac disease, 103 patients with parkinsonism were enrolled in the study. The heart to mediastinum ratio (H:M) of 123I-MIBG-derived radioactivity was calculated both for early (15 minutes) and delayed (4 hours after injection) images. The WR from early to delayed images was also calculated. Decreased early H:M was defined as ≤ 1.6 based on institutional control studies. The final diagnosis was confirmed based on clinical findings during more than 6 months of follow-up. Clinical diagnosis and findings such as age, tremor, rigidity, bradykinesia, and orthostatic hypotension (OH) were compared with respect to the 123I-MIBG WR. Among 71 patients with H:M > 1.6 (high H:M group), a WR > 30% had 72% sensitivity, 66% specificity, 72% positive predictive value, and 66% negative predictive value for the diagnosis of Lewy body disease (LBD), meaning PD or dementia with Lewy bodies. Multivariate analysis showed that WR was independently correlated with diagnosis of LBD, with age and orthostatic hypotension among the clinical parameters. The investigators concluded that the 123I-MIBG WR has an incremental diagnostic value to differentiate PD or DLB from other parkinsonian syndromes among patients with normal or borderline values for initial H:M; however, the WR is also increased in elderly subjects and OH patients without PD/DLB, which should be considered when this parameter is used.
Iijima et al. reported on olfactory function and its relation to cardiac 123I-MIBG scanning results in non-demented patients with Parkinson disease (PD). For 123I-MIBG, myocardial scintigraphy, planar imaging in the anterior view was done using a single head gamma camera 15 minutes (early) and 4 hours (delayed) after intravenous injection of 123I-MIBG (111 MBq). The smell identification test included 12 odorants. The subject sniffed odor and then chose 1 of 6 possible answers: 4 pictures of entities associated with the odors labeled with their names, 1 of which was correct, and 2 other ones (“unknown” and not detected”). The number of correct answers in the smell identification test correlated significantly with the H:M ratio of 123I-MIBG-derived radioactivity, in both the early and delayed phases, and with the radioactivity washout rate (p < 0.001). In contrast, there was no correlation between Unified Parkinson Disease Rating Scale scores and the number of correct answers, H:M ratios, or washout rate. The investigators concluded that the cardiac sympathetic innervation degenerates in parallel with the olfactory system in idiopathic PD.
Kashihara et al. studied patients with rapid eye movement sleep behavior disorder (RBD), which is common in PD. The investigators compared 123I-MIBG scanning results in groups with PD, RBD, and controls. When compared with controls, both the PD and RBD groups had decreased early and delayed H:M ratios of 123I-MIBG-derived radioactivity. In RBD, abnormal 123I-MIBG scanning results were consistent across patients, as indicated by low inter-individual variance.
Maeda et al. compared susceptibility-weighted imaging (SWI), a new method in magnetic resonance imaging, and cardiac 123I-MIBG scanning in PD patients. SWI is useful in identifying the substantia nigra (SN) and in evaluating SN signal intensity. An increase in SN signal correlates with clinical severity of parkinsonism. The H:M ratio of 123I-MIBG-derived radioactivity were decreased in all of 10 PD patients. The H:M ratio was lower in the higher signal intensity subgroup than in the lower signal intensity subgroup.
Raffel et al. presented results of preclinical investigations of 11C-phenethylguanidines as radiotracers for quantifying cardiac sympathetic nerve density by PET scanning (MIBG has a phenethylguanidine chemical structure.) In an isolated rat heart system, neuronal uptake rates (K up, mL/min/g wet) and neuronal retention times (T 1/2, min) of 11C-phenethylguanidines were measured. N-[11C]guanyl-meta-octopamine (GMO) showed the most favorable kinetics and provided high quality heart images.
In another report, Raffel et al. described PET measures of cardiac and nigrostriatal denervation in parkinsonian syndromes. The study tested the hypothesis that cardiac denervation can be used to differentiate idiopathic PD from MSA and progressive supranuclear palsy (PSP) by using the sympathetic neuroimaging agent 11C-meta-hydroxyephedrine (HED) PET scanning. Striatal presynaptic monoaminergic nerve density was measured with 11C-dihydroxytetrabenazine (DTBZ), to assess if nigrostriatal denervation correlated with cardiac denervation. Cardiac HED scans demonstrated extensive cardiac denervation in four of the nine IPD patients; however, global cardiac denervation was also seen in 2 of 10 MSA patients. Substantial regional denervation was also seen in 2 of 8 PSP patients. DTBZ studies demonstrated striatal denervation in all PD patients and in most MSA and PSP patients. No correlation was found between cardiac HED retention and striatal DTBZ binding. The investigators concluded that since cardiac denervation was observed not only in PD patients but also in some MSA and PSP patients, this clinical finding cannot be used independently to discriminate PD from other movement disorders.
Nagayama et al. carried out a longitudinal study of cardiac 123I-MIBG scintigraphy in patients with MSA. Approximately 30% of MSA patients had decreased 123I-MIBG uptake. There was no correlation with clinical features, and MIBG uptake remained unchanged over time.
Yoshita et al. compared cardiac 123I-MIBG scanning results in progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and idiopathic Parkinson disease (PD). There were 16 patients with PSP, 8 with CBD, and 10 with PD, and 10 controls. Heart:mediastinum (H:M) ratios of 123I-MIBG-derived radioactivity were lower in patients with PD than in patients with CBD or PSP and controls. Six patients with PSP had low H:M ratios, whereas the ratios were not different between patients with CBD and controls. The investigators concluded that PD is characterized by low H:M ratios of 123I-MIBG-derived radioactivity and that cardiac sympathetic denervation is found in some patients with PSP but not in CBD.
Nunomura et al. evaluated cardiac 123I-MIBG scintigraphy in patients with dementia. 123I-MIBG scintigraphy was performed in a series of 105 outpatients with symptoms and signs of progressive dementia. The cases were examined at the time of initial examination for the presence of important symptoms for diagnosing dementia with Lewy bodies (DLB), such as hallucinations, fainting or orthostatic hypotension, parkinsonism, and fluctuations in cognitive function during the follow-up observation period (1–3 years). There were 23 cases with decreased cardiac uptake of 123I-MIBG—more than 20% of the patients. Over half of the 23 cases were suspected of having DLB using diagnostic criteria upon initial examination. In addition, the majority of these 23 cases had findings characteristic of DLB during the follow-up observation period and were diagnosed as having probable or possible DLB. Nevertheless, some cases did not satisfy clinical diagnostic criteria for DLB, initially or during follow-up. 123I-MIBG scintigraphy, therefore, seems to be useful in diagnosing cases of dementia differentially; however, there are some cases for which it is difficult to diagnose DLB. It will therefore be necessary to conduct a more detailed study incorporating a longer observation period and adding postmortem pathological studies.
Furuhashi et al. reported that in patients with various chronic heart diseases and without chronic kidney disease, cardiac 123I-MIBG scanning results (specifically the delayed heart:mediastinum (H:M) ratio of 123I-MIBG-derived radioactivity) predicted cardiac events.
Emerging concepts
All studies to date agree that PD is associated with loss of cardiac sympathetic nerves. The loss is especially pronounced in PD with neurogenic orthostatic hypotension (NOH). In fact, at the roundtable discussion, when participants were canvassed about the results of cardiac sympathetic neuroimaging in PD + NOH, none could recall a single case in which there was intact cardiac sympathetic innervation. Most studies of cardiac sympathetic neuroimaging in PD, however, have not stratified patients in terms of NOH.
Based on the posters by Raffel et al., Yoshita et al., Nagayama et al., and Marumoto et al., a minority of MSA patients have neuroimaging evidence of cardiac sympathetic denervation. Given the postmortem neuropathologic findings described by Orimo, degeneration of the cardiac sympathetic nerves may occur in such patients. Moreover, such patients may have not only alpha-synuclein deposition in glial cells but also in catecholaminergic neurons. It will be important to follow patients with a clinical diagnosis of MSA and cardiac sympathetic denervation, to determine if their prognosis is worse than in patients with MSA and intact cardiac sympathetic innervation.
The presentations by Goldstein and Lee and poster by Iijima agree on a positive correlation between olfactory dysfunction and cardiac sympathetic denervation in parkinsonian syndromes, independently of the severity of the movement disorder or neuroimaging evidence of nigrostriatal dopaminergic denervation. Since the olfactory bulb, which is a site of early alpha-synucleinopathy in PD, receives scant dopaminergic denervation and extensive noradrenergic innervation derived from the locus ceruleus, it is possible that the correlation between olfaction and cardiac innervation reflects independent noradrenergic versus dopaminergic lesions in PD.
The striking neuropathologic findings reported by Orimo demonstrate an association between distal, centripetal alpha-synucleinopathy in cardiac sympathetic nerves as an early pathogenetic finding in PD. Future studies must examine in detail relationships between alpha-synuclein deposition and dying-back of catecholaminergic neurons in PD and related disorders.
Should cardiac sympathetic neuroimaging have a CPT code?
At the roundtable discussion following the oral presentations and poster session, participants were asked to consider whether the evidence about clinical utility of cardiac sympathetic neuroimaging is sufficient to justify this procedure having a current procedural terminology (CPT) code. CPT is a registered trademark of the American Medical Association (AMA).
It was pointed out that obtaining a CPT code enables billing for procedures, but the decision about third party payment is up to the payer. Having a CPT code also does not imply that there is scientific evidence proving the efficacy of the test.
According to the AMA’s website, Category I CPT codes describe a procedure or service identified with a five-digit CPT code and descriptor nomenclature. The inclusion of a descriptor and its associated specific five-digit identifying code number in this category of CPT codes is generally based upon the procedure being consistent with contemporary medical practice and being performed by many physicians in clinical practice in multiple locations. The Advisory Committee and the Editorial Panel require that the service/procedure receive approval from the Food and Drug Administration (FDA) for the specific use of devices or drugs; that the service/procedure is performed across the country in multiple locations; that many physicians or other health care professionals perform the service/procedure; and that the clinical efficacy of the service/procedure has been well established and documented. By these criteria, cardiac sympathetic neuroimaging, such as by 123I-MIBG scanning, is not a candidate for a Category I CPT code.
Category II CPT codes are intended to facilitate data collection by coding certain services and/or test results that are agreed upon as contributing to positive health outcomes and quality patient care. This category of CPT codes is a set of optional tracking codes for performance measurement. As such, this category seems not to be appropriate for cardiac sympathetic neuroimaging.
The most appropriate CPT coding category seems to be Category III, referring to Emerging Technology. The purpose of this category of codes is to facilitate data collection on and assessment of new services and procedures. These codes are intended to be used for data collection purposes to substantiate widespread usage or in the FDA approval process. As such, the Category III CPT codes may not conform to the usual CPT code requirements that services/procedures be performed by many health care professionals across the country; FDA approval be documented or be imminent within a given CPT cycle; and the service/procedure has proven clinical efficacy. Instead, the service/procedure must have relevance for research, either ongoing or planned.
Category III CPT codes will be assigned an alphanumeric identifier with a letter in the last field (e.g., 1234B). These codes will be located in a separate section of CPT, following the Medicine section. Introductory language will be placed in this code section to explain the purpose of these codes. These codes will be sunset after 5 years if the code has not been accepted for placement in the Category I section of CPT, unless demonstrated that a Category III code is still needed. These codes will not be reused.
Based on these considerations, successful application for Category I CPT coding for cardiac sympathetic neuroimaging may depend on the accumulation of sufficient experience with the procedure at multiple academic medical centers in the US to support FDA approval. Given the current climate of medical economics, such approval may require demonstration projects supported by industry. The presentations at the First International Symposium on Cardiac Sympathetic Neuroimaging may provide a springboard to convince domestic companies, such as manufacturers of 123I-MIBG, of the potential benefits of support this avenue or research.
Future trends
The finding of loss of cardiac noradrenergic innervation, as assessed by sympathetic neuroimaging, may identify Lewy body diseases such as Parkinson disease in a pre-symptomatic phase, when neuroprotective or neurorescue treatments would be expected to be most effective.
Combined assessments of nigrostriatal dopaminergic and cardiac noradrenergic innervation may confirm independence of central dopaminergic from peripheral sympathetic pathophysiology in PD.
Cardiac sympathetic neuroimaging offers the potential to track responses to putative neuroprotective or neurorescue treatments.
Reversible decreased uptake and increased washout of 123I-MIBG-derived radioactivity in conditions such as heart failure and cardiomyopathy may reflect a combination of increased cardiac sympathetic nerve traffic and decreased efficiency of energy requiring neuronal uptake and vesicular sequestration.
Greater understanding of interactions of alpha-synuclein with cytoplasmic catecholamines and determinants of distal-dominant, centripetal denervation may help elucidate pathogenetic mechanisms of autonomic failure syndromes and Parkinson disease.
References
Akashi YJ, Goldstein DS, Barbaro G, Ueyama T Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation (in press)
Dickson DW, Fujishiro H, DelleDonne A, Menke J, Ahmed Z, Klos KJ, Josephs KA, Frigerio R, Burnett M, Parisi JE, Ahlskog JE (2008) Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathologica 115:437–444
Goldstein DS, Holmes C, Bentho O, T. S, Moak J, Sharabi Y, Imrich R, Conant T, Eldadah BA Biomarkers to detect central dopamine deficiency and distinguish Parkinson disease from multiple system atrophy. Parkinsonism Relat Disord (in press)
Goldstein DS, Holmes C, Sato T, Bernson M, Mizrahi N, Imrich R, Carmona G, Sharabi Y, Vortmeyer AO (2008) Central dopamine deficiency in pure autonomic failure. Clin Auton Res (in press)
Goldstein DS, Sharabi Y, Karp BI, Bentho O, Saleem A, Pacak K, Eisenhofer G (2007) Cardiac sympathetic denervation preceding motor signs in Parkinson disease. Clin Auton Res 17:118–121
Kasama S, Toyama T, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, Kurabayashi M (2005) Effects of candesartan on cardiac sympathetic nerve activity in patients with congestive heart failure and preserved left ventricular ejection fraction. J Am Coll Cardiol 45:661–667
Kasama S, Toyama T, Sumino H, Nakazawa M, Matsumoto N, Sato Y, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, Kurabayashi M (2008) Prognostic value of serial cardiac 123I-MIBG imaging in patients with stabilized chronic heart failure and reduced left ventricular ejection fraction. J Nucl Med 49:907–914
Kioka H, Yamada T, Mine T, Morita T, Tsukamoto Y, Tamaki S, Masuda M, Okuda K, Hori M, Fukunami M (2007) Prediction of sudden death in patients with mild-to-moderate chronic heart failure by using cardiac iodine-123 metaiodobenzylguanidine imaging. Heart 93:1213–1218
Kollensperger M, Seppi K, Liener C, Boesch S, Heute D, Mair KJ, Mueller J, Sawires M, Scherfler C, Schocke MF, Donnemilier E, Virgolini I, Wenning GK, Poewe W (2007) Diffusion weighted imaging best discriminates PD from MSA-P: a comparison with tilt table testing and heart MIBG scintigraphy. Mov Disord
Kvetnansky R, Goldstein DS, Weise VK, Holmes C, Szemeredi K, Bagdy G, Kopin IJ (1992) Effects of handling or immobilization on plasma levels of 3, 4-dihydroxyphenylalanine, catecholamines, and metabolites in rats. J Neurochem 58:2296–2302
Lee PH, Yeo SH, Kim HJ, Youm HY (2006) Correlation between cardiac 123I-MIBG and odor identification in patients with Parkinson’s disease and multiple system atrophy. Mov Disord 21:1975–1977
Matsui T, Tsutamoto T, Maeda K, Kusukawa J, Kinoshita M (2002) Prognostic value of repeated 123I-metaiodobenzylguanidine imaging in patients with dilated cardiomyopathy with congestive heart failure before and after optimized treatments—comparison with neurohumoral factors. Circ J 66:537–543
Orimo S, Amino T, Itoh Y, Takahashi A, Kojo T, Uchihara T, Tsuchiya K, Mori F, Wakabayashi K, Takahashi H (2005) Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol (Berl) 109:583–588
Orimo S, Kanazawa T, Nakamura A, Uchihara T, Mori F, Kakita A, Wakabayashi K, Takahashi H (2007) Degeneration of cardiac sympathetic nerve can occur in multiple system atrophy. Acta Neuropathol (Berl) 113:81–86
Orimo S, Uchihara T, Nakamura A, Mori F, Kakita A, Wakabayashi K, Takahashi H (2008) Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain 131:642–650
Senard JM, Brefel-Courbon C, Rascol O, Montastruc JL (2001) Orthostatic hypotension in patients with Parkinson’s disease: pathophysiology and management. Drugs Aging 18:495–505
Sharabi Y, Imrich R, Holmes C, Pechnik S, Goldstein DS ((in press)) Generalized and neurotransmitter-selective noradrenergic denervation in Parkinson disease with orthostatic hypotension. Mov Disord
Ueyama T, Hano T, Kasamatsu K, Yamamoto K, Tsuruo Y, Nishio I (2003) Estrogen attenuates the emotional stress-induced cardiac responses in the animal model of Tako-tsubo (Ampulla) cardiomyopathy. J Cardiovasc Pharmacol 42(Suppl 1):S117–S119
Ueyama T, Tanioku T, Nuta J, Kujira K, Ito T, Nakai S, Tsuruo Y (2006) Estrogen alters c-Fos response to immobilization stress in the brain of ovariectomized rats. Brain Res 1084:67–79
Valli N, Labrousse L, Reant P, Dos-Santos P (2007) Significant improvement of cardiac sympathetic function following cardiac support device implantation: illustration by 123I-MIBG scintigraphy. Eur J Cardiothorac Surg 32:943–944
Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC (2005) Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 352:539–548
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goldstein, D.S., Orimo, S. Cardiac sympathetic neuroimaging: summary of the First International Symposium. Clin Auton Res 19, 137–148 (2009). https://doi.org/10.1007/s10286-009-0002-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-009-0002-9