Abstract
Pera is a neotropical genus that currently belongs to the family Peraceae. This circumscription resulted from an inclusion of the Rafflesiaceae between the old tribe Pereae and all other Euphorbiaceae, and wherein Pereae was elevated to family rank making Euphorbiaceae monophyletic again. These changes are necessary although Rafflesiaceae are holoparasitic with extremely reduced vegetative bodies and large flowers while Peraceae and Euphorbiaceae have well developed vegetative parts and reduced flowers. As the embryology of Peraceae was poorly known, and embryological processes are conservative, we studied the embryology of Pera glabrata, searching for similarities between Peraceae, Rafflesiaceae, and Euphorbiaceae that could support this grouping. Usual methods of light microscopy and scanning electron microscopy were utilised. The results show endothecium with reversed-T-shaped cells, prismatic crystals in the tapetum, and disintegrated aerenchymatous septum in the mature fruit as unique features for Peraceae and possibly apomorphies for the family. In addition to the unisexual flowers, porogamous fertilization is present and one ovule per carpel which may support the Peraceae–Rafflesiaceae–Euphorbiaceae clade. The comparative approach also suggests possible (syn-)apomorphies for linoids and phyllanthoids, only linoids, Rafflesiaceae, Euphorbiaceae, and Ixonanthaceae. The presence of a placental obturator found previously unknown in Peraceae emerged as a possible synapomorphy for the euphorbioids (including Ixonanthaceae, Linaceae, Phyllanthaceae, Picrodendraceae, Peraceae, Rafflesiaceae, and Euphorbiaceae), which appeared in a common ancestor of the group and has been lost in Rafflesiaceae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pera contains approximately 40 species occurring from Cuba to southern Brazil with higher diversity in the Amazon basin (Bigio and Secco 2012; Webster 1994). The genus is easily recognised in having a pseudanthium with reduced unisexual flowers enclosed in a saclike bract (Bigio and Secco 2012). Traditionally, Pera belonged to Euphorbiaceae and was included in the tribe Pereae together with Chaetocarpus, Clutia, Pogonophora, and Trigonopleura (Webster 1994). A DNA-based phylogenetic analysis placed Pera and its relatives as sister to Rafflesiaceae plus Euphorbiaceae, suggesting the elevation of Pereae to a family Peraceae, making Euphorbiaceae monophyletic again (Wurdack and Davis 2009). Rafflesiaceae are holoparasites with extremely reduced vegetative bodies and flowers reaching one meter in diameter (Meijer 1993; Nikolov et al. 2014b), whereas Peraceae and Euphorbiaceae vary from herbaceous to arborescent and their flowers are small with only one stamen in male flowers and a gynoecium in female flowers in taxa that possess pseudanthia, as in Pera (Bigio and Secco 2012; Webster 1994) and Euphorbieae (Radcliffe-Smith 2001; Webster 1994). Currently, only unisexual flowers morphologically support the clade comprising Peraceae, Rafflesiaceae, and Euphorbiaceae, but it is not exclusive and can be found in other Malpighiales families (Endress et al. 2013). Peraceae, Rafflesiaceae, and Euphorbiaceae are currently circumscribed in the euphorbioids together with Phyllanthaceae, Picrodendraceae, Linaceae, and Ixonanthaceae (Wurdack and Davis 2009; Xi et al. 2012).
Since Schnarf (1931), followed by Davis (1966) and Johri et al. (1992), features of embryology became important because of their stability in larger taxonomic groups. Despite not ranking species as the main objective, embryology has been used to solve taxonomic problems at various hierarchical levels (Cave 1953; Davis and Heywood 1963; Palser 1975; Stuessy 2009; Tobe 1989). Embryology of Peraceae is poorly understood, and only the seed coat was studied by Tokuoka and Tobe (2003). Anther development (including microsporogenesis and gametogenesis), ovule (including megasporogenesis and gametogenesis), fertilisation, endosperm, and embryo development are unknown. The embryology of Euphorbiaceae has been well studied, patterns for the family have been established (Davis 1966; Johri et al. 1992; Kapil and Bhatnagar 1994), and additional features were found (De-Paula and Sajo 2011; Tokuoka and Tobe 1998, 2002, 2003). In Rafflesiaceae, some aspects of embryology are known (Bouman and Meijer 1994; Ernst and Schmid 1909, 1913; Nikolov et al. 2014b). According to Endress et al. (2013), there are large gaps in the knowledge of Malpighiales embryology, and investigations on poorly known representatives are needed.
Thus, we studied embryologycal features of Pera glabrata, which is the most widely distributed member of neotropical Peraceae (Bigio and Secco 2012), to expand the data for the family, in which embryological features are virtually unknown. The data obtained were also compared with previously established patterns for Euphorbiaceae, Rafflesiaceae, and other euphorbioids, identifying shared features that might corroborate the current circumscription of Peraceae (Wurdack and Davis 2009; Xi et al. 2012). In addition to classical embryology, we studied fruit development and aimed to obtain more data that could improve the understanding of Malpighiales relationships.
Materials and methods
Fertile branches of Pera glabrata (Schott) Poepp. ex Baill. were collected in Uberlândia, Minas Gerais state, Brazil. The material was prepared and incorporated into the Herbarium Uberlandense (HUFU) under the numbers 67,405 and 67,406 for male and female specimens, respectively.
Inflorescences, fruits, and seeds at several stages of development (including mature) were fixed in FAA containing 50% (v/v) ethanol (Johansen 1940) for 48 h and stored in 50% ethanol (Berlyn and Miksche 1976).
For SEM analysis, fixed material was dissected, dehydrated in an ethanol series and processed in a CO2 critical point drier (EM CPD300, Leica Microsystems, Wetzlar, Germany). The dried samples were fixed on aluminium stubs, coated with gold using a sputter coater (EM SCD050, Leica Microsystems) and analysed in a scanning electron microscope (EVO MA10, Zeiss, Oberkochen, Germany). The most important and representative regions were recorded.
For anatomical studies, the fixed material was also dehydrated in an ethanol series and embedded with a Historesin Embedding Kit (Leica, Microsystems) according to the manufacturer’s instructions. The material was serially sectioned using a rotatory microtome (RM 2135, Leica Microsystems) with thicknesses varying from 4 to 10 µm. The produced sections were stained with 0.05% toluidine blue in acetate buffer at pH 4.7 (O’Brien et al. 1964 modified), and the slides were mounted with Entellan mounting medium. The slides were analysed with a light microscope (BX51, Olympus, Tokyo, Japan), and the most important and representative regions were recorded. Additionally, a polarising filter was used to highlight crystals and starch.
Results
Anther
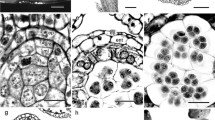
The primary parietal layer divides periclinally, forming two secondary parietal layers (Fig. 1a). Archesporial cells occur in the four anther lobes underlying the parietal layer (Fig. 1a, b). The connective has cells with phenolic content (Fig. 1a, b). A procambial strand is observed in the centre of the connective (Fig. 1b).
Anther and pollen grain development of Pera glabrata. Transverse sections (a–d, g–i, l, m), longitudinal sections (e, f), and SEM images (j, k). a Young anther. Note the archesporial cells. b Details of a young microsporangium showing the division of the secondary parietal layers. c Details of a microsporangium with the formation of the anther wall. d Detail of a microsporangium with meiocytes. Note the beginning of the accumulation of phenolic compounds in the endothecium stained in green and protruding toward the epidermis. e Details of the sporogenenous tissue with dyads and tetrads resulting from meiosis. Note that there is no cell wall formation among the nuclei, which are arranged tetrahedrally. f Details of the sporogenous tissue under polarised light. Note the prismatic and styloid crystals in the tapetum cells. g Details of an anther at pre-anthesis showing merger of the locules and the stomium where the anther will open and release pollen grains. The tissue of the connective on the left is torn by sectioning. h Details of the mature closed anther wall. Note that only the epidermis and endothecium remain. i Details of the mature wall in an open anther. Note the epidermis cells are wilting with a sinuous external wall. j Pollen grain with reticulated surface and one of the colpi. k Pollen grain germinated. l Mature pollen grain showing the generative and vegetative cells. m Pollen grains with pollen tubes formed within the closed anther. ac archesporial cell, arrow cell division, arrowhead secondary wall thickening, asterisk septa rupture between the locules, cr crystal, en endothecium, ep epidermis, gc generative cell, mm microspore mother cell (microsporocyte), ml middle layer, mi microspore, nu nucleus, pg pollen grain, pt pollen tube, sp septa, st stomium, ta tapetum, te tetrad, ts tapetum secretion, vc vegetative cell. Scale bars are 50 μm in g, 20 μm in a–f, h, i, 10 μm in l, m, 5 μm j, k
Later, the two secondary parietal layers divide periclinally forming four layers (Fig. 1b). The outermost layer gives rise to the endothecium, the innermost to the tapetum and the two intermediates to the middle layers (Basic type; Fig. 1b–d). Endothecium cells accumulate phenolics, and some protrude toward the epidermis (Fig. 1d).
In the mature anther, the epidermis is one-layered and interrupted by intruding endothecium cells. Its cells are ovoid without a lignin cell wall without noticeable amounts of cytoplasm. The endothecium cells are voluminous at the base and narrower towards the apex; the cell wall has longitudinal bands of lignin (fibrous), and the cytoplasm is filled with phenolic compounds (Fig. 1g, h). Internal to the endothecium, the middle layers disintegrate with the growth of the other layers, leaving remnants in mature anthers (Fig. 1d–g). The tapetum has cuboidal and juxtaposed cells with a dense cytoplasm (secretory tapetum) containing styloid and prismatic crystals and one or two nuclei with nucleoli (Fig. 1e, f).
The primary sporogenous cells (archesporial cells; Fig. 1a) divide, forming the secondary sporogenous cells (Fig. 1b). The secondary sporogenous cells differentiate into the microsporocytes (microspore mother cells; Fig. 1c). The microsporocytes undergo meiosis, resulting in microspores. Initially, a cell wall is not formed between stages I and II of meiosis between the daughter nuclei (simultaneous cytokinesis) (Fig. 1e). The configuration of the nuclei originating from meiosis is tetrahedral (Fig. 1e). Pollen grains are spheroidal and tricolporate, with a reticulate surface (Fig. 1i, j). The microspores divide unequally to give rise to a larger vegetative cell and a smaller generative cell (Fig. 1l). There are no further divisions, and the pollen grains are bicellular. Pollen is tricolporate (Fig. 1j–m). Some germinated pollen grains are observed inside the anther (Fig. 1k, m).
At the end of maturation, the septa among the sporangia disintegrate, uniting the two locules of the same theca (Fig. 1g) and each theca opens longitudinally.
Ovary
Pistillate flowers are tricarpellate and trilocular with a single ovule per carpel (Fig. 2a). The outer epidermis is one-layered with cuboid and juxtaposed cells and cytoplasm containing phenolic compounds (Fig. 2b). The mesophyll is parenchymatous with approximately 15 layers with two distinct regions. In the outer region the cells are larger and contain phenolics, in the inner region they are smaller, and only some contain phenolics (Fig. 2b). The inner ovary epidermis has one layer of anticlinally elongate cells (Fig. 2c). The septum tissue is similar to the ovary wall with a row of phenolic cells delimiting the prospective dehiscence line of each carpel (Fig. 2a).
Ovary of Pera glabrata. Transverse sections (a, b) and a longitudinal section (c). a General view. b, c Details of the ovary wall showing phenolic compounds (stained in green) in the outer epidermis, mesophyll, and inner epidermis. arrow prospective dehiscence line, ie inner ovary epidermis, me ovary mesophyll, oe outer ovary epidermis, ov ovule, ow ovary wall. Scale bars are 200 μm in a, and 20 μm in b, c
Ovule
The ovule is anatropous, bitegmic and has an axile placentation and short funiculus (Fig. 3a). The outer integument has four to six cell layers, the innermost with phenolic compounds (Fig. 3a–c). The inner integument has four to six layers of parenchymatous cells. The micropyle is formed by both integuments, but the exostomes and endostomes are not at the same level (zigzag micropyle; Fig. 3a, c). The ovule is vascularized by a procambial strand along the raphe and ending at the chalaza (Fig. 3a–b). The nucellus is approximately six cells in diameter, and the innermost layer is consumed by the developing megagametophyte (Fig. 3a–b). During integument differentiation, placental cells elongate radially, forming an obturator (Fig. 3c).
Ovule and megagametophyte development of Pera glabrata. A transverse section (b) and longitudinal sections (a, c–g). a, b General view of the ovule, showing phenolic compounds (stained in green) in the outer integument. Note that the vascular bundle is restricted to the raphe ending in the chalaza. c Details of the micropylar region of the ovule, showing that the endostome and exostome do not match (zigzag). Note that the endostome is blocked by a placental obturator. d–f Details of the ovule showing the development of the megaspores. d Megaspore mother cell. Note that there are four cells between the epidermis and previously mentioned cell (crassinucellate ovule). e Tetrad of megaspores arranged linearly. f Functional megaspore. g Megagametophyte Polygonum-type. an antipodal, arrow megaspore, ch chalaza, cc central cell, dm degenerating megaspores, fm functional megaspore, ii inner integument, mm megaspore mother cell, nd endostome, nu nucellus, ob obturator, oi outer integument, oo oosphere, rb raphal bundle, sy synergid, xo exostome. Scale bars are 50 μm in a–c, and 20 μm in d–g
Between the megaspore mother cell and nucellus epidermis, there are two to four cell layers (crassinucellate ovule; Fig. 3d). The megaspore mother cell undergoes meiosis, generating a linear tetrad of megaspores (Fig. 3e). From this tetrad, only the chalazal megaspore remains functional (Fig. 3f). The megagametophyte contains two synergids and an egg cell in the micropylar region, a binucleate central cell in the central portion, and three antipodals in the chalazal region (Polygonum type; Fig. 3g).
Fruit
The outer ovary epidermis gives rise to the exocarp, the ovary mesophyll produces the mesocarp, and the inner ovary epidermis the endocarp. The exocarp and outer mesocarp do not change during early fruit development (Fig. 4a, b). The middle mesocarp cells elongate radially, and the inner mesocarp periclinally, and both accumulate phenolic compounds (Fig. 4b). The septa become more voluminous in comparison to the ovary (compare with Fig. 2a) mainly because the intercellular spaces increase (Fig. 4a, b).
Fruit development of Pera glabrata. Transverse sections (a–c). a Postanthetic ovary. b Detail of a, showing the inner mesocarp cell elongation and intercellular spaces in the septal tissue. c Fruit at dehiscence. Note that the elongated layers of the young fruit form a sclerenchyma disposed perpendicularly to each other in the mature pericarp and that the intercellular spaces found in the young fruit septum are now more conspicuous, forming an aerenchyma in the mature fruit. asterisk intercellular space, er endocarp, ex exocarp, lo locule, mc mesocarp, pe pericarp, se seed, sp septum. Scale bars are 500 μm in a, 200 μm in c, and 100 μm in b
At the end of maturation, the exocarp has thick-walled cells (Fig. 4c). Some cells of the outermost mesocarp increase their volume. The middle region of the mesocarp has lignified and radially elongated cells, and the innermost mesocarp has periclinally elongate lignified cells (Fig. 4c). The endocarp cells are not lignified (Fig. 4c). The mesocarp in the septa has a conspicuous aerenchyma (Fig. 4c). In the fruit at dehiscence, the septa disintegrate, connecting the three locules.
Seed
After fertilisation, cellular divisions occur in both integuments only in the micropylar region. Idioblasts containing druses occur mainly in the outer integument (Fig. 5b, c). The endotegmen cells accumulate phenolic compounds (Fig. 5a–c). In the distal portion of the testa and tegmen, cellular divisions later result in the formation of a caruncle in the micropylar portion (Fig. 5c). The nucellus grows by cell divisions (Fig. 5a, b), accumulating starch in the micropylar portion (Fig. 5c) and crystals near the megagametophyte (Fig. 5d). The megagametophyte shows several vesicles in the cytoplasm (Fig. 5d).
Seed development of Pera glabrata. Longitudinal sections (a–g). a–d Post-anthesis. a Overview. Note the early development of a caruncle. b Details of the chalazal region of Fig. 5a, showing the cellular divisions of the nucellus. c Details of the micropylar region of Fig. 5a. Note the cellular divisions on the testa and tegmen, presence of pollen tube in endostome and starch accumulation in the micropylar portion of the nucellus. d Details of the central region of figure a showing crystals in the nucellus near the megagametophyte, nuclear endosperm, antipodals, synergids, and zygote. e–g Young seed. e Overview. f Details of the antiraphal portion. g Details of the seed coat. Note the radial elongation of exotesta and exotegmen. an antipodal, ap amyloplast, arrow cell division, arrowhead crystals, ca caruncle, ch chalaza, ed endosperm, eg exotegmen, em endotegmen, es endotesta, et exotesta, mg mesotegmen, mt mesotesta, nu nucellus, pt pollen tube, sc seed coat, sy synergid, te testa, tg tegmen, zy zygote. Scale bars are 200 μm in e, 100 μm in a and f, 50 μm in b–d, and 25 μm in g
At an intermediate stage, the caruncle develops in the seed coat (Fig. 5e). The seed coat increases the numbers of cell layers mainly in the most distal portion. The exotesta shows radial cell elongation (Fig. 5f, g). The tegmen and nucellus cells accumulate crystals (Fig. 5f). The endotegmen cells also periclinally elongate (Fig. 5f, g). The endosperm still divides without wall formation (free nuclei; Fig. 5f). There are no embryos in the studied seeds.
The mature seed is ovate (Fig. 6a–c) with an evident raphe (Fig. 6b) and has lateral constrictions close to the micropyle (Fig. 6c). The caruncle is fleshy and bifurcate (Fig. 6a) and originated from the testa near the micropyle (Fig. 6b, c). Some cells accumulate phenolic compounds in the chalaza, constituting the hypostase (Fig. 6e). The exotesta is elongated and lignified functioning as a mechanical layer of the seed coat (Fig. 6d, e). The mesotesta and endotesta do not show changes compared with young seeds. In the mesotegmen the cells and intercellular spaces increase, while the endotegmen has cells periclinally elongated with a phenolic content (Fig. 6d). The nucellus is partially consumed by endosperm development (Fig. 6e, f). The endosperm is concentrated in the chalazal and micropylar regions. A small part of the endosperm forms cell walls in small micropylar portions and the rest remains with free nuclei (Fig. 6e–g).
Mature seed of Pera glabrata. Transverse section (d), longitudinal sections (e–g), and SEM (a–c). a–c General view. a Raphal view. b Raphal view without the caruncle. c Antiraphal view. d Details of the median region, showing the elongation of the exotesta. Note that there is no increase of the number of layers in relation to the ovule integument (compare with Fig. 3). e Details of the chalazal region. Note the presence of cells with a phenolic content constituting the hypostase. f Details of the micropylar region. Note the cellularized endosperm part and the part that remains nuclear. g Details of the nuclear endosperm. ca caruncle, ch chalaza, ed endosperm, eg exotegmen, em endotegmen, es endotesta, et exotesta, mg mesotegmen, mt mesotesta, nu nucellus, ra raphe, te testa, tg tegmen. Scale bars are 1 mm in a–c, 100 μm in e, 50 μm in d and f, and 25 μm in g
Discussion
Comparative embryology of Peraceae and related families
Peraceae is circumscribed within the euphorbioids together with Euphorbiaceae, Ixonanthaceae, Linaceae, Phyllanthaceae, Picrodendraceae and Rafflesiaceae. These families are organised into two clades. One consists of Peraceae, Rafflesiaceae and Euphorbiaceae. The other clade contains two additional subclades, the phyllanthoids with Phyllanthaceae and Picrodendraceae and linoids with Linaceae and Ixonanthaceae (Fig. 7; Xi et al. 2012). In this discussion we compare the embryological features of Pera with the other representatives of euphorbioids, highlighting the similarities and differences between them. We added some data available for Picrodendraceae and include the family in the list of Malpighiales (Caryocaraceae, Centroplacaceae, Euphroniaceae, Goupiaceae, Ixonanthaceae, Lacistemataceae, Lophopyxidaceae, Malesherbiaceae, Medusagynaceae, Pandaceae, and Quiinaceae) with embryology widely unknown as mentioned by Endress et al. (2013).
Phylogenetic tree of Peraceae and related families (modified from Xi et al. 2012), showing possible (syn-)apomorphies indicated by solid rectangles and data requiring further studies are followed by question marks
The anther of Pera shares persistent epidermis at maturity; ephemeral middle layers, secretory tapetum, and tetrahedral tetrads with all euphorbioids (Ixonanthaceae, Linaceae, Phyllanthaceae, Picrodendraceae, Peraceae, Rafflesiaceae and Euphorbiaceae). Pera also shares tetrasporangiate anther with two thecae, binucleate tapetum, simultaneous cytokinesis, tetrahedral tetrads, pollen grain dispersal by longitudinal slits, and tricolporate pollen grains with Ixonanthaceae (Rao and Narayana 1965), Linaceae (Narayana 1964; Rao and Narayana 1965), Phyllanthaceae (Mukherjee and Padhye 1964), and most of Euphorbiaceae (Bhanwra 1987; Davis 1966; De-Paula and Sajo 2011; Johri et al. 1992; Kapil and Bhatnagar 1994; Liu et al. 2007; Mukherjee 1961; Nair and Maitreyi 1962; Nowicke 1994; Nowicke et al. 1998, 1999; Nowicke and Takahashi 2002; Rao 1964; Rao and Rao 1976; Takahashi et al. 2000). The anther of Rafflesiaceae differs in several aspects from all other euphorbioids. Polysporangiate anthers without a thecal organisation (Endress and Stumpf 1990; Nikolov et al. 2014a), tapetum with three to four layers (Nikolov et al. 2014a) and successive cytokinesis (Ernst and Schmid 1913) are unique character states of Rafflesiaceae. Endothecium without thickened cell walls occur in Rafflesiaceae (Ernst and Schmid 1913; Furness 2007; Nikolov et al. 2014a; Takhtajan et al. 1979) and Chrozophora rottleri, Euphorbiacecae (Sharma 1956). Inaperturate pollen grains occur in Rafflesiaceae (Ernst and Schmid 1913; Furness 2007; Nikolov et al. 2014a; Takhtajan et al. 1979) and in crotonoids, a monophyletic group within Euphorbiaceae (Nowicke 1994).
The only exclusive features of Pera anthers are the endothecium with reversed-T-shaped cells and tapetum with prismatic crystals. Styloid crystals also occur in Pera as in Croton and Astraea (Euphorbiaceae, De-Paula and Sajo 2011). The tapetal crystals may have been underestimated in Malpighiales and should be further studied to evaluate their occurrence. Other features of the anther such as its wall development and the number of the cells of pollen grains do not provide a clear pattern for the euphorbioids, varying even within some families (Bhanwra 1987; De-Paula and Sajo 2011; Ernst and Schmid 1913; Johri and Kapil 1953; Johri et al. 1992; Kapil and Bhatnagar 1994; Liu et al. 2007; Mukherjee and Padhye 1964; Nair and Maitreyi 1962; Narayana 1964; Rao and Narayana 1965).
The ovary of Pera is superior as in other Peraceae (Radcliffe-Smith 2001), Ixonanthaceae (Kubitzki 2014), Linaceae (Dressler et al. 2014), Picrodendraceae (Sutter et al. 2006), Phyllanthaceae (Radcliffe-Smith 2001), and Euphorbiaceae (Radcliffe-Smith 2001). Only Rafflesiaceae has an inferior ovary without carpel formation, and the ovules are formed in the schizogenous slits of a massive, morphologically undifferentiated gynoecium (Nikolov et al. 2014a). The number of ovules per carpel defined two major clades among families related to Peraceae. A group with two ovules per carpel comprising Ixonanthaceae (Kubitzki 2014), Linaceae (Dressler et al. 2014), Picrodendraceae (Sutter et al. 2006), and Phyllanthaceae (Radcliffe-Smith 2001). One ovule per carpel appeared in a common ancestor of Peraceae–Euphorbiaceae–Rafflesiaceae and changed in Rafflesiaceae to many ovules without carpel formation (Nikolov et al. 2014a).
In Pera, the ovule is anatropous, the megaspore tetrads are linear, the functional megaspore is chalazal, and the megagametophyte is monosporic and of the Polygonum type as in Ixonanthaceae (Rao and Narayana 1965), Linaceae (Narayana 1964; Rao and Narayana 1965), Picrodendraceae (Sutter et al. 2006), Phyllanthaceae (Mukherjee and Padhye 1964), Rafflesiaceae (Bouman and Meijer 1994; Ernst and Schmid 1909, 1913; Nikolov et al. 2014a), and most Euphorbiaceae (Davis 1966; De-Paula and Sajo 2011; Johri et al. 1992; Kapil and Bhatnagar 1994). The ovule is bitegmic in Pera and all related families (Davis 1966; De-Paula and Sajo 2011; Johri et al. 1992; Kapil and Bhatnagar 1994; Mukherjee and Padhye 1964; Narayana 1964; Rao and Narayana 1965; Sutter et al. 2006) except in Rafflesiaceae which is unitegmic (Bouman and Meijer 1994; Ernst and Schmid 1913; Nikolov et al. 2014a). The ovule vascular supply, nucellus, nucellar beak, and the micropyle does not provide a clear pattern for the euphorbioids, varying even within some families (Bouman and Meijer 1994; Corner 1976; Davis 1966; Gopinath and Gopalkrishnan 1949; Johri et al. 1992; Kapil and Bhatnagar 1994; Kool 1988; Mukherjee and Padhye 1964; Narayana 1964; Radcliffe-Smith 2001; Rao and Narayana 1965; Sutter et al. 2006; Tokuoka and Tobe 2001, 2002, 2003). Only in Rafflesiaceae, the ovules are not vascularized (Bouman and Meijer 1994; Igersheim and Endress 1998). An endothelium occurs in Linaceae and Ixonanthaceae (Narayana 1964; Rao and Narayana 1965) and may appear as a synapomorphy for the linoids (Ixonanthaceae and Linaceae).
The obturator of Pera has a placental origin as in Ixonanthaceae (Rao and Narayana 1965), Linaceae (Narayana 1964; Rao and Narayana 1965), Picrodendraceae (Sutter et al. 2006), Euphorbiaceae (Bhanwra 1987; Davis 1966; De-Paula and Sajo 2011; Johri et al. 1992; Landes 1946) and Phyllanthaceae (Sutter and Endress 1995). In Rafflesiaceae, there is no obturator (Bouman and Meijer 1994).
There are large gaps in the knowledge of fruit anatomy and development of the families related to Peraceae making it difficult to compare the groups. The absence of septa in the fruits is reported for other genera of Peraceae (Radcliffe-Smith 2001), but it was unclear how the septum disappears during development. In this study, we observed that the septum is aerenchymatic and their intercellular spaces increase during development culminating in its disintegration in mature fruits. The loss of the septum is an exclusive feature for Peraceae and not reported for any related family. Only in Rafflesiaceae, fruits are a kind of berry (Bouman and Meijer 1994) and it is also a unique feature for the family.
Pera has porogamous fertilisation, which also occurs in Euphorbiaceae (Mukherjee 1961; Rao 1964) and Rafflesiaceae (Ernst and Schmid 1909, 1913). This feature supports Peraceae–Rafflesiaceae–Euphorbiaceae clade, but this character is unknown in all other euphorbioids and must be studied in phyllanthoids (Phyllanthaceae and Picrodendraceae) and linoids (Ixonanthaceae and Linaceae) to evaluate its evolution in Malpighiales.
The seed coat in Linaceae (Corner 1976), Rafflesiaceae (Bouman and Meijer 1994), Euphorbiaceae is exotegmic (Bhatnagar 1994; Corner 1976; Davis 1966; Johri et al. 1992; Kapil and Bhatnagar 1994; Tokuoka and Tobe 1998, 2002, 2003). Phyllanthaceae has exotegmic, rarely endotegmic or endotestal seeds (Tokuoka and Tobe 2001). Ixonanthaceae has exotestal and exotegmic seeds (Boesewinkel 1988; Corner 1976). In Picrodendraceae, the seed coat is unknown. Exotegmic seeds are a plesiomorphic character state in euphorbioids and would have been modified to endotegmic or endotestal in Phyllanthaceae. Only Ixonanthaceae have exotestal and exotegmic seeds (Boesewinkel 1988; Corner 1976) and also can represent an apomorphy for the family. Rafflesiaceae has exotesta with u-shaped thickening (Bouman and Meijer 1994), also a possible apomorphy.
In Peraceae, Pera, Clutia and Chaetocarpus have exotestal seeds, Pogonophora is exotegmic and in Trigonopleura the seed coat is unknown (Tokuoka and Tobe 2003). In a phylogenetic analysis proposed by Wurkack et al. (2005), Pogonophora is the sister genus of Pera, Clutia and Chaetocarpus, and Trigonopleura was not included in their analysis. Therefore exotestal seeds would have emerged as a synapomorphy in Pera–Clutia–Chaetocarpus clade.
The seeds of Pera share nuclear and persistent endosperm in the mature seed (albuminous seed) with all other euphorbioids (Berg 1975; Bouman and Meijer 1994; Davis 1966; Ernst and Schmid 1913; Johri et al. 1992; Kapil and Bhatnagar 1994; Mukherjee and Padhye 1964; Narayana 1964) except with Picrodendraceae, which is unknown. Besides, these character states also occur in other Malpighiales as Salicaceae, Passifloraceae, and Violaceae (Johri et al. 1992) and cannot be a synapomorphy for the euphorbioids.
Implications of Pera embryology in systematics and evolution of Malpighiales
Based on the comparative data, the embryological features were placed in the current phylogenetic tree (Wurdack and Davis 2009; Xi et al. 2012), proposing morphological support for clades and hypotheses of how embryology evolved in this group.
An endothecium with reversed-T-shaped cells, prismatic crystals in the tapetum and disintegrated aerenchymatous septum in the mature fruit, were character state found in Pera but not in other euphorbioids. All of the listed features are potential autapomorphies for Peraceae; however, most species of Pera and more Peraceae should be studied to confirm this. An aerenchymatic septum is a character states previously described for other species of Pera and other genera of Peraceae (Radcliffe-Smith 2001), supporting its autapomorphy status for Peraceae (Fig. 7).
The results show that Peraceae and Euphorbiaceae have more features in common compared to Rafflesiaceae. In addition to the unisexual flowers, chosen as a morphological feature supporting the Peraceae–Rafflesiaceae–Euphorbiaceae clade by Xi et al. (2012), we raised the porogamous fertilisation and one ovule per carpel supporting the clade. However, the porogamous fertilisation is very common in the angiosperms and should be studied in other euphorbioids to confirm its presence and to evaluate its evolution in Malpighiales. The presence of an ovule per carpel would have appeared in the Peraceae–Rafflesiaceae–Euphorbiaceae ancestor, and changed in Rafflesiaceae, which has many ovules and has no carpel. Furthermore, Rafflesiaceae, has several apomorphies as polysporangiate and poricidal anther, nonfibrous endothecium, multilayered tapetum, successive cytokinesis, inaperturate pollen, greatly modified gynoecium, inferior ovary, many ovules, unitegmic ovules, ovules without vascularization, loss of obturator, berry, U-shaped exotegmic cells, and lacking embryo differentiation appear as possible apomorphies for the family (Fig. 7).
Our comparative approach shows some possible synapomorphies such as Solanad embryogenesis and two ovules per carpel for the phyllanthoids and linoids, and the endothelium for linoids. Moreover, some apomorphies such as exotestal and endotestal seed for Ixonanthaceae, and Onagrad embryogenesis for Euphorbiaceae (Fig. 7).
The presence of placental obturator previously unknown in Peraceae reinforces the status of synapomorphy for euphorbioids mentioned by Xi et al. (2012) being lost in Rafflesiaceae (Fig. 7).
References
Berg R (1975) Fruit, seed and myrmecochorous dispersal in Micrantheum (Euphorbiaceae). Norw J Bot 22:173–194
Berlyn GP, Miksche JP (1976) Botanical microtechnique and cytochemistry. Iowa State University Press, Ames
Bhanwra R (1987) Embryology of Euphorbia maddeni and Euphorbia nivulia. Curr Sci 56:1062–1064
Bigio N, Secco R (2012) As espécies de Pera (Euphorbiaceae s.s) na Amazônia brasileira. Rodriguésia 63:163–207. doi:10.1590/S2175-78602012000100012
Boesewinkel FD (1988) Development of ovule and testa of Linum usitatissimum L. Acta Bot Neerl 29:17–32. doi:10.1111/j.1438-8677.1980.tb01185.x
Bouman F, Meijer W (1994) Comparative structure of ovules and seeds in Rafflesiaceae. Plant Syst Evol 193:187–212. doi:10.1007/BF00983550
Cave MS (1953) Cytology and embryology in the delimitation of genera. Chron Bot 14:140–153
Corner EJH (1976) The seeds of dicotyledons, vol 1 and 2. Cambridge University Press, Cambridge
Davis GL (1966) Systematic embryology of the angiosperms. Wiley, New York
Davis PH, Heywood VH (1963) Principles of angiosperm taxonomy. Van Nostrand, Princeton
De-Paula OC, Sajo MG (2011) Morphology and development of anthers and ovules in Croton and Astraea (Euphorbiaceae). Nord J Bot 29:505–511. doi:10.1111/j.1756-1051.2011.01072.x
Dressler S, Repplinger M, Bayer C (2014) Linaceae. In: Kubitzki K (ed) Eudicots—malpighiales, vol. 11. The families and genera of vascular plants. Springer, Berlin, pp 237–246. doi:10.1007/978-3-642-39417-1
Endress PK, Stumpf S (1990) Non-tetrasporangiate stamens in the angiosperms: structure, systematic distribution and evolutionary aspects. Bot Jahrb Syst 112:193–240
Endress PK, Davis CC, Matthews ML (2013) Advances in the floral structural characterization of the major subclades of Malpighiales, one of the largest orders of flowering plants. Ann Bot 111:961–985. doi:10.1093/aob/mct056
Ernst A, Schmid E (1909) Embryosackentwicklung und Befruchtung bei Rafflesia patma Bl. Ber Deut Bot Ges 27:176–186. doi:10.1111/j.1438-8677.1909.tb06784.x
Ernst A, Schmid E (1913) Über Blüte und Frucht von Rafflesia. Morphologisch-biologische Beobachtungen und entwicklungsgeschichtlich-zytologische Untersuchungen. Ann Jard Bot Buitenzorg 27:1–55
Furness CA (2007) Why does some pollen lack apertures? A review of inaperturate pollen in eudicots. Bot J Linn Soc 155:28–48. doi:10.1111/j.1095-8339.2007.00694.x
Gopinath D, Gopalkrishnan K (1949) The ovule and the development of the female gametophyte in Homonoia retusa Muell, and Euphorbia oreophila Miquel. Am Midl Nat 41:759–764
Igersheim A, Endress PK (1998) Gynoecium diversity and systematics of the paleoherbs. Bot J Linn Soc 127:289–370. doi:10.1006/bojl.1998.0180
Johansen DA (1940) Plant microtechnique. McGraw-Hill Book Company, New York
Johri BM, Kapil RN (1953) Contribution to the morphology and life history of Acalypha indica L. Phytomorphology 3:137–151
Johri BM, Ambegaokar KB, Srivastava PS (1992) Comparative embryology of angiosperms. Springer, Berlin. doi:10.1007/978-3-642-76395-3
Kapil RN, Bhatnagar AK (1994) The contribution of embryology to the systematics of the Euphorbiaceae. Ann Mo Bot Gard 81:145–159. doi:10.2307/2992091
Kool R (1988) A taxonomic revision of the genus Ixonanthes (Linaceae). Blumea 26:191–204
Kubitzki K (2014) Ixonanthaceae. In: Kubitzki K (ed) Eudicots—malpighiales, vol. 11. The families and genera of vascular plants. Springer, Berlin, pp 233–236. doi:10.1007/978-3-642-39417-1
Landes M (1946) Seed development in Acalypha rhomboidea and some other Euphorbiaceae. Am J Bot 33:562–568
Liu HF, Kirchoff BK, Wu GJW, Liao JP (2007) Microsporogenesis and male gametogenesis in Jatropha curcas L. (Euphorbiaceae). J Torrey Bot Soc 134:335–343. doi:10.3159/1095-5674(2007)134[335:MAMGIJ]2.0.CO;2
Meijer W (1993) Rafflesiaceae. In: Kubitzki K, Rohwer JG, Bittrich V (eds) Dicotyledons—magnoliid, hamamelid and caryophyllid, vol 2. The families and genera of vascular plants. Springer, Berlin, pp 557–563. doi:10.1007/978-3-662-02899-5
Mukherjee P (1961) Embryology of two Euphorbiaceae. Proc Indian Acad Sci B 53:217–229
Mukherjee PK, Padhye MD (1964) Contribution to the embryology of the genus Phyllanthus Linn. Proc Natl Acad Sci India B 34:117–128
Nair N, Maitreyi M (1962) Morphology and embryology of Sebastiania chamaelea. Bot Gaz 124:58–68
Narayana LL (1964) A contribution to the floral anatomy and embryology of Linaceae. J Indian Bot Soc 43:343–357
Nikolov LA, Staedler YM, Manickam S, Schönenberger J, Endress PK, Kramer EM, Davis CC (2014a) Floral structure and development in Rafflesiaceae with emphasis on their exceptional gynoecia. Am J Bot 101:225–243. doi:10.3732/ajb.1400009
Nikolov LA, Tomlinson PB, Manickam S, Endress PK, Kramer EM, Davis CC (2014b) Holoparasitic Rafflesiaceae possess the most reduced endophytes and yet give rise to the world’s largest flowers. Ann Bot 114:233–242. doi:10.1093/aob/mcu114
Nowicke JW (1994) A palynological study of Crotonoideae (Euphorbiaceae). Ann Missouri Bot Gard 81:245–269. doi:10.2307/2992096
Nowicke JW, Takahashi M (2002) Pollen morphology, exine structure and systematics of Acalyphoideae (Euphorbiaceae), Part 4: Tribes Acalypheae pro parte (Erythrococca, Claoxylon, Claoxylopsis, Mareya, Mareyopsis, Discoclaoxylon, Micrococca, Amyrea, Lobanilia, Mallotus, Deuteromallotus, Cordemoya, Coccoceras, Trewia, Neotrewia, Rockinghamia, Octospermum, Acalypha, Lasiococca, Spathiostemon, Homonoia), Plukenetieae (Haematostemon, Astrococcus, Angostyles, Romanoa, Eleutherostigma, Plukenetia, Vigia, Cnesmone, Megistostigma, Sphaerostylis, Tragiella, Platygyna, Tragia, Acidoton, Pachystylidium, Dalechampia), Omphaleae (Omphalea), and discussion and summary of the complete subfamily. Rev Palaeobot Palynol 121:231–336. doi:10.1016/S0034-6667(02)00087-8
Nowicke JW, Takahashi M, Webster GL (1998) Pollen morphology, exine structure and systematics of Acalyphoideae (Euphorbiaceae) Part 1. Tribes Clutieae (Clutia), Pogonophoreae (Pogonophora), Chaetocarpeae (Chaetocarpus, Trigonopleura), Pereae (Pera), Cheiloseae (Cheilosa, Neoscortechinia), Erismantheae pro parte (Erismanthus, Moultonianthus), Dicoelieae (Dicoelia), Galearieae (Galearia, Microdesmis, Panda) and Ampereeae (Amperea, Monotaxis). Rev Palaeobot Palynol 102:115–152. doi:10.1016/S0034-6667(98)80001-8
Nowicke JW, Takahashi M, Webster GL (1999) Pollen morphology, exine structure and systematics of Acalyphoideae (Euphorbiaceae): Part 2. Tribes Agrostistachydeae (Agrostistachys, Pseudagrostistachys, Cyttaranthus, Chondrostylis), Chrozophoreae (Speranskia, Caperonia, Philyra, Ditaxis, Argythamnia, Chiropetalum, Doryxylon, Sumbaviopsis, Thyrsanthera, Melanolepis, Chrozophora), Caryodendreae (Caryodendron, Discoglypremna, Alchorneopsis), Bernardieae (Bernardia, Necepsia, Paranecepsia, Discocleidion, Adenophaedra) and Pycnocomeae (Pycnocoma, Droceloncia, Argomuellera, Blumeodendron, Podadenia, Ptychopyxis, Botryophora). Rev Palaeobot Palynol 105:1–62. doi:10.1016/S0034-6667(98)00069-4
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373. doi:10.1007/BF01248568
Palser BF (1975) The bases of angiosperm phylogeny: embryology. Ann Mo Bot Gard 62:621–646
Radcliffe-Smith A (2001) Genera Euphorbiacearum. Royal Botanic Gardens, Kew
Rao A (1964) Notes on the embryology of Hevea brasiliensis Muell. Curr Sci 33:739–740
Rao D, Narayana LL (1965) Embryology of Linaceae. Curr Sci 34:92–93
Rao PN, Rao DS (1976) Embryology of cassava. Proc Indian Acad Sci Sect B Biol Sci 42:111–116
Schnarf K (1931) Vergleichende Embryologie der Angiospermen. Bornträger, Berlin
Sharma G (1956) Studies in the family Euphorbiaceae 1. The gametophytes of Chrozophora rottleri A. Juss. J Indian Bot Soc 35:189–193
Stuessy TF (2009) Plant taxonomy: the systematic evaluation of comparative data. Columbia University Press, New York
Sutter D, Endress PK (1995) Aspects of gynoecium structure and macrosystematics in Euphorbiaceae. Bot Jahrb Syst 116:517–536
Sutter DM, Forster PI, Endress PK (2006) Female flowers and systematic position of Picrodendraceae (Euphorbiaceae s.l., Malpighiales). Pl Syst Evol 261:187–215. doi:10.1007/s00606-006-0414-0
Takahashi M, Nowicke JW, Webster GL, Orli SS, Yankowski S (2000) Pollen morphology, exine structure, and systematics of Acalyphoideae (Euphorbiaceae), part 3: Tribes Epiprineae (Epiprinus, Symphyllia, Adenochlaena, Cleidiocarpon, Koilodepas, Cladogynos, Cephalocrotonopsis, Cephalocroton, Cephalomappa), Adelieae (Adelia, Crotonogynopsis, Enriquebeltrania, Lasiocroton, Leucocroton), Alchorneae (Orfilea, Alchornea, Coelebogyne, Aparisthmium, Bocquillonia, Conceveiba, Gavarretia), Acalypheae pro parte (Ricinus, Adriana, Mercurialis, Leidesia, Dysopsis, Wetria, Cleidion, Sampantaea, Macaranga). Rev Palaeobot Palynol 110:1–66. doi:10.1016/S0034-6667(99)00061-5
Takhtajan AL, Meyer NR, Kosenko VN (1979) Morphology of pollen grains of the family Hydnoraceae in relation to its systematic position. Bot Zhurn (Moscow &Leningrad) 64:1774–1777
Tobe H (1989) The embryology of angiosperms: its broad application to the systematic and evolutionary study. Bot Mag Tokyo 102:351–367. doi:10.1007/BF02488572
Tokuoka T, Tobe H (1998) Ovules and seeds in Crotonoideae (Euphorbiaceae): structure and systematic implications. Bot Jahrb Syst 120:164–186
Tokuoka T, Tobe H (2001) Ovules and seeds in subfamily Phyllanthoideae (Euphorbiaceae): structure and systematic implications. J Plant Res 114:75–79. doi:10.1007/PL00013970
Tokuoka T, Tobe H (2002) Ovules and seeds in Euphorbioideae (Euphorbiaceae): structure and systematic implications. J Plant Res 115:361–374. doi:10.1007/s10265-002-0047-5
Tokuoka T, Tobe H (2003) Ovules and seeds in Acalyphoideae (Euphorbiaceae): structure and systematic implications. J Plant Res 116:355–380. doi:10.1007/s10265-003-0116-4
Webster GL (1994) Classification of the Euphorbiaceae. Ann Mo Bot Gard 81:3–32. doi:10.2307/2399908
Wurdack KJ, Davis CC (2009) Malpighiales phylogenetics: gaining ground on one of the most recalcitrant clades in the angiosperm tree of life. Am J Bot 96:1551–1570. doi:10.3732/ajb.0800207
Xi Z, Ruhfel BR, Schaefer H, Amorim AM, Sugumaran M, Wurdack KJ, Endress PK, Matthews ML, Stevens PF, Mathews S, Davis CC (2012) Phylogenomics and a posteriori data partitioning resolve the Cretaceous angiosperm radiation Malpighiales. Proc Natl Acad Sci USA 109:17519–17524. doi:10.1073/pnas.1205818109
Acknowledgements
The study was supported by Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq no. 484376/2013-6). The authors thank Glein Monteiro de Araújo for help in locating the species and the Laboratório Multiusuário de Microscopia Eletrônica of the Faculdade de Engenharia Química (UFU) for technical support with the SEM. We also thank two anonymous reviewers for their comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Olivera Franca, R., De-Paula, O.C. Embryology of Pera (Peraceae, Malpighiales): systematics and evolutionary implications. J Plant Res 130, 709–721 (2017). https://doi.org/10.1007/s10265-017-0916-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-017-0916-6