Abstract
Most species of Sapindaceae are described as monoecious, sharing the presence of staminate flowers and flowers that are morphologically perfect but functionally pistillate. Duodichogamy has also been recorded in the family, but with few evaluations of the structures of different flower types or the functionality of reproductive structures in the different genera analyzed. Cupania L. is a monophyletic genus that occurs in tropical and subtropical America, consisting of monoecious duodichogamous trees with functionally unisexual, actinomorphic, and nectariferous flowers. Cupania emarginata Cambess. is endemic to Brazil, occurring in the northeast and southeast regions, mainly on sandy coastal plains (restingas). This study described and analyzed the flowers of this species based on morphometry and anatomy, and evaluated the functionality of the reproductive whorls and nectaries in the different floral types. Staminate flowers from the first and third flowering phases differ morpho-structurally; the gynoecium (pistillode) in first-flowering staminate flowers is markedly more collapsed than the gynoecium of third-flowering staminate flowers, in which the ovules may differentiate and contain embryo sacs with hypertrophied cells; mature anthers of pistillate flowers contain pollen grains at the stage of two-celled microgametophytes, similar to those of mature anthers of staminate flowers; and anthers of pistillate flowers are indehiscent, probably due to ineffective dehydration, as suggested by the persistence of the septum, which remains intact. The floral nectary is functional in all three flowering phases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Species of Sapindaceae generally have unisexual, actinomorphic, or zygomorphic and dichlamydeous, heterochlamydeous, hypogynous and nectariferous flowers. Pistillate flowers have staminodes and staminate flowers have pistillodes (Somner et al. 2009a). The corollas vary widely in detail, as in the vascularized ventral appendages. These appendages have different shapes and may have different colors, functioning as nectar guides or, when arched over the floral center, may protect against nectar thieves or prevent nectar dilution or evaporation (Endress and Matthews 2005). Perfect flowers have been recorded for Delavaya toxocarpa Franch and for some species of the genera Acer L., Aesculus L., Dodonaea Mill., Bizonula Pellerg., Exothea Macfad. and Handeliodendron Rehder (Acevedo-Rodríguez et al. 2011; Cao et al. 2014).
Most members of Sapindaceae are described as monoecious, sharing the presence of staminate flowers and morphologically perfect but functionally pistillate flowers (Acevedo-Rodríguez et al. 2011). The occurrence of dichogamy and duodichogamy is known in the family, even in unrelated taxa (Aluri et al. 1998; Avalos et al. 2019a; Bawa 1977; Lima et al. 2016; Rathcke and Kass 2003; Renner et al. 2007), but structural evaluations of the flowers as well as the functionality of reproductive structures in the different genera are uncommon.
Duodichogamy consists of a sequential three-phase flowering with alternation between female and male morphs (Acevedo-Rodríguez et al. 2011). The most common pattern a male-female-male flowering sequence, as recorded in Cupania guatemalensis Radlk., Cardiospermum grandiflorum Sw., and Urvillea chacoensis Hunz. (Bawa 1977; Solís et al. 2010). Although rare, the female-male-female flowering sequence has been recorded in Koelreuteria elegans (Seem.) A. C. Sm. subsp. formosana (Hayata) F.G. Mey. (Avalos et al. 2019a).
In flowering plants, several mechanisms promote cross-pollination, such as sterility of one of the reproductive whorls (Dellaporta and Calderon-Urrea 1993). Cross-pollination avoids the deleterious effects of inbreeding and promotes genetic variability, increasing the chances of survival and adaptation of a species (Dellaporta and Calderon-Urrea 1993). The phenotypic manifestations of male sterility are diverse and include complete absence of male organs, failure of normal development of sporogenous tissue, abortion of pollen grains at some stage of their development, indehiscence of anthers or inability of the microgametophyte to germinate on a compatible stigma (Budar and Pelletier 2001). Phenotypic manifestations of female sterility may be related to the absence or atrophy of the gynoecium or the ovules, or even to characteristics of the megagametophyte (Diggle et al. 2011; Pannell 2017). Studies of floral anatomy in representatives of Sapindaceae are mostly related to taxonomic and phylogenetic discussions, while the sterility and functionality of the reproductive whorls are rarely addressed (Avalos et al. 2019a, b; González et al. 2014, 2017; Solís et al. 2010; Weckerle and Rutishauser 2005; Zini et al. 2012; ).
Cupania is a monophyletic genus, distributed in tropical and subtropical America and includes approximately 50 species (Acevedo-Rodríguez et al. 2011; Buerki et al. 2009). The species are monoecious duodichogamous trees (Acevedo Rodríguez et al. 2011; Somner et al. 2009b).
Bawa (1977), studying the reproductive biology of C. guatemalensis, reported three phases of flowering: two phases in which staminate flowers are displayed, separated by a single phase in which pistillate flowers are displayed. Staminate flowers of the first phase have fertile stamens and a rudimentary gynoecium. Pistillate flowers have stamens with short filaments and indehiscent anthers, but with apparently viable pollen grains. Staminate flowers of the third phase appear after fertilization of pistillate flowers, are smaller and have a non-functional gynoecium. Based on the behavior of floral visitors, Bawa (1977) also stated that in C. guatemalensis the nectaries are non-functional and pollen is the reward for pollinators.
Cupania emarginata Cambess. is an endemic species, occurring in the Atlantic Forest domain in northeastern and southeastern Brazil (Cupania in Flora do Brasil 2020). In Rio de Janeiro State (southeastern Brazil) it occurs mainly on the sandy coastal plains, known as restingas (Somner et al. 2009a).
Considering the importance of floral anatomy for understanding reproduction in Sapindaceae, the present study described and analyzed the flowers of C. emarginata using morphometry and structural observations to evaluate the functionality of the reproductive whorls and nectaries of the different flower types. We addressed the following questions: (i) Do staminate flowers differ morpho-structurally between the first and third flowering phases? (ii) Does the gynoecium differ morpho-structurally between staminate flowers from the first and third flowering phases? (iii) Are the anthers from pistillate flowers indehiscent? (iv) Do the mature anthers of pistillate flowers have pollen grains? and (v) Are the floral nectaries of Cupania emarginata functional?
2 Materials and methods
Study site –
The study was conducted at the Restinga da Marambaia in the municipalities of Rio de Janeiro, Itaguaí and Mangaratiba (Rio de Janeiro State, southeastern Brazil, between 23°04'–23°02'S and 44°00'–44°34'W). Observations and collections were carried out between December 2017 and April 2018. Young inflorescences and pistillate and staminate flower buds and flowers were collected from five adult individuals of Cupania emarginata. Collections took place at intervals of 7 to 20 days throughout the flowering period (Table 1). The third flowering phase was not observed for individual c1.
Voucher specimens are deposited in the herbaria of the Museu Nacional (R) and of the Federal Rural University of Rio de Janeiro (RBR).
Morphometry –
For morphometric evaluation, 30 flowers were chosen at random and measured (length and greatest width) from each of 5 individuals, consisting of 10 flowers in each of the three flowering phases from each plant, totaling 140 flowers [in the third flowering phase no collection was made for individual c1 (see Table 1)]. Measurements were taken using a Digimess digital caliper with 0.01 mm resolution and 0–150 mm/6″ range (Digimess, São Paulo, Brazil) and a Leica EZ4 stereoscopic microscope (Leica, Wetzlar, Germany). The length and greatest width of the sepals and petals, and the length of the stamens and gynoecium were also measured (Dafni et al. 2005; Faegri and van der Pijl 1979). The differences between the observed means were tested using the t (Student) test, using the software Statistica 8.0 (2008). Differences corresponding to values of t with a probability < 0.05 under the null hypothesis were considered significant.
Structural analysis –
For scanning electron microscopy (SEM), pistillate and staminate floral buds and flowers were fixed in 4% formaldehyde + 2.5% glutaraldehyde in 0.05 M sodium-phosphate buffer pH 7.2 (Gahan 1984) and subjected to low pressure. The samples were dehydrated in an ethanol series, post-fixed with 0.5% osmium tetroxide in the same buffer, critical-point dried using a Bal-Tec CPD 020 (Bal-Tec, Pfäffikon, Switzerland) critical-point dryer with CO2, gold-coated using a Bal-Tec SCD 050 vacuum sputter-coater, and examined using an FEI QUANTA 200 (FEI, Hillsboro, OR, USA) scanning electron microscope.
For light microscopy, pistillate and staminate flower buds and flowers were fixed in 4% formaldehyde + 2.5% glutaraldehyde in 0.05 M sodium-phosphate buffer pH 7.2 (Gahan 1984) and subjected to low pressure. All samples were dehydrated in an ethanol series and embedded in Leica Historesin® following the manufacturer’s recommended procedure, and sectioned with glass knives at a thickness of 1–3 μm, with a Spencer 820 rotary microtome (American Optical Co, Buffalo, NY, USA). The sections were stained with 0.05% toluidine blue O (Feder and O'Brien 1968). Additionally, sections of embedded samples, obtained in a rotary microtome, were treated with: (a) Lugol, for starch detection (Langeron 1949); (b) potassium dichromate, for phenolic compounds (Gabe 1968); or (c) periodic acid + Schiff's reagent, for polysaccharides (Taboga and Vilamaior 2013). Measurements and photomicrographs were obtained using the LAS EZ software (version 3.0.0, Leica Microsystems) and a Leica ICC50 digital camera attached to a Leica DM500 microscope.
For callose observation, sections were stained with Aniline Blue (0.1%) in K2HPO4, 0.15 M (Martin 1959), refrigerated for 2 h, and observed under UV light in an Olympus BX-51 epifluorescence microscope (Olympus Co. Ltd., Tokyo, Japan), with a Q color 5 camera and Image-Pro Express software.
All images were processed using Adobe Photoshop extended CS4.
3 Results
Morphological description of staminate and pistillate flowers –
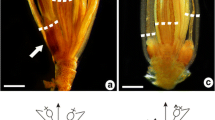
Cupania emarginata is a monoecious, duodichogamous tree with unisexual flowers (Fig. 1b, c) arranged in terminal or axillary panicle-shaped thyrses (Fig. 1a). Staminate flowers have pistillodes (Fig. 2d) and pistillate flowers have staminodes (Fig. 2e). The flowers are nectariferous, actinomorphic, pedicellate, dichlamydeous, heterochlamydeous, and hypogynous. The calyx has 5 free sepals, which are ovate, concave, imbricate, arranged in a whorl, and pubescent with ciliate margins. The petals are yellowish white and obovate with bifid appendages (Fig. 2c) that are equal in length than the villous petals themselves. In the receptacle, positioned between the corolla and the androecium, is a 5-lobed, annular nectariferous disk (Fig. 2d, e). In the staminate flower, the androecium is composed of 8 stamens of different sizes (Fig. 2a, b, d); the filaments are pubescent, with trichomes occurring for up to 3/4 of their length; and the pistillode is ovoid (Fig. 2d). Staminate flowers from the first flowering phase (Fig. 2a) are smaller than those from the third phase (Fig. 2b). The pistillate flower has an androecium with 8 staminodes and a syncarpous, bicarpellate, and puberulent gynoecium (Fig. 2e); the ovary is ovoid (Fig. 2e), superior, with 2 uniovulate locules; placentation is axial; the style is long and the stigma is bilobed.
Cupania emarginata flowers: a, staminate flower from the first flowering phase; b, staminate flower from the third flowering phase; c, petal in ventral view, showing the appendage; d, staminate flower, 1 sepal and petals removed, showing nectary, stamens and pistillode; e, pistillate flower, 1 sepal and petals removed, showing nectary, staminodes and gynoecium. ap = appendage; pe = petal
Morphometry –
The morphometric results are shown in Table 2. The t test indicated a statistically significant difference between the means of the measurements of the staminate flowers from phases 1 and 3, as well as between the means of the measurements of the different structures of these flowers. The staminate flowers from the third flowering phase are larger than the staminate flowers from the first flowering phase (t test: P = 0.007688; significance < 0.01).
Floral structure –
The 5 sepals are hypostomatic. In dorsal view, they show rectangular epidermal cells (Fig. 3a) covered with a striated cuticle, with unicellular non-glandular trichomes at the margins (Fig. 3b). In cross section (Fig. 3c) the sepals show a one-layered epidermis on both surfaces. The epidermal cells are rectangular in outline (adaxial surface) or quadrangular to rounded (abaxial surface), and larger on the abaxial surface (in relation to those of the adaxial surface). The stomata, present on the abaxial surface, project above the level of ordinary epidermal cells, giving the surface a wavy appearance. Uni- or bicellular trichomes with phenolic content occur more densely on the adaxial surface (Fig. 3d), and are covered with a striated cuticle. The mesophyll is composed of 3 to 9 parenchyma layers. The innermost layers have cells with larger dimensions than those in the outermost layers. In the mesophyll there are collateral vascular bundles (Fig. 3e), laticifers and idioblasts containing druses or phenolic compounds. The sepal margin has an epidermis with uni- or bicellular trichomes and 1 to 2 parenchyma layers with idioblasts containing druses (Fig. 3f).
Cupania emarginata sepal in dorsal view (SEM, a, b); in cross section (c, d, e, f): a, general view; b, trichomes on the margin; c, overview; d, trichome on the adaxial surface; e, collateral vascular bundle (arrow head = phloem; ⃰ = xylem); f, sepal margin. (dr = druse; s = stomata; tr = trichome). Scale bars = 100 µm in a; 20 µm in b, c; 10 µm in d, e, f
The 5 petals have vascularized ventral appendages. Petals and appendages are fused together for up to 1/3 of their length. The petal, in dorsal view, shows rectangular to rounded epidermal cells and non-glandular trichomes located mainly on the margins (Fig. 4a). The trichomes are covered with an ornamented cuticle (Fig. 4b). In cross section, the petal shows a one-layered epidermis on both surfaces, consisting of cells with a quadrangular to rounded outline, and uni- or bicellular trichomes with phenolic content (Fig. 4c). The mesophyll has 3 to 8 parenchyma layers. In this region, there are collateral vascular bundles and idioblasts containing druses (Fig. 4d). The number of parenchyma layers decreases toward the margins, which are composed of epidermis with trichomes and 1 or 2 parenchyma layers.
Cupania emarginata petal, in dorsal view (SEM, a, b) and in longitudinal section (c, d): a, abaxial surface; b, trichomes; c, proximal portion of a trichome with phenolic content, on the adaxial surface; d, mesophyll showing collateral vascular bundle (arrow head = phloem; ⃰ = xylem). (dr = druse). Scale bars = 200 µm in a; 50 µm in b; 10 µm in c, d
The androecium, in both staminate and pistillate flowers, is composed of 8 free stamens (staminate flowers, Fig. 5a) or staminodes (pistillate flowers, Fig. 5b), positioned between the nectary and the gynoecium. The filament has an epidermis with axially elongated rectangular cells, and numerous non-glandular trichomes covered with an ornamented cuticle (Fig. 5c). In cross section (Fig. 5d), the filament has an ellipsoid outline, and an epidermis with rectangular to rounded cells and uni- or bicellular trichomes, 6 to 8 parenchyma layers, and a central collateral vascular bundle. The parenchyma cells may have starch grains (Fig. 5e). The parenchyma, epidermal and trichome cells sometimes contain phenolic compounds.
Cupania emarginata androecium, under scanning electron microscopy (a, b, c, f) and light microscopy (d, e): a, staminate flower; b, pistillate flower; c, filament; d, filament, in cross section; e, starch grains (arrows) in parenchyma cells of the filament; f, anther surface in dorsal view, with trichome and stomata (arrows in the highlight). (an = androecium; gy = gynoecium). Scale bars = 500 µm in a, b; 250 µm in f; 100 µm in c; 50 µm in fand in the highlight (f); 10 µm in e
The anthers are dorsifixed, bithecate, tetralocular, dehisce by means of a longitudinal slit, and may have sparsely distributed trichomes (Fig. 5f). In surface view, they show an epidermis with polygonal to rounded cells, with slightly convex outer periclinal walls, and stomata (Fig. 5f, highlight).
In staminate flower buds measuring between 0.76 and 0.80 mm long, the septum is intact and shows drusiferous and phenolic idioblasts (Fig. 6a). The anther wall has an epidermis, 4 or 5 undifferentiated parietal layers, a secretory tapetum and sporogenous tissue (Fig. 6b). The cells of the epidermal and subepidermal layers have phenolic compounds.
Cupania emarginata staminate flower bud anther, in longitudinal (c, e, f), oblique (a, b) and cross (d) sections: a, septum; b, detail of a, showing an anther locule; c, anther locule showing epidermis, endothecium, middle layers, binucleate tapetum (white arrow), and two-celled microgametophytes (black arrows); d, anther locule with two-celled microgametophyte (arrow); e, anther submitted to potassium dichromate test, showing phenolic content; f, anther submitted to lugol test, showing starch grains. (en = endothecium; ep = epidermis; ml = middle layer; s = septum; st = sporogenous tissue; tp = tapetum; ups = undifferentiated parietal strata). Scale bars = 20 µm in a, c, e, f; 10 µm in b, d
In staminate flower buds measuring 1.9–2.0 mm long, microsporogenesis has already occurred, as well as the first mitosis of microgametogenesis (Fig. 6c). The anther wall is composed of an epidermis, an endothecium with bar thickenings, 2 middle layers with collapsed cells and a secretory tapetum (Fig. 6c, d). The epidermal cells have convex outer periclinal walls, are covered with a striated cuticle, and contain phenolic compounds (Fig. 6e). These compounds are also present in cells of the endothecium, interlocular septum and connective. The free uni- or binucleate microspores contain dense cytoplasm and starch grains (Fig. 6f).
The mature anther, in frontal view, shows a papillose epidermis, covered with striated cuticle (Fig. 7a). In cross-section, the mature anther wall has an epidermis with rounded to elliptical cells, rich in phenolic compounds (Fig. 7b) and with stomata (Fig. 7c), one layer of endothecium with bar thickenings (Fig. 7b) and collapsed middle layers. The septum is degenerate (Fig. 7d). The connective shows idioblasts with phenolic and drusiferous content and a vascular bundle (Fig. 7d). The triaperturate pollen grains are released as bicellular monads.
Cupania emarginata staminate flower anther, in surface view (SEM, a) and in cross sections (b–f); staminate flower from the first flowering phase (a–d); staminate flower from the third flowering phase (e, f): a, epidermis; b, anther wall; c, stomata; d, general view; e, anther with collapsed microspores; f, anther locules with hypertrophied tapetum and collapsed microspores. (en = endothecium; ep = epidermis; ml = middle layer). Scale bars = 200 µm in e; 10 µm in d; 50 µm in f; 20 µm in a; 10 µm in b, b
Some third-flowering staminate flowers have the anthers with collapsed pollen grains and a non-degenerate tapetum with hypertrophied cells (Fig. 7e, f).
In pistillate floral buds measuring between 0.53 and 0.68 mm long, the parenchymatous septum is intact and shows idioblasts with phenolic content or druses (Fig. 8a). The anther wall comprises epidermis, 4 or 5 undifferentiated parietal strata and secretory tapetum. The locules show microspore tetrads (Fig. 8b). Epidermal and subepidermal layer cells contain phenolic compounds. In some locules the callose wall surrounds the 4 microspores and there is no wall between them (Fig. 8c, e); in other locules, there is already wall formation between the microspores (Fig. 8d, e), indicating that microsporogenesis is simultaneous. The connective tissue contains idioblasts with phenolic or drusiferous contents and a vascular bundle (Fig. 8f).
Cupania emarginata pistillate flower bud anther, in oblique (a, c–e) or cross (b, f) sections under light (a–c, f) and epifluorescence (d, e) microscopy: a, septum; b, anther wall; c, tetrads surrounded by a callose wall; d, tetrads with microspores separated by the callose wall; e, locules of the same anther showing different stages of callose wall deposition; f, connective detail. (dr = druse; ep = epidermis; te = tetrads; tp = tapetum; s = septum; ups = undifferentiated parietal strata; vb = vascular bundle). Scale bars = 50 µm in a, e,f; 20 µm in b, c, d
In pistillate floral buds 1.21–1.56 mm long, the microspores are free. The anther wall comprises an epidermis, subepidermal layer, 2 middle layers with collapsed cells, and a secretory tapetum (Fig. 9a). Epidermal cells have convex outer periclinal walls, covered with a striated cuticle and containing phenolic compounds, the latter also present in cells of the subepidermal layer, septum and connective.
Pistillate flower bud anther (a) and pistillate flower anther (b–d) of Cupania emarginata, in cross (a, c) and oblique (d) sections and in surface view (SEM, b): a, anther wall; b, dehiscence line (arrows); c, anther wall; d, septum and microgametophytes (arrows). (en = endothecium; ep = epidermis; ml = middle layers; tp = tapetum; s = septum). Scale bars = 50 µm in b; 20 µm in c, d; 10 µm in a
In pistillate flowers, the anthers are indehiscent, with a slight separation in the region of dehiscence (Fig. 9b). The mature anther in surface view has a papillose epidermis covered with a striated cuticle. In longitudinal section, the mature anther wall shows an epidermis with rounded to elliptical cells and stomata, in addition to a layer of endothecium with bar thickenings (Fig. 9c), 2 collapsed middle layers and tapetum remnants. The septum is intact (Fig. 9d). The connective tissue contains idioblasts with phenolic or drusiferous contents and a vascular bundle. The pollen grains are triaperturate and bicellular.
The gynoecium is syncarpous and bicarpellate. The carpel primordia (Fig. 10a) in cross section are composed of a one-layered epidermis and parenchyma layers. The subepidermal layer facing the abaxial surface has cells with phenolic contents.
Gynoecium in pistillate floral buds and pistillate flowers of Cupania emarginata, in cross (a) and longitudinal (b–f) sections: a, young carpels; b, ovary and ovule primordia; c, ovule integuments and megasporocyte; d, ovarian wall; e, micropyle; f, ovule and obturator. (at = anther; gy = gynoecium; ii = inner integument; la = laticifer; mg = megasporocyte; ob = obturator; oi = outer integument; ov = ovule; pe = petal). Scale bars = 50 µm in a–d, f; 20 µm in e
In pistillate buds 0.65 mm long, the ovary wall in longitudinal section (Fig. 10b) has an external epidermis with rectangular cells containing phenolic compounds and trichomes. The cells of the subepidermal parenchyma layers (2 to 3) have phenolic compounds and are followed by about 6 parenchyma layers in which laticifers and vascular bundles occur. The inner epidermis has rectangular cells. At this stage, the ovule primordia with differentiating integuments and megasporocyte can be seen (Fig. 10c). Between the megasporocyte and the nucellar epidermis are 3–5 cell layers, characterizing the ovule as crassinucellate.
In pistillate flowers, the ovary is bilocular and uniovulate per locule. The ovary wall (Fig. 10d), in longitudinal section, has a one-layered external epidermis, with quadrangular cells and trichomes, both rich in phenolic compounds. The epidermis is followed by 3 to 5 hypodermal layers of parenchyma cells with phenolic content in addition to 15 strata of parenchyma with idioblasts containing druses or phenolic compounds. Laticifers occur between the hypodermal layers and the remaining parenchyma layers. The inner epidermis is one-layered. Ovules are anatropous, bitegmic and with axile placentation. The integuments are equal in length, but only the inner integument forms the micropyle (Fig. 10e). The obturator (Fig. 10f), a structure of placental origin, is in contact with the micropyle of the mature ovule. The obturator has an epidermis with axially elongated cells and a subepidermal layer with phenolic compounds.
The style surface has an epidermis with elongated cells, stomata and trichomes (Fig. 11a). In longitudinal section, the style shows an epidermis with rectangular cells and trichomes, both containing phenolic compounds. The epidermis is followed by parenchyma layers where laticifers and idioblasts with phenolic or drusiferous content and vascular bundles are found. The transmitting tissue is centrally positioned and has sometimes loosely arranged axially elongated cells with dense cytoplasm (Fig. 11b).
The stigmas, positioned above the anthers, are papillose (Fig. 12a) and moist. In surface view, a secretion is visible on the papillae can be verified (Fig. 12b). In longitudinal section, the epidermis of the adaxial surface is one-layered with papillose cells having a secretory appearance: dense cytoplasm, conspicuous nucleus and vacuoma composed of small vacuoles (Fig. 12c). The three subepidermal layers (Fig. 12d) have cells with dense cytoplasm and a similarly secretory appearance.
Cupania emarginata pistillate flower stigma, in surface view (SEM, a, b) and in longitudinal section (c–d): a, general view; b, secretion (arrows) on papillae surface; c, papillae; d, epidermis and subepidermal layers. (n = nucleus; v = vacuole). Scale bars = 100 µm in a; 50 µm in d; 20 µm in b; 10 µm in c
The pistillodes of staminate flowers are smaller than pistils in pistillate flowers.
In staminate flowers of the first flowering phase, fusion of the 2 carpels is incomplete, restricted to the portion corresponding to the ovary (Fig. 13a, b). Styles are short and unfused (Fig. 13a). Stigmas have few papillae. The ovary wall in longitudinal section (Fig. 13b) has the outer epidermis and subepidermal strata with cells containing phenolic compounds, followed by laticifers and collapsed cellular layers; the inner epidermis has rectangular cells. The axile placentae, obturators, and ovules have collapsed tissues making them difficult to distinguish (Fig. 13c).
In staminate flowers of the third flowering phase, the identity of the placenta, obturator and ovule is clearer, despite the presence of collapsed tissues (Fig. 13d). Ovules may have differentiated integuments, nucellus and an embryo sac (Fig. 14a). Embryo sac cells are hypertrophied and with imprecise identity (Fig. 14b-i).
The 5-lobed annular nectary is positioned between the corolla and the androecium (Fig. 15a), and is vascularized only by phloem (Fig. 15b).
Floral nectary of Cupania emarginata, in surface view (SEM, c–d), in oblique section (b) and in longitudinal sections (a, e–f): a, c, overview; b, phloem (arrows); d, stomata and secretion on the nectary surface (arrows); e, epidermis with stomata (arrows); f, secretory parenchyma and laticifers. (fi = filament; la = laticifer; pe = petal). Scale bars = 200 µm in c; 100 µm in a; 25 µm in d; 20 µm in b; 10 µm in e, f
In surface view (Fig. 15c), the epidermal cells have varied outlines, rounded, rectangular or polygonal. Stomata occur over the entire surface, and a secretion is visible (Fig. 15d).
In longitudinal section, the nectary has a one-layered epidermis with stomata (Fig. 15e) and secretory parenchyma layers (Fig. 15f). The epidermal and subepidermal layers have cells rich in phenolic compounds (Table 3). Parenchyma cells also contain starch grains (Table 3; Fig. 16a) and polysaccharides (Table 3; Fig. 16b). In the parenchymatous portion there are laticifers (Fig. 16c), and idioblasts containing druses (Fig. 16d) or phenolic compounds.
4 Discussion
Unisexual flowers have evolved independently in numerous lineages and the arrest of reproductive organs occurs at all stages of development. The morphological and cellular mechanisms of reproductive organ abortion vary and are regulated by complex hormonal, genetic and epigenetic processes (Diggle et al. 2011).
Cupania emarginata has small flowers (3–64.9 mm) arranged in a panicle-shaped thyrse. The flowering period in the study population occurred from the beginning of February to the beginning of April, and comprised three flowering phases: two staminate phases intercalated with a pistillate phase. During this period, the first staminate phase overlapped with the pistillate phase. In the third flowering phase, the pistillate flowers, fruits and staminate flowers co-occurred. In the flowering period in Cupania vernalis Cambess., Ferreira (2010) also reported some overlap of the sequential staminate and pistillate phases, resulting in a temporal distinction between male and female phases. In Sapindaceae, the most common duodichogamous pattern seems to be a male-female-male flowering sequence, as recorded here for Cupania emarginata, and also for C. guatemalensis Radlk., Cardiospermum grandiflorum Sw. and Urvillea chacoensis Hunz. (Bawa 1977; Solís et al. 2010). The case of Koelreuteria elegans subsp. formosana, studied by Avalos et al. (2019a), is the only reported instance in Sapindaceae of a duodichogamous flowering pattern with two female flowering phases separated by a single male flowering phase. Avalos et al. (2019a) suggested that possible benefits of this female-male-female duodichogamy are an increase in the number of ovules that may mature to seeds, the provision of two opportunities for fertilizing the ovules and an increase in the genetic variability of the progeny, since the ovules of the different female phases are fertilized by pollen grains originating from different plants than to those concerned in the male phase.
Although the reproductive biology of C. emarginata was not studied here, the morpho-structural analysis of the flowers, as well as the monitoring of the flowering phases, indicated predominant cross-pollination, since these flowers are functionally unisexual and mature at different times. Thus, the activity of pollinating agents that visit staminate flowers in the first and third flowering phases, when pollen grains are available and viable and pistillate flowers during the second flowering phase, when the stigmas are receptive, seems to be extremely important. Bawa (1977) reported that staminate and pistillate flowers of Cupania guatemalensis showed no sign of nectar, although both floral types had a nectariferous disk. The author suggested that these flowers produce nectar in quantities too small to measure and that the floral resource would be pollen foraged by Trigona species. In C. emarginata, the histochemical analysis evidenced the presence of starch and polysaccharides in the nectariferous parenchyma cells, as well as stomata covered with a secretion on the surface of the floral nectaries. During field work, insects visits, especially by bees, were always observed. These insects held the thyrsus with their legs, curved the front part of the body toward the center of the flowers and inserted their tongue to suck the accumulated nectar.
The general effect of (duo)dichogamy is that monoecious plants behave similarly to dioecious plants, through temporal separation of the flowers (Welzen 1989). This temporal pattern, as well as the occurrence of synchrony in the flowering of morphs within the inflorescence, promotes a spatial and temporal heterogeneity in the quality and quantity of available floral resources. This dynamic fosters the movement of pollinators and the occurrence of xenogamy (Bawa 1977).
In studies of the floral anatomy of the Sapindaceae, reports of indehiscent anthers with fertile pollen grains are not uncommon. In Xanthoceras sorbifolium Bunge, viable pollen grains are produced in staminodes whose anthers are mostly indehiscent, but a small proportion of them dehisces, releasing pollen grains that germinate and emit a pollen tube (Zhou and Liu 2012). In Xerospermum intermedium Radlk., anthers of morphologically hermaphrodite flowers are fertile and indehiscent, but at the end of anthesis they break down, promoting self-pollination and consequent fruit production (Appanah 1982). Cupania guatemalensis has well-developed but indehiscent anthers in pistillate flowers that apparently contain viable pollen grains (Bawa 1977). Similar anthers, containing pollen grains at the stage of two-celled microgametophytes, occur in indehiscent anthers of pistillate flowers of C. emarginata. In these last two species, pollen grains contained in indehiscent anthers are not available to pollinators. In C. emarginata, Magonia pubescens A. St.-Hil. (González et al. 2017) and Xerospermum intermedium, the septum in mature anthers of pistillate flowers does not degenerate and the stomium is non-functional, leading to non-dehiscence of the anthers. The failure in programmed death of the septum cells seems to be a common pattern found in indehiscent anthers in Sapindaceae species (Yadav et al. 2018). In M. pubescens (González et al. 2017) the inner middle layer collapses, while the outer middle layer remains intact, unlike in C. emarginata, where the two middle layers collapse but do not degenerate. Bawa and Opler (1975) suggested that these flowers represent a transitory stage in the evolution of unisexuality, indicating that the presence of anthers in pistillate flowers, in most species, is adaptive and probably increases their attractiveness for pollinators.
In the case of C. guatemalensis, two types of staminate flowers have been described (Bawa 1977): the first type appears and matures before the pistillate flowers, and has a rudimentary pistil. The second type has smaller dimensions and matures after the pistillate flowers have been pollinated. In C. emarginata, the staminate flowers from the third flowering phase were larger overall, as were the different structures of these flowers. In this species, in addition to morphometric differences, there were structural differences between the anthers and pistillodes of staminate flowers from the first and third flowering phases. The latter can have stamens with anthers with a tapetum with hypertrophied cells and collapsed pollen grains, in addition to larger pistillodes, and ovules with differentiated integuments, nucellus and embryo sac. Embryo sac cells, however, were hypertrophied.
In pistillate flowers of some species of Sapindaceae, the sterility of pollen grains has been associated with partial or total persistence of tapetum cells throughout microsporogenesis (González et al. 2014, 2017; Solís et al. 2010; Zini et al. 2012). The programmed cell death of tapetum cells (Kawanabe et al. 2006; Wu and Cheung 2000) generally occurs at the microspore tetrad stage (Kawanabe et al. 2006). When this process is premature or late, pollen grains development is interrupted (González-Melendi et al. 2008; Haddad et al. 2018). Our observations of the C. emarginata staminate flower structure suggest that, in anthers of some flowers from the third flowering phase, the process of programmed cell death of tapetum cells is delayed, leading to inviability of pollen grains and male sterility.
Female sterility phenotypes have also been observed in some Sapindaceae species. The absence of ovules was observed in staminate flowers of Acer oblongum Wall. ex DC. and their abortion after formation in Allophylus edulis (A. St.-Hil.) Radlk. (González et al. 2014; Yadav et al. 2018). In species such as Koelreuteria elegans subsp. formosana, Magonia pubescens and Handeliodendron bodinieri (H.Lév.) Rehder, disruption of ovule development and degeneration of the nucellus and integuments were observed (Avalos et al. 2019b; Cao et al. 2008; González et al. 2017). In Xanthoceras sorbifolium, ovule abortion occurs even before integument formation (Zhou and Liu 2012). In staminate flowers from the first stage of flowering of C. emarginata, the ovules were completely collapsed, whereas in staminate flowers from the third stage of flowering, the ovules could have differentiated integuments, nucellus, and an embryo sac, despite the presence of collapsed tissues and hypertrophied cells. When studying the development of the megagametophyte and female sterility in Monteverdia obtusifolia (Mart.) Biral (refered to as Maytenus obtusifolia Mart. Celastraceae), Haddad et al. (2019) demonstrated the structural and ultrastructural bases of these processes, associating female sterility with the absence or delay in the development of the megagametophyte or with the programmed death of its cells. The phenotypes observed in ovules from staminate flowers of C. emarginata are similar to those described by Haddad et al. (2019), i.e., lack of megagametophyte development in ovules from staminate flowers of the first flowering phase and hypertrophy of megagametophyte cells in ovules from staminate flowers of the third flowering phase. These findings suggested that both types of staminate flowers lack a functional ovary/ovules and thus do not contribute to fruit set. The male and female sterility phenotypes mentioned here occur in different genera of Sapindaceae, which are phylogenetically unrelated (e.g. Cupania, Bawa 1977; Handeliodendron, Cao et al. 2008; Allophylus, González et al. 2014; Magonia, González et al. 2017; Acer, Yadav et al. 2018; and Koelreuteria, Avalos et al. 2019b) suggesting that these characteristics have arisen independently in different lineages of the family.
Considering the questions posed initially, we can conclude that: (i) staminate flowers from the first flowering phase differ morpho-structurally from those of the third flowering phase; (ii) the gynoecium (pistillode) in staminate flowers of the first flowering phase is markedly collapsed in relation to that of staminate flowers of the third flowering phase, which may have differentiated ovules containing embryo sacs with hypertrophied cells; (iii) mature anthers of pistillate flowers are indehiscent, probably due to ineffective dehydration, suggested by the permanence of the septum which remains intact; while in staminate flowers, mature anthers are dehiscent and show a degenerate septum; (iv) mature anthers of pistillate flowers contain pollen grains at the stage of two-celled microgametophytes, similar to those of mature anthers of staminate flowers; and (v) the nectaries of flowers from the three flowering phases are functional.
These findings suggest that investigation of the anatomy of the different floral types produced by monoecious species, with alternating cycles of staminate and pistillate flowers during the flowering period, is important for understanding the phenotypic variation of sterility in Sapindaceae. This information, together with studies of reproductive biology, will enable interesting and enlightening interpretations of the family’s reproductive strategies.
References
Acevedo-Rodríguez P, van Welsen PC, Adema F, van Der Ham RWJM (2011) Sapindaceae. In: Kubitzki K (ed) The families and genera of vascular plants. X. Flowering Plants. Eudicots: Sapindales, Cucurbitales, Myrtaceae. Springer, Berlin, pp 357–407
Aluri JSR, Reddi CS, Das KR (1998) Temporal dioecism and pollination by wasps and bees in Allophylus serratus (Roxb.) Radlk. (Sapindaceae). Plant Species Biol 13:1–5. https://doi.org/10.1111/j.1442-1984.1998.tb00242.x
Appanah S (1982) Pollination of Androdioecious Xerospermum intermedium Radlk. (Sapindaceae) in a rain forest. Biol J Linn Soc 18:11–34. https://doi.org/10.1111/j.1095-8312.1982.tb02031.x
Avalos AA, Lattar EC, Ferrucci MS, Torretta JP (2019a) Reproductive biology of duodichogamous Koelreuteria elegans (Sapindaceae): the rare case of a female-male-female flowering sequence. Aust J Bot 67:149–158. https://doi.org/10.1071/BT18159
Avalos AA, Zini LM, Ferrucci MS, Lattar EC (2019b) Anther and gynoecium structure and development of male and female gametophytes of Koelreuteria elegans subsp. formosana (Sapindaceae): phylogenetic implications. Flora 255:98–109. https://doi.org/10.1016/j.flora.2019.04.003
Bawa KS (1977) The reproductive biology of Cupania guatemalensis Radlk. (Sapindaceae). Evolution 31:52–63. https://doi.org/10.1111/j.1558-5646.1977.tb00981.x
Bawa KS, Opler PA (1975) Dioecism in tropical forest trees. Evolution 29:167–179. https://doi.org/10.2307/2407150
Budar F, Pelletier G (2001) Male sterility in plants: occurrence, determinism, significance and use. Comptes Rendus De L’académie Des Sci Ser III Sci De La Vie 324:543–550. https://doi.org/10.1016/S0764-4469(01)01324-5
Buerki S, Forest F, Acevedo-Rodrígues P, Callmander MW, Nylander JAA, Harrington M, Sanmartim I, Kupfer P, Alvarez N (2009) Plastid and nuclear DNA markers reveal intricate relationships at subfamilial and tribal levels in the soapberry family (Sapindaceae). Mol Phylogenet Evol 51:238–258. https://doi.org/10.1016/j.ympev.2009.01.012
Cao LM, Xia NH, Deng YF (2008) Embryology of Handeliodendron bodinieri (Sapindaceae) and its systematic value: development of male and female gametophytes. Plant Syst Evol 274:17–23. https://doi.org/10.1007/s00606-008-0024-0
Cao LM, Cao M, Liu JH, Wang ZX, Lin Q, Xia NH (2014) Sporogenesis and gametogenesis of Delavaya toxocarpa (Sapindaceae) and their systematic implications. J Syst Evol 52:533–539. https://doi.org/10.1111/jse.12083
Cupania in Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB20891. Accessed from 4 Jun 2021
Dafni A, Kevan PG, Husband BC (2005) Practical pollination biology. Ontario, Cambridge
Dellaporta SL, Calderon-Urrea A (1993) Sex determination in flowering plants. Plant Cell 5:1241–1251. https://doi.org/10.1105/tpc.5.10.1241
Diggle PK, Di Stilio VS, Gschwend AR, Golenberg EM, Moore RC, Russell JRW, Sinclair JP (2011) Multiple developmental processes underlie sex differentiation in angiosperms. Trends Genet 27:368–376. https://doi.org/10.1016/j.tig.2011.05.003
Endress PK, Matthews ML (2005) Elaborate petals and staminodes in eudicots: diversity, function, and evolution. Org Divers Evol 6:257–293. https://doi.org/10.1016/j.ode.2005.09.005
Faegri K, van der Pijl L (1979) The principles of pollination ecology. Pergamon Press, London
Feder N, O’Brien TP (1968) Plant microtechnique: some principles and new methods. Am J Bot 55:123–142. https://doi.org/10.1002/j.1537-2197.1968.tb06952.x
Ferreira DL (2010) Interações entre Cupania vernalis Camb. (Sapindaceae) e insetos antófilos em fragmentos florestais no sul do Brasil. Dissertation, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre
Gabe M (1968) Techiniques histologiques. Masson, Paris
Gahan PB (1984) Plant histochemistry and cytochemistry: an introduction. Academic Press Inc., London
González VV, Solís SM, Ferrucci MS (2014) Anatomía reproductiva en flores estaminadas y pistiladas de Allophylus edulis (Sapindaceae). Bol Soc Argent Bot 49:207–216
González VV, Solís SM, Ferrucci MS (2017) Embryological studies of Magonia pubescens (Dodonaeaeae, Sapindaceae): development of male and female gametophytes in both floral morphs and its phylogenetic implications. Aust Syst Bot 30:279–289. https://doi.org/10.1071/SB17021
González-Melendi P, Uyttewaal M, Morcillo CN, Mora JRH, Fajardo S, Budar F, Lucas MM (2008) A light and electron microscopy analysis of the events leading to male sterility in Ogu-INRA CMS of rapeseed (Brassica napus). J Exp Bot 59:827–838. https://doi.org/10.1093/jxb/erm365
Haddad IVN, Santiago-Fernandes LDR, Machado SR (2018) Autophagy is associated with male sterility in pistillate flowers of Maytenus obtusifolia (Celastraceae). Aust J Bot 66:108–115. https://doi.org/10.1071/BT17174
Haddad IVN, Sá-Haiad B, Santiago-Fernandes LDR, Machado SR (2019) Megagametophyte development and female sterility in Maytenus obtusifolia Mart. (Celastraceae). Protoplasma 256:1667–1680. https://doi.org/10.1007/s00709-019-01413-y
Kawanabe T, Ariizumi T, Kawai-Yamada M, Uchimiya H, Toriyama K (2006) Abolition of the tapetum suicide program ruins microsporogenesis. Plant Cell Physiol 47:784–787. https://doi.org/10.1093/pcp/pcj039
Langeron M (1949) Précis de microscopie. Masson et Cie, Paris
Lima HA, Somner GV, Giulietti AM (2016) Duodichogamy and sex lability in Sapindaceae: the case of Paullinia weinmanniifolia. Plant Syst Evol 302:109–120. https://doi.org/10.1007/s00606-015-1247-5
Martin FW (1959) Staining and observing pollen tubes in the style by means of fluorescence. Stain Technol 34:125–128. https://doi.org/10.3109/10520295909114663
Pannell JR (2017) Plant sex determination. Curr Biol 27:R191–R197. https://doi.org/10.1016/j.cub.2017.01.052
Rathcke BJ, Kass LB (2003) Temporal dioecy in Three Fingers, Thouinia discolor (Sapindaceae), a medicinal shrub endemic to the Bahamas. In: Smith DL, Smith S (eds) Proceedings of the Ninth Symposium on the Natural History of the Bahamas. San Salvador, Bahamas, pp 28–33
Renner SS, Beenken L, Grimm GW, Kocyan A, Ricklefs RE (2007) The evolution of dioecy, heterodichogamy, and labile sex expression in Acer. Evolution 61:2701–2719. https://doi.org/10.1111/j.1558-5646.2007.00221.x
Solís SM, Galati B, Ferrucci MS (2010) Microsporogenesis and microgametogenesis of Cardiospermum grandiflorum and Urvillea chacoensis (Sapindaceae, Paullinieae). Aust J Bot 58:597–604. https://doi.org/10.1071/BT10162
Somner GV, Carvalho ALG, Siqueira CT (2009a) Sapindaceae da restinga da Marambaia, Rio de Janeiro, Brasil. Rodriguesia 60:485–507. https://doi.org/10.1590/2175-7860200960303
Somner GV, Ferrucci MS, Rosa MMT (2009b) Cupania. In: Somner GV (coord) Sapindaceae. In: Wanderley MGL, Shepherd GJ, Melhem TS, Giulietti AM, Martins SE (coords). Flora Fanerogâmica do Estado de São Paulo 6 In: Martins SE, Wanderley MGL, Shepherd GJ, Giulietti AM, Melhem TS (eds). Instituto de Botânica/ Fapesp/Imprensa Oficial, São Paulo, pp 202–207
Statistica for Windows: Version 8.0 (2008) Statsoft Institute Corporation, Tulsa
Taboga SR, Vilamaior PSL (2013) Citoquímica. In: Carvalho HF, Recco-Pimentel SM (eds) A célula. Manole, Barueri, pp 42–50
Weckerle CS, Rutishauser R (2005) Gynoecium, fruit and seed structure of Paullinieae (Sapindaceae). Bot J Linn Soc 147:159–189. https://doi.org/10.1111/j.1095-8339.2005.00365.x
Welzen PC (1989) Guioa Cav. (Sapindaceae): taxonomy, phylogeny, and historical biogeography. Leiden Bot Ser 12:1–314
Wu H, Cheung AY (2000) Programmed cell death in plant reproduction. In: Lam E, Fukuda H, Greenberg J (eds) Programmed cell death in higher plants. Springer, Dordrecht
Yadav N, Pandey AK, Bhatnagar AK (2018) Comparative anther and pistil anatomy of three flowering morphs of andromonoecious Acer oblongum (Sapindaceae s.l.) and its adaptive significance. Nord J Bot 36:1–9. https://doi.org/10.1111/njb.01572
Zhou QY, Liu GS (2012) The embryology of Xanthoceras and its phylogenetic implications. Plant Syst Evol 298:457–468. https://doi.org/10.1007/s00606-011-0558-4
Zini LM, Galati GB, Solís SM, Ferrucci MS (2012) Anther structure and pollen development in Melicoccus lepidopetalus (Sapindaceae): an evolutionary approach to dioecy in the family. Flora 207:712–720. https://doi.org/10.1016/j.flora.2012.07.003
Acknowledgements
We are grateful to Dr. Simon Mayo for valuable comments on the manuscript and linguistic editing. This study forms part of the master’s thesis of VCS, which was carried out in the Programa de Pós-graduação em Ciências Biológicas (Botânica), Museu Nacional, Universidade Federal do Rio de Janeiro (UFRJ).
Funding
RASS was supported by PIBIC (Programa Institucional de Bolsas de Iniciação Científica) granted by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). BSH was supported by research grant from the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (Proc. E-26/200.088/2019).
Author information
Authors and Affiliations
Contributions
BSH, GVS and VCS contributed to the study conception and design. Material preparation, data collection and analysis were performed by VCS and RASS. The first draft of the manuscript was written by VCS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
dos Santos, V.C., Silva, R.d., Somner, G.V. et al. Floral anatomy of Cupania emarginata, a duodichogamous tree. Braz. J. Bot 45, 463–483 (2022). https://doi.org/10.1007/s40415-021-00758-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-021-00758-0