Abstract
The effects of hypergravity on elongation growth and lignin deposition in secondary cell walls of the Arabidopsis thaliana (L.) Heynh. inflorescence stem were examined in plants grown for 3 days after exposure to hypergravity in the direction from shoot to root at 300 g for 24 h. The content of acetylbromide-extractable lignins in a secondary cell wall fraction prepared by enzyme digestion of inflorescence stem segments removing primary cell wall components was significantly increased by the hypergravity stimulus. Xylem vessels, particularly in a region closer to the base of the inflorescence stem, increased in number. Gadolinium chloride at 0.1 mM, a blocker of mechanoreceptors, partially suppressed the effect of hypergravity on lignin deposition in the secondary cell wall fraction. These results suggest that mechanoreceptors are responsible for hypergravity-induced lignin deposition in secondary cell walls in A. thaliana inflorescence stems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary and secondary cell walls play a major role in supporting the aerial parts of terrestrial plants under 1 g on Earth. The chemical and mechanical properties of cell walls are known to be modified under altered gravity conditions. In fact, under hypergravity conditions, the content of matrix and cellulosic polysaccharides in shoots increases per unit length (Hoson et al. 1996; Soga et al. 1999a, b). Under microgravity conditions in space, on the other hand, the content of some cell wall components such as cellulose (Cowles et al. 1984; Nedukha 1996) and matrix polysaccharides (Hoson et al. 2002) decreases.
The mechanical properties of primary cell walls are determined by the physicochemical properties of matrix polysaccharides such as xyloglucan and β-1,3-1,4-glucan (Sakurai 1991). The mass-molecular weight of these polysaccharides has been reported to increase under hypergravity conditions, and to decrease under microgravity conditions (Hoson et al. 2002; Soga et al. 2002), resulting in a decrease in cell wall extensibility under hypergravity conditions and in its increase under microgravity conditions.
In addition to the above polysaccharides in cell walls, lignin in the secondary cell wall imparts rigidity to the plant body. There are several reports indicating that the formation of lignin is suppressed under microgravity conditions in space (Cowles et al. 1984; Nedukha 1996). Clinorotation suppresses the formation of the xylem (Nakamura et al. 1999; Yoneyama et al. 2004), a tissue with intensively lignified secondary cell walls. These facts suggest that hypergravity conditions should stimulate lignin formation in secondary cell walls.
The mechanism of graviperception in growth inhibition of plant shoots under hypergravity conditions is independent of that in gravitropism because, in A. thaliana plants, the hypergravity stimulus suppresses hypocotyl elongation in wild-type Columbia plants as well as in the gravitropic mutants sgr1-1 and pgm1 (Soga et al. 2004). The inhibitory effect of the hypergravity stimulus on hypocotyl elongation in A. thaliana was eliminated by the application of La3+ or Gd3+ (Soga et al. 2004). These ions are known to be blockers of stretch-activated mechanosensitive ion channels (mechanoreceptors) (Kanzaki et al. 1999). There are lines of experimental evidence suggesting the existence of GdCl3-sensitive mechanoreceptors in plants. Gd3+ blocks calcium release from ion channels in touch-sensitive tendrils of Bryonia dioca, and a tendril’s reaction to touch is also suppressed by Gd3+ (Klüsener et al. 1995). GdCl3 inhibits the mechano-stimulated chloroplast relocation (Sato et al. 2001, 2003). The Atmid1 gene identified in A. thaliana is considered to be a candidate for a stretch-activated mechano-sensitive ion channel, although its function in gravity sensing is unknown at present (H. Iida et al. unpublished data). Thus, the effects of GdCl3 on changes in lignin formation under hypergravity were examined to test whether mechanoreceptors are involved in this process.
In the present study, these possibilities were examined using inflorescence stems of A. thaliana plants grown under hypergravity conditions.
Materials and methods
Plant material and hypergravity treatment
Arabidopsis thaliana (L.) Heynh. ecotype Columbia was used for the experiments. Seeds were surface sterilized with 95% (v/v) ethanol for 10 s and planted on 1.0% (w/v) agar consisting of 4 ml Murashige and Skoog medium (Wako, Tokyo, Japan) with 2% (w/v) sucrose in a test tube (15×105 mm). After 3 days at 4°C, plants were allowed to grow at 22°C for 20–26 days under continuous white light provided with fluorescence tubes (FL20SS·N/18, Toshiba, Tokyo, Japan), giving an intensity of 130 μmol m−2 s−1 at plant level. Plants with inflorescence stems of 5 mm in length, i.e., at A. thaliana growth stage number 5 (according to Boyes et al. 2001), were selected and exposed to hypergravity at 300 g in the shoot to root direction for 24 h at 25°C in the dark using a centrifuge (SL-05A, Sakuma, Tokyo, Japan). After centrifugation, plants were grown at 22°C for another 3 days in continuous light. For the 1 g control, test tubes containing plants with 5 mm inflorescence stems were placed in the dark without centrifugation, and then incubated for another 3 days in continuous light.
Lignin quantification
Inflorescence stem length of the 1 g control and plants subjected to centrifugation at 300 g was measured before sampling of inflorescence stems and rosette leaves. Each organ was dried overnight at 60°C. After measuring its dry weight with a S4 Supermicro (Sartorius, Goettingen, Germany), each organ was cut into pieces with a razor blade and suspended in an aqueous solution of cell wall-degrading enzymes containing 1% (w/v) Sumizyme C (Shin Nihon Chemical, Aichi, Japan) and 0.05% (w/v) pectolyase (Kikkoman, Tokyo, Japan) for 5 days at room temperature to digest the primary cell wall. After enzyme digestion, the suspension was centrifuged at 1,500 g for 10 min. The pellet, defined as the secondary cell wall fraction, was washed with distilled deionized water. The pellet was dried overnight at 60°C, and its dry weight determined. The quantification of acetylbromide-extractable lignins was carried out according to the methods described by Morrison (1972) and Jaegher et al. (1985).
Phloroglucinol staining
Phloroglucinol-HCl reagent was prepared by mixing two volumes of 2% (w/v) phloroglucinol (Merck, Frankfurt, Germany) in 95% (v/v) ethanol with one volume of 30% (v/v) HCl. Inflorescence stems were cut at 10, 20, and 30 mm from the base and the three segments were stained with phloroglucinol-HCl for 5 min and observed under a dissecting microscope (SZH-10, Olympus, Tokyo, Japan). Dark-field micrographs were taken with a microscope equipped with a digital camera (HC300Z, Fuji Photo Film, Tokyo, Japan).
Application of gadolinium chloride
A. thaliana plants grown for 22 days having an inflorescence stem of 5 mm length were selected to examine the effect of gadolinium chloride (GdCl3) on lignin formation. A 10 μl aliquot of an aqueous solution containing 40 mM GdCl3 was added to the agar medium resulting in a final concentration of 0.1 mM; 10 μl deionized distilled water was used in controls. At 3 h after GdCl3 application, plants were exposed to hypergravity at 300 g in the shoot to root direction for 24 h at 25°C in the dark using a centrifuge (see above). After centrifugation, the plants were grown at 22°C for another 3 days under continuous white light as described above. Finally, inflorescence stems were cut at 10 mm from the base. The content of acetylbromide-extractable lignins in five inflorescence stem segments was analyzed according to the methods described by Morrison (1972) and Jaegher et al. (1985).
Light microscopy
Following previously described methods (Karahara and Shibaoka 1992), frozen cross sections of 40 μm thickness were cut at 1 mm above the base of the inflorescence stems and observed using a fluorescence microscope (BX-50, Olympus, Tokyo, Japan) with UV excitation. Samples stained with phloroglucinol were observed by bright-field microscopy. Fluorescence and bright-field micrographs were taken with a digital camera (Coolsnap cf, Nippon Roper, Tokyo, Japan) connected to the microscope.
Results
Growth and lignin formation
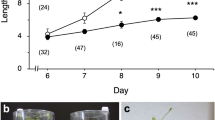
Elongation growth of the inflorescence stems of A. thaliana plants subjected to centrifugation at 300 g for 24 h in the shoot to root direction and subsequently grown for another 3 days in the light, was inhibited by the hypergravity stimulus (Fig. 1a). Dry weight of inflorescence stems increased in response to the hypergravity stimulus (Fig. 1b).
Effect of hypergravity on elongation growth of A. thaliana inflorescence stems. Plants with a 5 mm inflorescence stem were centrifuged at 300 g for 24 h in the dark, and then grown for another 3 days in the light. a Length of inflorescence stems. Values are mean ±SE (n=33–34). *z=−3.183 (Mann–Whitney U-test), P<0.05. b Dry weight of inflorescence stems and all of the rosette leaves. Values are mean ±SE (n=11). *z=−2.2, P<0.05
There was no significant effect of hypergravity on the content of secondary cell walls in an inflorescence stem or any of the rosette leaves (milligram per gram dry weight of organs; Fig. 2). However, in response to hypergravity at 300 g, the content of acetylbromide-extractable lignins in the secondary cell wall fraction substantially increased in the inflorescence stems, but not in rosette leaves (Fig. 3). In the case of rosette leaves, all rosette leaves were measured in this study, which may have masked differences that occurred in individual leaves.
Effect of hypergravity on changes in the content of the secondary cell wall fraction in inflorescence stems and all of the rosette leaves (milligram per gram dry weight of organs). A. thaliana plants were grown and subjected to centrifugation as indicated in Fig. 1. The secondary cell wall fractions were prepared by enzymatic degradation of the primary cell wall components in inflorescence stems and rosette leaves (see Materials and methods). Values are mean ±SE (n=7)
Effect of hypergravity on changes in the content of acetylbromide-extractable lignins (milligram per gram dry weight of organs) in secondary cell wall fractions obtained from inflorescence stems and all of the rosette leaves. A. thaliana plants were grown and subjected to centrifugation as indicated in Fig. 1. Secondary cell wall fractions were prepared by enzymatic digestion from inflorescence stems and rosette leaves (see Materials and methods). Values are mean ±SE (n=7). *z=−2.108, P<0.05
An increase in the content of acetylbromide-extractable lignins in 10 mm segments excised from the basal part of inflorescence stems was partially decreased by 0.1 mM GdCl3 (Table 1). We tested 0.1 mM because GdCl3 at this concentration was previously reported to be sufficient to inhibit hypergravity-induced phenomena in most plants tested (Sato et al. 2003; Soga et al. 2004).
Histochemical and microscopic analysis
Histochemical detection of lignin deposition in the inflorescence stems was monitored by microscopy after staining with phloroglucinol-HCl. Only those segments excised from the basal region (i.e., 0–10 mm from the base) of the inflorescence stem showed an intense lignin staining with phloroglucinol-HCl (Fig. 4), while other regions did not. There were no visible differences in staining pattern of lignin deposition, namely, the basipetal gradient of the staining, between 1 and 300 g on whole mount specimens.
Dark-field micrographs of inflorescence stem segments stained with phloroglucinol-HCl reagent. Three segments were excised from an inflorescence stem of A. thaliana plants grown without centrifugation. The A. thaliana growth stage was 5 (Boyes et al. 2001). a 0–10 mm; b 10–20 mm; c 20–30 mm from the base. t Top side; b bottom side. Bar 5 mm
However, the number of xylem vessels in cross sections cut at 1 mm above the base of the inflorescence stem that had been subjected to hypergravity, was greater compared to the controls (Figs. 5, 6). These results suggest that lignin formation, particularly in the basal region of the inflorescence stem, was promoted and that the number of xylem vessels was increased under hypergravity conditions.
Discussion
Hypergravity is known to suppress shoot elongation in pea (Pisum sativum L.) epicotyls (Waldron and Brett 1990), in radish and cucumber (Cucumis sativus L.) hypocotyls (Kasahara et al. 1995), in cress (Lepidium sativum L.) hypocotyls (Hoson et al. 1996), in azuki bean (Vigna angularis Ohwi et Ohashi) epicotyls (Soga et al. 1999a), in maize (Zea mayz L.) coleoptiles (Soga et al. 1999b), and in A. thaliana hypocotyls (Soga et al. 2001). In the present study, we found that this is also the case for elongation growth of A. thaliana inflorescence stems (Fig. 1a). Although hypergravity conditions decreased elongation growth of inflorescence stems in A. thaliana plants (Fig. 1a), the dry weight of inflorescence stems increased under hypergravity conditions (Fig. 1b). Hypergravity caused an increase in the content of cell wall polysaccharides in cress hypocotyls (Hoson et al. 1996), and in azuki bean epicotyls (Soga et al. 1999a). Alterations in plant growth by prolonged hypergravity treatment should be explained by the hypergravity-induced modification of primary cell wall since primary growth is regulated through modification of the primary cell wall. Thus, we calculated the change in the content of primary cell wall per unit length to discuss the inhibition of elongation growth, as was done in previous studies (Soga et al. 1999a, b). Since the major component of the dry mass in plant tissues is formed by the cell walls in general, the content of primary cell wall was estimated by subtracting the dry weight of secondary cell wall from the dry weight of the stem. The content of primary cell wall per unit length was 47.9±3.0 μg/mm (mean ±SE, n=7) in the control and 73.3±6.7 μg/mm (mean ±SE, n=7) in the hypergravity-treated sample. The content of primary cell wall also significantly increased under hypergravity (Mann–Whitney U-test, P=0.0253, z=−2.236, two-tailed), which is consistent with results of shorter periods of hypergravity treatment in previous reports (Soga et al. 1999a, b). Taken together, the prolonged hypergravity treatment is suggested to cause growth inhibition by thickening primary cell walls, as do shorter periods of hypergravity treatment.
In the present study, we attempted to examine the effect of hypergravity on secondary cell wall formation by weighing the secondary cell wall fractions prepared by enzymatic digestion of primary cell wall components. However, there was no significant effect of hypergravity on the content of secondary cell walls in an inflorescence stem or all of the rosette leaves (milligram per gram dry weight of organs; Fig. 2). On the other hand, we calculated the change in the content of secondary wall per unit length. The content of secondary cell wall per unit length was 8.6±1.1 μg/mm (mean ±SE, n=7) in the control and 13.8±1.1 μg/mm (mean ±SE, n=7) in the hypergravity-treated sample. Thus, the content of secondary cell wall, as well as that of primary cell wall, increased significantly per unit length under hypergravity (Mann–Whitney U-test, P=0.0088, z=−2.619, two-tailed).
The content of acetylbromide-extractable lignins in the secondary cell wall fractions increased significantly under hypergravity conditions (Fig. 3). Lignin formation has been shown to be inhibited under microgravity conditions in space (Cowles et al. 1984; Nedukha 1996). Our results suggest that lignin formation in secondary cell walls was stimulated under hypergravity conditions. This effect on lignin formation as well as on the content of primary and secondary wall per unit length will contribute to increasing the rigidity of the inflorescence stem. However, it remains possible that rigidity of the secondary wall is increased not by affecting its amount but by modifying its composition. Measurements of the physical properties and composition of the secondary wall will be necessary in the future.
Referring to the magnitude of gravity, a linear relationship was shown between the logarithm of the magnitude of gravity and physiological phenomena, e.g., elongation of azuki bean roots, at least up to 300 g (Soga et al. 2005). Elongation of azuki bean roots at 300 g for 5 h was approximately 50% of that under 1 g conditions (Soga et al. 2005). This clearly indicates that the 300 g stimulus itself is not an extreme condition. In addition, the effect of hypergravity on cell wall extensibility was reported to be reversible in azuki bean epicotyls, and in maize coleoptiles and mesocotyls within few hours after growth under hypergravity conditions at 300 g for 3–5 h (Soga et al. 2003). This reversible decrease in cell wall extensibility, in turn, suggests less possibility of enhancement of lignification since a decrease in cell wall extensibility would become irreversible once lignification is enhanced. This is why we chose 300 g for 24 h as the hypergravity condition. As stated above, the hypergravity condition used in this study is not necessarily an excessive treatment. Also, the inhibition of elongation of azuki bean roots at 300 g for 5 h was previously shown to be a normal physiological response (Soga et al. 2005). Taken together, these statements indicate that the responses observed in this study are not general stress responses.
A basipetal gradient of lignification exists in Arabidopsis inflorescence stems (Roger and Campbell 2004). An intensive lignin staining with phloroglucinol-HCl reagent was observed at the basal segment of A. thaliana inflorescence stems under either 300 or 1 g conditions (Fig. 4). Roger and Campbell (2004) demonstrated that in a cross section near to the apex of stalks, lignin staining is limited to xylem vessels, while in a cross section closer to the base of the floral stalk, xylem vessels and interfascicular fibers can be stained. The present study showed that the number of xylem vessels at the base of inflorescence stems increased under hypergravity conditions compared to the 1 g controls (Figs. 5, 6). These results suggest that the hypergravity stimulus promoted lignin deposition by increasing the number of xylem vessels in A. thaliana inflorescence stems. This possibility may be supported by experimental data that xylem vessel formation in Prunus tree stems was suppressed by simulated microgravity conditions on a three dimensional clinostat with two rotating axes (Nakamura et al. 1999).
The inhibitory effect of the hypergravity stimulus on the hypocotyl elongation in A. thaliana was eliminated by the application of La3+ or Gd3+ (Soga et al. 2004). Our study reveals that hypergravity-induced lignin deposition in the basal region of the inflorescence stems was indeed partially inhibited by Gd3+ (Table 1). Thus, our findings suggest that mechanoreceptors are involved in hypergravity-induced xylem vessel formation in A. thaliana inflorescence stems.
Even under nearly equal 1 g conditions on Earth, every plant needs to modify its body shape, e.g., length or height of its stem and shapes of its branches, to a given environment against gravity. The evidence that lignin deposition is regulated by the magnitude of gravity, and the involvement of a mechano-sensing system in this phenomenon, suggest that these mechanisms play an important role in the maintenance and modification of a plant’s body shape by sensing its body weight. A basipetal gradient of lignin deposition (Fig. 4; Ko et al. 2004) might be a consequence of the regulation of lignin deposition by sensing body weight, which should be tested under microgravity conditions in the future.
References
Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis K, Gorlach J (2001) Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13:1499–1510
Cowles JR, Scheld HW, Lemay R, Peterson C (1984) Growth and lignification in seedlings exposed to eight days of microgravity. Ann Bot 54:33–48
Hoson T, Nishitani K, Miyamoto K, Ueda J, Kamisaka S, Yamamoto R, Masuda Y (1996) Effect of hypergravity on growth and cell wall properties of cress hypocotyls. J Exp Bot 47:513–517
Hoson T, Soga K, Mori R, Saiki M, Nakamura Y, Wakabayashi K, Kamisaka S (2002) Stimulation of elongation growth and cell wall loosening in rice coleoptiles under microgravity conditions in space. Plant Cell Physiol 43:1067–1071
Jaegher G, Boyer N, Gaspar T (1985) Thigmomorphogenesis in Bryonia diocica: changes in soluble and bound peroxidase, phenylalanine ammonia-lyase activity, cellulose, lignin content and monomeric constituents. Plant Growth Regul 3:133–148
Kanzaki M, Nagasawa M, Kojima I, Sato C, Naruse K, Sokabe M, Iida H (1999) Molecular identification of a eukaryotic stretch-activated nonselective cation channel. Science 285:882–886
Kasahara H, Shiwa M, Takeuchi Y, Yamada M (1995) Effects of hypergravity on elongation growth in radish and cucumber hypocotyls. J Plant Res 108:59–64
Karahara I, Shibaoka H (1992) Isolation of Casparian strips from pea roots. Plant Cell Physiol 33:555–561
Ko JH, Han KH, Park S, Yang J (2004) Plant body weight-induced secondary growth in Arabidopsis and its transcription phenotype revealed by whole-transcriptome profiling. Plant Physiol 135:1069–1083
Klüsener B, Boheim G, Liß H, Engelberth J, Weiler EW (1995) Gadolinium-sensitive, voltage-dependent calcium release channels in the endoplasmic reticulum of a higher plant mechanoreceptor organ. EMBO J 14:2708–2714
Morrison IM (1972) A semi-micro method for the determination of lignin and its use in predicting the digestibility of forage crops. J Sci Food Agric 23:455–463
Nakamura T, Sassa N, Kuroiwa E, Negishi Y, Hashimoto A, Yamashita M, Yamada M (1999) Growth of Prunus tree stems under simulated microgravity conditions. Adv Space Res 23:2017–2020
Nedukha EM (1996) Possible mechanisms of plant cell wall changes at microgravity. Adv Space Res 17:37–45
Roger LA, Campbell MM (2004) The genetic control of lignin deposition during plant growth and development. New Phytol 164:17–30
Sakurai N (1991) Cell wall functions in growth and development. A physical and chemical point of view. Bot Mag Tokyo 104:235–251
Sato Y, Wada M, Kadota A (2001) External Ca2+ is essential for chloroplast movement induced by mechanical stimulation but not by light stimulation. Plant Physiol 127:497–450
Sato Y, Wada M, Kadota A (2003) Accumulation response of chloroplasts induced by mechanical stimulation in bryophyte cells. Planta 216:772–777
Soga K, Wakabayashi K, Hoson T, Kamisaka S (1999a) Hypergravity increases the molecular size of xyloglucans by decreasing xyloglucan-degrading activity in azuki bean epicotyls. Plant Cell Physiol 40:581–585
Soga K, Harada K, Wakabayashi K, Hoson T, Kamisaka S (1999b) Increased molecular mass of hemicellulosic polysaccharides is involved in growth inhibition of maize coleoptiles and mesocotyls under hypergravity conditions. J Plant Res 112:273–278
Soga K, Wakabayashi K, Hoson T, Kamisaka S (2001) Gravitational force regulates elongation growth of Arabidopsis hypocotyls by modifying xyloglucan metabolism. Adv Space Res 27:1011–1016
Soga K, Wakabayashi K, Kamisaka S, Hoson T (2002) Stimulation of elongation growth and xyloglucan breakdown in Arabidopsis hypocotyls under microgravity conditions in space. Planta 215:1040–1046
Soga K, Wakabayashi K, Kamisaka S, Hoson T (2003) Growth restoration in Azuki bean and maize seedlings by removal of hypergravity stimuli. Adv Space Res 31:2269–2274
Soga K, Wakabayashi K, Kamisaka S, Hoson T (2004) Graviperception in growth inhibition of plant shoots under hypergravity condition produced by centrifugation is independent of that in gravitropism and may involve mechanoreceptors. Planta 218:1054–1061
Soga K, Wakabayashi K, Kamisaka S, Hoson T (2005) Mechanoreceptors rather than sedimentable amyloplasts perceive the gravity signal in hypergravity-induced inhibition of root growth in azuki bean. Funct Plant Biol 32:175–179
Yoneyama E, Ishimoto-Negishi Y, Sano Y, Funada R, Yamada M, Nakamura T (2004) Morphological changes in woody stem of Prunus jamasakura under simulated microgravity. Bio Sci Space 18:3–6
Waldron KW, Brett CT (1990) Effect of extreme acceleration on the germination, growth and cell wall composition of pea epicotyls. J Exp Bot 41:71–77
Acknowledgements
This study was supported by “Ground-Based Research Announcement for Space Utilization” founded by Japan Space Forum and in part by a grant from The First Bank of Toyama. We thank Shin Nihon Chemical Co. for kindly providing Sumizyme C. We also thank to Dr. Seiji Yamaguchi of Toyama University for his helpful advice in using a supermicro chemical balance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamaoki, D., Karahara, I., Schreiber, L. et al. Effects of hypergravity conditions on elongation growth and lignin formation in the inflorescence stem of Arabidopsis thaliana. J Plant Res 119, 79–84 (2006). https://doi.org/10.1007/s10265-005-0243-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-005-0243-1