Abstract
Yacon (Smallanthus sonchifolius, Asteraceae) is an ancient andean crop that has numerous dietary and medicinal properties. Morphological and anatomical features and developmental changes of the capitulum were studied. A ray floret is a pistillate, female flower, while a disc floret is a staminate male flower, and the former opens before the latter, being pseudanthium protogynous. The capitulum presents interesting attributes for pollinators such as flower structure, nectaries and pollenkitt. Gynoecial nectaries were found on undeveloped ovary in the disc floret, but not in the ray floret. Glandular trichomes were observed on the abaxial epidermis of corolla in the ray floret, but not in the disc floret. Capitulum development was divided into eight stages. Stigma receptivity varied with these stages. Pollen viability was low (15%). In accordance with low viability, pollen grains exhibit diverse sizes and shapes, reduction in length of spines, and abnormal protoplasm. Examination of ovary development in the ray floret showed that a mature ovule was formed, but fertilization did not occur. In advanced developmental stages, the capitulum showed proliferation of the endothelium, degeneration of the embryo sac, and all harvested cypselae had aborted seeds. Problems found in pollen viability and aborted cypselae could be the result of a history of vegetative propagation in the domestication process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Smallanthus sonchifolius (Poepp. et Endl.) H. Robinson (= Polymnia sonchifolia Poepp. & Endl.) an allopolyploid species (2n = 6 A + 2B = 42 + 16 = 58) commonly named yacon, is a perennial semidomesticated herb of the Asteraceae family (Dempewolf et al. 2008; Grau and Rea 1997). It is an ancient Andean crop whose tuberous roots are traditionally consumed raw as “fruit” (Grau and Rea 1997), and attracting worldwide interest due to dietary and medicinal properties. Tuberous roots are rich in fructooligosaccharides (Ohyama et al. 1990) compounds potentially useful as prebiotics, natural sweetener, functional food, and dietary supplements (Genta et al. 2005, 2009; Seminario et al. 2003). Leaves are used as medicinal tea with hypoglycemic properties (Aybar et al. 2001; Genta et al. 2010). However, efficiency of sexual propagation by seeds of this plant is generally low, which hampers its breeding programs.
Asteraceae family is characterized by a inflorescence composed by florets arranged on a receptacle surrounded by protective bracts, a capitulum; by anthers fused in the margins conforming a tube where the pollen is pushed out by the style; and by achenes (cypselae) as fruit (Funk et al. 2009). A frequent arrangement of Asteraceae capitulum consists of peripherally ray florets that attract pollinators and disc florets with reproductive function (Bello et al. 2013). Usually ray florets are female and disc florets are bisexual. Smallanthus sonchifolius presented a heterogamous capitulum, with outer female or pistillate ray florets and inner male or staminate disc florets (Grau and Rea 1997; Seminario et al. 2003). Only few morphological characters have been reported on sexual reproductive organs, such as number of ray and disc florets; shape, length and width of the ligule; length of the stigma (Grau and Rea 1997; Seminario et al. 2003). Little is known about the flower structure of the capitulum, floret anatomy and developmental changes of flower organs.
We aimed to investigate external and internal flower structure, and characterize stages of development of the capitulum in order to better understand the reproductive features of this plant and to elucidate the cause of low efficiency of seed propagation.
Materials and methods
Plant material
An accession of S. sonchifolius (UNT–LIEY 97–1) from the collection of Universidad Nacional de Tucumán was studied. Plant material was cultivated in experimental plots at Centro Universitario Horco Molle (CUHM), Tucumán, Argentina (26° 47′ S, 65° 19′ W. 547 m.a.s.l.). Relevant meteorogical data during the experimental period were: February: rainfall 303 mm, mean 22.9 °C, max 36.5 °C, min 15.0 °C; March: 604 mm, 21.2 °C, 31.6 °C, 13.8 °C; April: 44 mm, 28.2 °C, 31.6 °C, 12.8 °C; May: 56 mm; 15.0 °C, 24.1 °C, 10.2 °C; June: 0 mm, 17.4 °C, 25.3 °C, 5.1 °C. Samples were obtained in June 2015. Voucher specimens were deposited in the Herbarium of Fundación Miguel Lillo, Tucumán, Argentina (LIL 607173, 607174). Morphological, anatomical and developmental characterization of the capitulum.
Developmental stages of capitulum were visually identified according to the maturity of ray and disc florets and involucral bracts. Three capitula per stage (stage 1 to 7) were fixed in FAA (formalin: ethanol: acetic acid: water, 100:500:50:350 v/v/v/v). Equatorial diameter of each capitulum and quantitative morphological characters of ray and disc florets were registered. Disc florets were differentiated in three sectors (peripheral, middle and central), each equal to 1/3 of the capitulum radius. Measurements were made in ten ray florets per capitulum and five disc florets per sector.
For light microscopy (LM), three capitula of each stage were dehydrated and embedded in paraffin. Sections of 20 µm were made with a rotary microtome (Microm HM315), stained with safranin and fast green mounted in Canada balsam (Zarlavsky 2014).
The permanent sections and fresh material were visualized on an Olympus SZ61 and a Zeiss STemi 2000-C stereoscopic microscopes and a Carl Zeiss Lab A1 Axiolab light microscope equipped with a Zeiss Axiocam ERc 5 s digital camera. Measurements were made using software AxioVision version 4.8.2 (Carl Zeiss Ltd, Herts, UK).
Data sets were tested with Shapiro Wilk’s test for normality and Levene’s test for homoscedasticity. Values are presented as mean ± standard deviation and were compared by Kruskal Wallis test (non-parametric). A probability value < 0.05 was considered statistically significant. InfoStat Statistical software was used to analyze all data (Di Rienzo et al. 2008).
For scanning electron microscopy (SEM) samples were fixed in 5% glutaraldehyde buffered with 0.1 M sodium cacodylate at pH 7, and post-fixed in 1.5% osmium tetroxide buffered with 0.1 M sodium cacodylate at pH 7.2. The samples were dehydrated in a graded acetone series, dried by CO2 critical point drying method and covered with a thin gold layer (200 Å) by using an ion sputter and examined in a Jeol JSM 35 Scanning Electron Microcope at CIME (Centro Integral de Microcopía Electrónica), Tucumán, Argentina.
Pollen viability
Pollen viability was indirectly estimated (Alexander 1969). This methodology stains cell wall and protoplast green and pink, respectively. In stage 6, three florets per capitulum were used for each pollen sample. Three replicates per capitulum were evaluated of three different plants. Pollen was observed under a light microscope. Four hundred pollen grains were examined per sample (n = 3600 pollen grains). Spherical and fully stained, deep pink pollen grains were considered viable. Pollen viability was obtained from average percentages of viability for all samples. Pollen diameter was measured in viable grains (n = 25).
Stigma receptivity
Stigma receptivity was tested in three stages (4, 5 and 6), using a 6% hydrogen peroxide solution that react in the presence of peroxide enzyme (Dafni 1992). Three stigmas from each capitulum from five different plants were evaluated for each stage. Vigorous bubbling was considered as receptive, whereas not bubbling was assumed as non–receptive.
Fruit and seed production
At stage 8, all fruits from one capitulum for ten different plants were evaluated for seed production. Number and filling of seeds were recorded.
Results
Capitulum morphology
The inflorescence of S. sonchifolius is a heterogamous capitulum with an external whorl of pistillate ligulated ray florets and central whorls of tubular staminate disc florets. The numbers of ray florets and disc florets per capitulum are 11–19 and 62–116, respectively (Fig. 1a, b). The receptacle has an inverted bell-shape and 5–6 free phyllaries. Each floret has a palea (Fig. 1b, c). Anthesis of the inflorescence is acropetal, starting with the external female whorl followed by the male whorls, making the pseudanthium protogynous (Table 1).
Capitulum of Smallanthus sonchifolius. a Capitulum with an external whorl of pistillate ray florets and central whorls of staminate disc florets. b Longitudinal section of capitulum at stage 6 with an inverted bell-shaped receptacle. c LM image of longitudinal section of capitulum at stage 1 showing parenchyma with procambial strands. df disc florets, pal palea, ph phyllary, ps procambial strands, rf ray florets. Scale bars are 10 mm in a, 5 mm in b, 1 mm in c
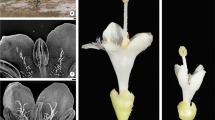
Ray florets are pistillate and zygomorphic with a yellow-orange corolla formed by a short tube and an oblong 2 or 3-toothed ligule (Fig. 2a). Abaxial epidermis of the ligule has multicellular uniseriate non-glandular trichomes and multicelullar biseriate glandular trichomes similar to those previously described for the leaf (Duarte et al. 2008; Mercado et al. 2006, 2012), and a third type two-celled uniseriate non-glandular trichome with bulbous base and rounded apex (Fig. 3). Adaxial epidermis of the ligule has conical papillae. Base of corolla is externally surrounded by a white pappus (Fig. 2a). The style ends in a dry bilobed stigma with a lip covered in both surfaces by short conical papillae where pollen grains are deposited (Fig. 2c, d). The ovary is inferior, purple and pyramidal (Fig. 2a). Cypselae are brown with thin fruit wall and pyramidal in shape (Fig. 11a).
Ray floret of Smallanthus sonchifolius. a Pistillate floret. b SEM image of bilobed stigma with a lip in both surfaces. c Detail of the lip with pollen grains (arrowheads), receptive stigmal area. d LM image of transverse section of lip with conical papillae. cp conical papillae, li ligule, ov ovary, pa pappus, st stigma. Scale bars are 2 mm in a, 300 µm in b, 200 µm in c, 100 µm in d
SEM image of abaxial epidermis of the ligule with trichomes of Smallanthus sonchifolius. a Lateral view. b Top view. Single arrowheads multicellular uniseriate non-glandular trichomes, double arrowheads multicelullar biseriate glandular trichomes, triple arrowheads two-celled uniseriate trichomes with bulbous base and rounded apex. Scale bars are 100 µm
Disc florets are staminate and actinomorphic, they present a yellow tubular corolla with five equal lobes (Fig. 4a). Abaxial epidermis of the lobes shows multicellular uniseriate non-glandular trichomes and the adaxial epidermis exhibits conical papillae (Fig. 4b, c). Disc florets have five stamens, with free filaments attached to the base of the tubular corolla and connated apiculate anthers forming a tube (syngenesious stamens) (Fig. 4a). Atrophied style and stigmas are located inside the anther tube. Ovary is not developed (Fig. 4a, d). A gynoecial nectary on the top of the undeveloped ovary surrounds the base of the style (Fig. 4d). Pollen grains are yellow, spherical, triporate, with sharp spines (Fig. 5).
Disc floret of Smallanthus sonchifolius. a Staminate florets. b, c SEM images of lobes of the tubular corolla. b Abaxial epidermis with multicellular uniseriate non-glandular trichomes (single arrowheads). c Adaxial epidermis with conical cells (double arrowheads). d LM image of longitudinal section of floret with nectaries at the base of the atrophied style. ast atrophied stigma, asty atrophied style, caa connated apiculate anthers, fa filaments attached, ff free filaments, nc nectaries, pal palea, syn syngenesious stamens, tc tubular corolla, uo undeveloped ovary. Scale bars are 2 mm in a, 200 µm in b, 100 µm in c, d
Pollen grains of Smallanthus sonchifolius. a SEM image of normal pollen grains, b, c LM image of abnormalities in pollen grains stained by Alexander method. b Pollen grains with different shapes and/or abnormal protoplasm surrounded by pollenkitt connecting pollen grains. c Pollen grains of different sizes with short spines. arrowheads pore, n normal pollen grain. Scale bars are 10 µm in a, 20 µm in b, c
Morphological stages of development of the capitulum
Eight developmental stages are differentiated from bud to maturity stage (Table 2; Fig. 6). Sizes of various floret parts were measured in stage 1 to 6 (Table 2). In ray florets, there is a statistically significant increase in the growth of all characters until stage 4. At stages 5 and 6, ovary height and style reach both the greatest size, while stigma and ovary width grow gradually between stage 4 and stage 6 (Table 2; Fig. 7).
Developmental stages of capitulum of Smallanthus sonchifolius. Stage: S1 involucral bracts close, S2 involucral bracts half close, S3 ligules emerging (capitulum after and before removing phyllaries), S4 ligules open and stigmas close, S5 stigmas open and 33% of disc florets open, S6 stigmas open and 100% of disc florets open, S7 ray and disc florets senescent, S8 mature capitulum. S1– S8 stage 1– stage 8. Scale bar is 1 cm
At stage 6, the peripheral whorl of disc florets shows smaller size of florets and anthers have not emerged (Fig. 8a, b). Anthers and filaments are statistically smaller than the rest of the whorls (Table 2, capital letter). In peripheral, middle and central sections of the capitulum, anthers reach their greatest size at stage 4, maintaining it in stages 5 and 6.
Anatomical development of the capitulum
The receptacle presents a parenchyma vascularized with procambial strands. Phyllaries have a uniseriate epidermis, multicellular uniseriate non-glandular trichomes in the abaxial surface, and 4–7 layers of homogeneous mesophyll and vascular bundles (Fig. 1c).
In ray florets, the ovary is unilocular and contains one ovule with basal placentation (Fig. 9a, d, g, j). In the cross section of stage 1, the ovary wall is undifferentiated (Fig. 9b). In stage 2, the parenchyma and the inner and outer epidermis can be noticed (Fig. 9e). From stage 3 to 6, ovary wall consists in an outer epidermis composed by a single layer of cubical and juxtaposed cells. The parenchyma is heterogeneous and has two regions. The outer parenchyma shows 4 to 7 layers of cubical cells of varying diameters. The inner parenchyma is composed by 10 to 12 layers of narrow periclinally elongated thin walled cells. The inner epidermis is multiseriate and constituted by similar cells to those described for the inner parenchyma but more elongated (Fig. 9h). The ovary walls undergo changes that culminate in the 7th stage, in which the outer epidermal cells remain cubical, the external parenchyma decreases to 4 layers of cells, the inner parenchyma presents cells with lobated walls, and the inner epidermis is absent due to a breakup process that begins at stage 3 (Fig. 9j, k).
Ovary and ovule development in ray florets of Smallanthus sonchifolius. a, d, g, j Longitudinal section of ovary in ray florets showing unilocular cavity and ovule with basal placentation. Stage 1: a ovary with unitegmic ovule, b undifferentiated ovary wall, c primordium of the ovule. Stage 2: d ovule anatropous and tenuicellate with embryo sac, e ovary wall differentiated in the inner and outer epidermis and the parenchyma, f mature embryo sac surrounded by one layer of endothelium. Stage 6: g cavity between ovary wall and ovule, h ovary wall with an outer epidermis composed of a single layer of cubical and juxtaposed cells, a heterogenous parenchyma and multiseriate inner epidermis, i embryo sac with a vacuolated central cell showing one of its two polar nuclei. Stage 7: j increase in cavity size, k ovary walls with an outer epidermis, an outer parenchyma that decreases the number of layers, the inner parenchyma whose cells have changed to cells with lobed walls, and no inner epidermis, l proliferation of endothelium and degeneration of embryo sac. cc central cell, ch chalaza, ec egg cell, en endothelium, es embryo sac, fu funiculus, ie inner epidermis, ip inner parenchyma, mp micropyle, nu nucellus, oe outer epidermis, op outer parenchyma, ov ovule, ow ovary wall, p parenchyma, pn polar nucleus, sy synergid, v vacuole. Scale bars are 20 µm in f, 50 µm in b, i, l, 100 µm in a, c, e, h, k, 200 µm in d, 500 µm in g, j
The ovule is anatropous, unitegmic, tenuinucellate, and has a short funicle (Fig. 9a, d). At stage 1, the ovary presents a primordium of the ovule with undifferentiated cells (Fig. 9c). In stage 2, the embryo sac has been formed. In Fig. 9f, two synergid cells, the egg cell, and the central cell can be distinguished. The integumentary tapetum or endothelium constituted by one layer of anticlinally elongated cells with dense cytoplasm and large nuclei, surrounds the embryo sac. Inside the endothelium at the micropylar region, nucellar tissue is differentiated (Fig. 9f). At stage 6, the embryo sac has increased in size, the endothelium cells become enlarged, and the nucellar tissue is consumed. The ovary and the ovule do not increase simultaneously, resulting a cavity between them (Fig. 9g, j). Figure 9i shows a vacuolated central cell with one of its two polar nuclei. We could not find any sign of fertilization in the serial sections of embryo sac through the stage 4 to stage 6, during which fertilization would be expected to normally occur. At stage 7, the endothelium undergoes mitotic divisions that increase the number of cells around the embryo sac, which appears detached from the endothelium. The embryo sac begins to degenerate (Fig. 9l).
In disc florets at stage 1, anthers show microsporocytes undergoing meioses and surrounded by callose (Fig. 10a). Tapetum is composed of a single layer of uninucleate or binucleate cells. The tapetal cells have a larger volume, dense cytoplasm and larger spherical nuclei (Fig. 10a). In stage 2, young microspores are surrounded by a periplasmodial tapetum (Fig. 10b). From stage 3, ornamentation of the exine becomes notable (Fig. 10c). Mature pollen grains with abnormalities, described below, are observed in stages 5 and 6. In both stages, the tapetum is consumed (Fig. 10d).
Pollen grains development in disc florets of Smallanthus sonchifolius. a Stage 1: Microsporocytes undergo meioses and tapetum is compose of a single layer of uninucleate or binucleate cells. b Stage 2: Young microspores surrounded by a periplasmodial tapetum. c Stage 3: Young pollens with notable exine ornamentation. d Stage 5: Mature normal and abnormal (arrowheads) pollen grains. mp microsporocyte, tp tapetum. Scale bar is 25 µm
Pollen viability
The viability of mature pollen determined by Alexander staining is as low as 15%. Pollen grains present diverse sizes and shapes, reduction in length of spines, and abnormalities in protoplasm (Fig. 5b, c). The protoplasm takes several forms and visually varied densities. The diameter of viable pollen is 24 ± 2 µm. Pollenkitt, an amorphous yellow substance that surrounds and connects pollen grains is observed (Fig. 5).
Stigma receptivity
Different stigmatic receptivity is observed depending on the stages of the capitulum: stage 4 and 6 show 40%, whereas stage 5 presents 87% of receptivity.
Fruit and seed production
At stage 8, all the evaluated capitula show underdeveloped and different sizes of cypselae (Fig. 11a). All cypselae present aborted seeds (Fig. 11b).
Discussion
In the present study of Smallanthus sonchifolius, the heterogamous capitulum with pistillate ray florets and disc florets functionally staminate is confirmed. Other genera of Asteraceae as Stachycephalum sp., Narvalina sp. and Unxia sp. present the same arrangement (Anderberg et al. 2007). Our study recognized eight stages for capitulum development and confirmed the protogynous nature of the pseudanthium.
The studied accession of yacon (UNT-LIEY 97–1) presents a wider range of number of ray and disc florets in comparison with other clones of yacon (Mansilla et al. 2010; Polanco Puerta 2011; Seminario et al. 2003). When all whorls of disc florets are open (stage 6), these can be classified in two groups, that is, the marginal outer whorl, with smaller florets and anthers that do not emerge, and the interior whorls.
Combination of diverse floral attributes made the capitulum interesting to pollinators in order to improve the chances of reproductive success than does a single flower (Jeffrey 2009; cited by; Bello et al. 2013). The color, shape and size of ligule make visually attractive ray florets for insect visitors in yacon (Mansilla et al. 2010). Conical papillae on the adaxial epidermis of ligule of ray florets and lobes of disc florets described in this study, may also be involved in attracting pollinators. Nectaries, pollenkitt and spined pollen grains observed in disc florets in the present work, not only offer nutrition reward to insect visitors but also help in dispersion of pollen to other inflorescences. Seminario et al. (2003) described sugary secretion in yacon disc florets and viscosity in pollen grains, but he did not mention the presence of nectaries, as in many other dioecious Asteraceae (Mani and Saravanan 1999). Pollenkitt has been previously reported in some Asteraceae genera as Artemisia, Ambrosia and Echinacea (Anderberg et al. 2007; Pacini and Hesse 2005; Wist and Davis 2008). On the other hand, glandular and non-glandular trichomes observed on the abaxial epidermis of ligule of ray florets and non-glandular trichomes on the abaxial epidermis of disc florets lobes, may be involved in chemical and mechanical defense of the plant against herbivores and pathogens as demonstrated in Salvia officinalis (Corsi and Bottega 1999).
Yacon female ray florets show dry bilobed stigma, as many Asteraceae (Heslop-Harrison and Shivanna 1977; Mani and Saravanan 1999). The reaction of bubbling of the viability test was vigorous in the stigmatic lip suggesting that this area corresponded with the receptive area of the stigma. Mansilla et al. (2010) evaluated the receptivity in the branches of opened stigma but did not mention the specific area of the reaction. The highest receptivity was observed at stage 5 where stigma and 33% of disc florets whorls were opened, agreeing with Mansilla et al. (2010).
Yacon is a species characterized by poor sexual reproduction (Grau and Rea 1997). This can be the result of different causes: pollen sterility, environmental conditions during seed germination and subsequent growth or allopolyploid hybrid origin (Grau and Rea 1997; Manrique et al. 2014; Seminario et al. 2003).
In accordance with our results, Grau and Slanis (1996), Grau and Rea (1997), and Ibañez and Zannier (2015) in Argentina, and Mansilla et al. (2010) in Perú, reported low viability of pollen by the technique of staining and germination in vitro. However, Soto Fernández (1998, cited by Seminario et al. 2003) obtained high values of viability in Perú by staining. Differences could be the result of different environmental conditions, or most likely, different genotypic materials (Mansilla et al. 2010).
Lozzia et al. (2000) reported in a clone from New Zealand of yacon, irregular and deformed pollen grains as in the present work, but with higher values of equatorial diameter pollen. Similarly, Fisher and Wells (1962) and Wells (1971) reported different pollen shapes and sizes, and reduction in the length of the spines in other Smallanthus species. Frías et al. (2000) described some irregularities in the meiosis as laggard chromosomes with or without bridge, dyads or tetrads with micronucleus; in addition Grau and Rea (1997) suggested that abnormal pollen can arise from irregular meiosis.
Manrique et al. (2014) made cross-pollinations between six accesions of yacon. They obtained 4.5% of filled seeds in one location and no seed in other locality, suggesting that this could be because of environmental conditions. Also, they suggested that a percentage of seeds obtained could be the result of self or cross-pollination before artificial crosses or by apomixis. They pollinated when ray florets were well expanded and the bifid stigma well opened but may be not receptive. Percentage of stigma receptivity was low for our stage 4, which is approximately the same stage reported for Manrique et al. (2014) for artificial crosses. They did not make a previous isolation in order to avoid self-pollination or foreign pollen contamination.
In our study, all harvested cypselae have aborted seeds only. In anatomical observation of stage 7, the endothelium shows an increase in the number of cells and embryo sac degeneration. This kind of abnormal endothelial proliferation in conjunction with ovule abortion has been observed in other Asteraceae as Cichorium intybus, Flaveria, Sigesbeckia, Chrysanthemum, and non-Asteraceae genus as Lycopersicon, Datura, Arachis; as a result of failure of fertilization, and/or as evidence of an inter- or intraspecific incompatibility reaction (Chican and Palser 1982). In yacon poor seed setting and low seed viability were reported by Meza Zela (1995), Grau and Rea (1997), Sugiura et al. (2007) and Mansilla et al. (2010). However, Chicata (1998, cited in Seminario et al. 2003) and Manrique et al. (2014) obtained a high value of viability in filled seeds. Seminario et al. (2003) suggested a predominance of partially filled or empty seeds under field conditions, and concluded that there are not only problems in pollination and fertilization, but also in seed development and germination.
Yacon has been described as a semidomesticated species for two reasons: it is a crop cultivated since prehistoric times suggesting a long history of mass selection, and because it is a polyploidy species that has suffered an ancient interspecific hybridization (Dempewolf et al. 2008). Ancestrally, yacon has been selected for its tuberous roots attributes, and has been vegetative propagated by rhizome (Grau and Rea 1997). In domestication, crops maintained by asexual propagation exhibit most drastic disruption of their flowering and fruiting systems, and the most abnormal chromosomal situations (Zohary 2004). Problems found in pollen viability and aborted cypselae in yacon, may be the result of the type of propagation and the domestication process.
Even though yacon propagates clonally, some phenotypic and genetic diversity has been reported (Lebeda et al. 2011; Mansilla et al. 2006; Mercado et al. 2014; Milella et al. 2005; Svobodová et al. 2013; Valentová et al. 2006). Future works that study pollen-pistil interactions of each clone and between various accessions (clones) of yacon, as well as wild related species, must be done.
Many crops as soybean, corn, wheat and sunflower have a guide for their developmental stages (Fehr et al. 1971; Ritchie and Hanway 1982; Schneiter and Miller 1981; Zadoks et al. 1974). Our work presents illustrated descriptions of eight reproductive stages of the capitulum of yacon, which can be easily identified in the field. Though further studies are needed to confirm that these stages are invariably recognized under various environmental conditions, such as temperature, and for different genotypes, by morphological and anatomical observations, as well as tests for pollen viability and stigma maturation, stages could be used to establish artificial crosses: stage 1 and 2 when florets are close—for isolation; stage 3 when ray florets are easily identifiable and disc florets are still close—for emasculation; stage 5 when stigma receptivity is highest, the style reaches the greatest size and embryo sac is fully develop—for artificial pollination; stage 5 and 6 when pollen grains are mature—for pollen collection; and stage 8 when capitulum is brown and physiologically mature—for fruit harvest.
References
Alexander MP (1969) Differential staining of aborted and non-aborted pollen. Stain Technol 44:117–122
Anderberg AA, Baldwin BG, Bayer RG et al (2007) Compositae. In: Kadereit JW, Jeffrey C (eds) The families and genera of vascular plants, flowering plants eudicots, Asterales, 8. Springer, Berlin, pp 61–621
Aybar MJ, Riera ANS, Grau A, Sanchez SS (2001) Hypoglycemic effect of the water extract of Smallantus sonchifolius (yacon) leaves in normal and diabetic rats. J Ethnopharmacol 74:125–132
Bello MA, Álvarez I, Torices R, Fuertes-Aguilar J (2013) Floral development and evolution of capitulum structure in Anacyclus (Anthemideae, Asteraceae). Ann Bot 112:1597–1612. doi:10.1093/aob/mcs301
Chican MA, Palser BF (1982) Development of normal and seedless achenes in Cicorium intibus (Compositae). Am J Bot 69:885–895
Chicata N (1998) Variabilidad de la semilla botánica y comparación de la progenie y clones provenientes de germoplasma de yacon (Polymnia sonchifolia). Tesis de Grado. Universidad de San Antonio Abad del Cuzco, Perú. (in Spanish)
Corsi G, Bottega S (1999) Glandular trichomes of Salvia officinalis: new data on morphology localization and histochemistry in relation to function. Ann Bot 84:657–664
Dafni A (1992) A Pollination ecology: a practical approach. IRL, New York
Dempewolf H, Rieseberg LH, Cronk QC (2008) Crop domestication in the compositae: a family-wide trait assessment. Genet Resour Crop Evol 55:1141–1157
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2008) InfoStat, version 2008. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina
Duarte MR, Wolf S, Paula BG (2008) Smallanthus sonchifolius (Poepp.) H. Rob. (yacón): identificação microscópica de folha e caule para o controle de qualidade farmacognóstico. Braz J Pharmaceutical Sci 44:157–164 (in Portuguese with English abstract)
Fehr WR, Caviness CF, Burmood DT, Pennington JS (1971) Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop Sci 11:929–931
Fisher TR, Wells JR (1962) Heteromorphich pollen grains in Polymnia. Rhodora 64:336–339
Frías AM, Caro MS, Lozzia ME, Grau A (2000) Estudio citológico del Yacon (Smallanthus sonchifolius) y Yacon del campo (Smallantus macrocyphus). Lilloa 40:115–125 (in Spanish with English abstract)
Funk VA, Susanna A, Steussy TF, Robinson HE (2009) Classification of Compositae. In: Funk VA, Susana A, Stuessy TF, Bayer RJ (eds) Systematics, evolution, and biogeography of Compositae. IAPT, Vienna, pp 171–189
Genta S, Cabrera WM, Grau A, Sanchez SS (2005) Subchronic 4-month oral toxicity study of dried Smallanthus sonchifolius (yacon) roots as a diet supplement in rats. Food Chem Toxicol 43:1657–1665
Genta S, Cabrera WM, Habib N, Pons J, Carillo IM, Grau A, Sánchez S (2009) Yacon syrup: beneficial effects on obesity and insulin resistance in humans. Clin Nutr 28:182–187
Genta S, Cabrera WM, Mercado MI, Grau A, Catalan C, Sanchez S (2010) Hypoglycemic activity of leaf organic extracts from Smallanthus sonchifolius: constituents of the most active fractions. Chem Biol Interact 185:143–152
Grau A, Rea J (1997) Yacón. Smallanthus sonchifolius. In: Hermann M, Heller J (eds) Andean root and tubers: Ahipa, arracacha, maca and yacón. IPGRI, Rome, pp 199–240
Grau A, Slanis A (1996) Is Polymnia sylphioides var. perennis a wild ancestor of yacon? Resumos I Congresso Latino Americano de Raízes Tropicais. CERAT-UNESP, São Pedro, Brasil
Heslop-Harrison Y, Shivanna RC (1977) The receptive surface of the angiosperm stigma. Ann Bot 41:1233–1258
Ibañez MS, Zannier M (2015) Viabilidad polínica de tres clones de yacón (Smallanthus sonchifolius) y una especie emparentada (Smallanthus siegesbeckius). JBAG 26 Suppl 1:155 (in Spanish)
Jeffrey C (2009) Evolution of Compositae flowers. In: Funk VA, Susana A, Stuessy TF, Bayer RJ (eds) Systematics, evolution, and biogeography of Compositae. IAPT, Vienna, pp 131–138
Lebeda A, Dolezalová I, Fernández CM, Viehmannová I (2011) Yacon (Asteraceae, Smallanthus sonchifolius). In: Singh R (ed) Genetic resources, chromosome engineering, and crop improvement series, vol 6—medicinal crops. CRC Press, Boca Raton, pp 642–702
Lozzia ME, García ME, Caro MS, Frías AM, Grau A (2000) La morfología del polen de Yakón y Yakón del campo. Sus relaciones citológicas. Lilloa 40:127–132 (in Spanish with English abstract)
Mani MS, Saravanan JM (1999) Pollination ecology and evolution in Compositae (Asteraceae). N.H. Science Publishers, Enfield
Manrique I, Gonzales R, Valladolid A, Blas R, Lizárraga L (2014) Producción de semillas en yacón (Smallanthus sonchifolius (Poepp. & Endl.)) mediante técnicas de polinización controladas. Ecol Apl 13:135–145 (in Spanish with English abstract)
Mansilla R, López BC, Blas SR, Chia JW, Baudoin J (2006) Análisis de la Variabilidad molecular de una colección peruana de Smallanthus sonchifolius (Poepp. & Endl.) H. Robinson, ‘‘yacon’’. Ecol Apl 5:75–80 (in Spanish with English abstract)
Mansilla R, López C, Flores M, Espejo R (2010) Estudios de la biología reproductiva en cinco accesiones de Smallanthus sonchifolius (Poepp. & Endl.) Robinson. Ecol Apl 9:167–175 (in Spanish with English abstract)
Mercado MI, Ponessa GI, Grau A (2006) Morfología y anatomía foliar de yacón Smallanthus sonchifolius (Asteraceae) con fines de control de calidad. Acta Farm Bonaerense 25:526–532 (in Spanish with English abstract)
Mercado MI, Coll Aráoz MV, Ruiz AI, Grau A, Ponessa GI (2012) Tricomas glandulares de yacon, Smallanthus sonchifolius (Asteraceae). Desarrollo ontogenético análisis estructural y ultraestructural. Lilloa 49:40–51 (in Spanish with English abstract)
Mercado MI, Coll Aráoz MV, Manrique I, Grau A, Catalán CAN (2014) Variability in sesquiterpene lactones from the leaves of yacon (Smallanthus sonchifolius) accessions of different geographic origin. Genet Resour Crop Ev 61:1209–1217
Meza Zela G (1995) Variedades nativas de Llacon (Polymnia sonchifolia Ker Gawler) en Cusco. UNSAAC-CICA, CIP-COTESU, Kayra. (in Spanish with English abstract)
Milella L, Salava J, Martelli G, Greco I, Cusimamani EF, Viehmannová I (2005) Genetic diversity between yacon landraces from different countries based on random amplified polymorphic DNAs. Czech J Genet Plant Breed 41:73–78
Ohyama T, Ito O, Yasoyoshi S, Ikarashi T, Minamisawa K, Kubota M, Tsukihashi T, Asami T (1990) Composition of storage carbohydrate in tubers of yacon (Polymnia sonchifolia). Soil Sci Plant Nutr 31:167–171
Pacini E, Hesse M (2005) Pollenkitt – its composition, forms and functions. Flora:399–415
Polanco Puerta MF (2011) Caracterización morfológica y molecular de materiales de yacón (Smallanthus sonchifolius Poep. & Endl) H. Robinsón colectados en la eco región eje cafetero de Colombia. Tesis magister en Ciencias Agrícolas con énfasis en Fitomejoramiento. Universidad Nacional de Colombia, Facultad de Ciencias Agropecuarias. (in Spanish with English abstract)
Ritchie S, Hanway JJ (1982) How a corn plant develops. Iowa, Iowa State Univ of Science and Technology. Coop. Ext. Service
Schneiter AA, Miller JF (1981) Description of sunflower growth stages. Crop Sci 21:901–903
Seminario J, Valderrama M, Manrique I (2003) El yacon: fundamentos para el aprovechamiento de un recurso promisorio. Centro Internacional de la Papa (CIP), Universidad Nacional de Cajamarca, Agencia Suiza para el Desarrollo y la Cooperación (COSUDE), Lima, Perú. (in Spanish)
Soto Fernández R (1998) Estudio de la biología floral del germoplasma regional del yacon. Tesis de Grado. Universidad Nacional de Cajamarca, Perú. (in Spanish with English abstract)
Sugiura M, Nakanishi T, Kameno T, Doi Y, Fujino M (2007) A new yacon cultivar: Sarada Otome. Bull NARC West Reg Agric Res Cent 6:1–13
Svobodová E, Dvoráková Z, Cepková P, Viehmannová I, Havlícková L, Fernández Cusimamani E, Russo D, Meza Zela G (2013) Genetic diversity of yacon (Smallanthus sonchifolius (Poepp. & Endl.) H. Robinson) and its wild relatives as revealed by ISSR markers. Biochem Syst Ecol 50:383–389
Valentová K, Lebeda A, Dolezalova I, Jirovsky D, Simonovska B, Vovk I, Kosina P, Gasmanova N, Dziechciarkova M, Ulrichová J (2006) The biological and chemical variability of yacon. J Agric Food Chem 54:1347–1352
Wells JR (1971) Variation in Polymnia pollen. Am J Bot 38:124–130
Wist TJ, Davis AR (2008) Floral structure and dynamics of nectar production in Echinacea pallida var. angustifolia (Asteraceae). Int J Plant Sci 169:708–722
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for growth stages of cereals. Weed Res 14:415–421
Zarlavsky GE (2014) Histología vegetal: técnicas simples y complejas, 1a ed. Sociedad Argentina de Botánica, Buenos Aires. (in Spanish)
Zohary D (2004) Unconscious selection and the evolution of domesticated plants. Econ Bot 58:5–10
Acknowledgements
The authors are thankful to Prof. PhD. Elsa Camadro for assistance in microsporogenesis analysis; and to Lab of genetics and Lab of Nematology of INTA Balcarce for providing necessary lab facilities during the work. This research was supported by ANPCyT (Grant PICTO 2004-503, PICT 2011-1871), CIUNT A26/403 and CONICET from Argentina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ibañez, M.S., Mercado, M.I., Coll Aráoz, M.V. et al. Flower structure and developmental stages of the capitulum of Smallanthus sonchifolius (Asteraceae): reproductive implications. J Plant Res 130, 327–337 (2017). https://doi.org/10.1007/s10265-017-0904-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-017-0904-x