Abstract
Hypergravity caused by centrifugation inhibits elongation growth of shoots by decreasing the cell wall extensibility via suppression of xyloglucan breakdown as well as by the thickening of cell walls. The mechanism of graviperception in hypergravity-induced growth inhibition was investigated in Arabidopsis [A. thaliana (L.) Heynh.] hypocotyls and azuki bean (Vigna angularis Ohwi et Ohashi) epicotyls. Hypergravity caused growth suppression in both sgr1-1 and pgm1, which are Arabidopsis mutants deprived of gravitropism, as in wild-type plants, suggesting that the graviperception in hypergravity-induced growth inhibition of shoots is independent of that in gravitropism. Hypergravity had no effects on growth of azuki bean epicotyls or Arabidopsis hypocotyls in the presence of lanthanum or gadolinium, which are blockers of mechanoreceptors. Moreover, lanthanum or gadolinium at the same concentration had no influence on gravitropism of azuki bean epicotyls and Arabidopsis hypocotyls. Hypergravity had no effects on cell wall extensibility and affected neither xyloglucan metabolism nor the thickness of cell walls in the lanthanum- or gadolinium-treated azuki bean epicotyls. Lanthanum or gadolinium inhibited the hypergravity-induced increase in the pH of the apoplastic fluid in the epicotyls, which is involved in the processes of the suppression of xyloglucan breakdown due to hypergravity. These findings suggest that plants perceive the hypergravity stimuli by mechanoreceptors in the plasma membrane, and utilize the perceived signal to regulate the growth rate of their shoots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
On earth, gravity has been present throughout the evolution of plants and they have utilized gravity for regulation of growth and development. In order to clarify the role of gravity in regulating plant growth, it is effective to change the magnitude of the gravitational force on plants. In this context, hypergravity conditions produced by centrifugation have provided us with useful experimental systems. Hypergravity stimuli have been shown to inhibit elongation growth of pea epicotyls (Waldron and Brett 1990), radish and cucumber hypocotyls (Kasahara et al. 1995), cress hypocotyls (Hoson et al. 1996), azuki bean epicotyls (Soga et al. 1999a), Arabidopsis hypocotyls (Soga et al. 2001) and coleoptiles and mesocotyls of maize (Soga et al. 1999b). Hypergravity-induced growth inhibition is supposed to be caused by a decrease in the mechanical extensibility of cell walls (Hoson et al. 1996; Soga et al. 1999a, 1999b, 2001). In dicotyledonous plants, suppression of xyloglucan breakdown and the thickening of cell walls are involved in the decrease in the cell wall extensibility under hypergravity conditions (Soga et al. 1999a, 2001). A hypergravity-induced increase in the pH in the apoplastic fluid of epicotyls is involved in the suppression of xyloglucan breakdown (Soga et al. 2000). However, the mechanism for perception of gravity stimuli in the hypergravity-induced growth inhibition and the cell wall changes is not known.
Gravitropism has been recognized as the principal gravity response of plants, and has been intensively investigated (Chen et al. 1999; Hemmersbach et al. 1999; Kiss 2000; Tasaka et al. 2001; Boonsirichai et al. 2002). For graviperception of gravitropism, the starch-statolith hypothesis has been proposed and supported. Plants have special cells, so-called statocytes, for sensing of gravity in gravitropic responses. In shoots, starch sheath cells or endodermal cells have been recognized as statocytes. According to the starch-statolith hypothesis, dense organelles that sediment, usually amyloplasts (statoliths), trigger gravitropic sensing (Chen et al. 1999; Kiss 2000; Tasaka et al. 2001). The alternative to the starch-statolith hypothesis, which is referred to as the gravitational pressure model, was reintroduced by Wayne and Staves (1997) and Staves (1997). In this hypothesis, the entire protoplast, not particular organelles, acts as the gravity sensor. It has been proposed that both gravisusceptors, the amyloplast and the protoplast, exert a mechanical effect on the plasma membrane and regulate the activity of stretch-activated mechanosensitive ion channels (mechanoreceptors; Sievers et al. 1991; Wayne and Staves 1997; Sack 1997; Hemmersbach et al. 1999; Boonsirichai et al. 2002). Are these components also involved in the gravity perception mechanism in hypergravity-induced growth inhibition of shoots?
In Arabidopsis, inflorescence stems and hypocotyls of sgr1 (shoot gravitropism 1) lack the endodermal cell layer containing sedimentable amyloplasts and show no gravitropic responses (Fukaki et al. 1998). In addition, the pgm1 (phosphoglucomutase 1) mutant shows reduced gravitropic responses in inflorescence stems and hypocotyls, because of reduced numbers of sedimentable amyloplasts (Caspar et al. 1985). If gravity perception in hypergravity-induced growth inhibition of shoots is mediated by sedimentable amyloplasts in endodermal cells, both sgr1 and pgm1 mutants are expected not to respond, or to respond only weakly, to hypergravity stimuli. In order to examine this possibility, we measured the growth of both sgr1 and pgm1 mutants under hypergravity conditions. On the other hand, if mechanoreceptors are involved in the graviperception in hypergravity-induced growth inhibition, the shoots are expected not to respond to hypergravity stimuli under conditions in which mechanoreceptors do not act. To ascertain this possibility, we also examined the effects of lanthanum and gadolinium, blockers of stretch-activate mechanosensitive ion channels (mechanoreceptors), on growth and cell wall properties of azuki bean epicotyls and Arabidopsis hypocotyls.
Materials and methods
Plant material and hypergravity experiments
Wild-type Arabidopsis thaliana (L.) Heynh. (ecotype Columbia) and two gravitropic mutants (sgr1-1 and pgm1) were used in the present experiments. Seeds of Arabidopsis were sterilized in 95% ethanol for 4 min and then in 1.6% sodium hypochlorite solution for 8 min. Sterilized seeds were planted on 1% agar medium placed in a centrifuge tube (25 mm in diameter, 110 mm in height) and kept at 4°C for 2 days, and then they were allowed to germinate at 25°C in the dark. After 1.5 days, Arabidopsis seedlings that had a hypocotyl ca. 2 mm long were selected, and the centrifuge tube containing the seedlings was then exposed to basipetal hypergravity by centrifugation at 300 g for 1 day in the dark (centrifuge model 90-22; Sakuma, Tokyo, Japan). After the incubation, hypocotyl lengths were measured using a scale.
Azuki bean (Vigna angularis Ohwi et Ohashi cv. Erimowase) was also used in these experiments. Seeds of azuki bean were soaked in running tap water for 1 day at 30°C and they were allowed to germinate on gauze spread on a plastic dish filled with water at 25°C in the dark. After 5 days, seedlings that had an epicotyl 30–35 mm long were selected, and then transplanted into a plastic dish filled with 1 mM MES–KOH buffer (pH 6.0) with or without various concentrations of lanthanum chloride (LaCl3) or gadolinium chloride (GdCl3). After 2 h, 10 mm of the subhook region (3–13 mm below the hook) was marked with India ink. The marked seedlings were transplanted into test tubes (16 mm in diameter, 100 mm in length) containing 1.5 ml of 1 mM MES–KOH buffer (pH 6.0) with or without various concentrations of lanthanum chloride or gadolinium chloride, and then exposed to basipetal hypergravity by centrifugation for 5 h at 25°C in the dark. After the incubation, the lengths of the marked regions were measured using a scale, and then the regions were excised. All manipulations were done under dim green light (ca. 0.02 W m−2 at handling level).
Measurement of gravitropic curvature
As described above, azuki bean seedlings that had an epicotyl 30–35 mm long were selected and pretreated with lanthanum chloride or gadolinium chloride for 2 h. They were fixed by pinning the seeds on a plastic plate. Seeds and roots were moisturized using cotton wool containing 1 mM MES–KOH buffer solution (pH 6.0) with or without 0.1 mM lanthanum chloride or gadolinium chloride. The time course of curvature was determined by rotating plastic plates containing vertically fixed seedlings 90° at 25°C in the dark and then measuring the curvature of epicotyls after various times. All manipulations were done under dim green light (ca. 0.02 W m−2 at handling level).
Measurement of the mechanical properties of cell walls
The marked regions excised from azuki bean epicotyls were immediately boiled in methanol for 10 min and stored in fresh methanol until use. Before measurement of the cell wall extensibility, the methanol-killed segments were rehydrated for 3 h at 4°C with several changes of water. The cell wall extensibility was measured with a tensile tester (Tensilon RTM-25; Toyo Baldwin, Tokyo, Japan; Parvez et al. 1996). Segments were fixed between two clamps (the distance between the clamps was 5 mm) and stretched by lowering the bottom clamp at a speed of 20 mm min−1 until a load of 10 g was produced. The total extensibility was measured using the first load–extension curve immediately before the load of 10 g was produced, whereas the reversible extensibility was measured using the second load–extension curve. The apparent irreversible extensibility was calculated as the difference in slopes between the two extensions.
Determination of the amounts of cell wall polysaccharides
The marked regions excised from azuki bean epicotyls were immediately boiled in methanol as described above. Before the fractionation, segments were rehydrated with water. Cell wall polysaccharides were fractionated as previously reported (Soga et al. 1999a). The rehydrated segments were homogenized in water in a mortar with a pestle, washed with water, acetone and a methanol:chloroform mixture (1:1, v/v) and then treated with 2 units ml−1 of porcine pancreatic α-amylase (EC 3.2.1.1; type I-A; Sigma, St. Louis, MO, USA) in 50 mM sodium acetate buffer (pH 6.5) at 37°C for 3 h. After amylase treatment, pectic substances were extracted from the cell wall materials three times (15 min each) with 50 mM EDTA (pH 6.8) at 95°C. Then hemicellulose was successively extracted three times (12 h each) with 4% (w/v) KOH and three times (12 h each) with 24% (w/v) KOH containing 0.02% NaBH4 at 25°C. The fractions extracted with 4% and 24% KOH were designated as hemicellulose-I (HC-I) and hemicellulose-II (HC-II), respectively. The HC-I and the HC-II fractions were neutralized with acetic acid and then dialyzed against water. The alkali-insoluble fraction (cellulose fraction) was washed successively with 0.03 M acetic acid and ethanol, and dried at 40°C. The cellulose fraction was dissolved in 72% (v/v) sulfuric acid for 1 h at 25°C, and then diluted with a 29-fold volume of water. Total sugar content in each fraction was determined by the phenol–sulfuric acid method (Dubois et al. 1956) and expressed as glucose equivalents.
Determination of the molecular mass of xyloglucans
For determination of the molecular mass of xyloglucans, the HC-II fraction was lyophilized, and then samples were dissolved in 50 mM potassium phosphate buffer (pH 7.2), and a portion of the solution was injected into the gel permeation column (TSK-GEL 5000 PW; Tosoh, Tokyo, Japan) of an HPLC system (LC-6A; Shimadzu, Kyoto, Japan) equipped with a refractive index detector (RID-6A; Shimadzu). The sample was eluted with 50 mM potassium phosphate buffer (pH 7.2) at a flow rate of 1 ml min−1. The elution pattern was monitored by a refractive-index detector. Fractions were collected with a fraction collector (model-203; Gilson, Middleton, WI, USA) at 0.5-min intervals. The xyloglucan content in each fraction was determined by the iodine staining method (Kooiman 1960). The weight-average molecular mass of xyloglucans was calculated from the equation reported by Nishitani and Masuda (1981). Dextrans (Sigma) of 10, 40, 70, 120 and 500 kDa were used as molecular mass markers.
Measurement of the xyloglucan-degrading activity
The marked regions excised from azuki bean epicotyls were immediately frozen with liquid nitrogen and kept at −80°C until use. Extraction and assay of the xyloglucan-degrading activity were carried out essentially by the methods of Hoson et al. (1995) and Tabuchi et al. (1997). The frozen segments (ca. 400 mg FW) were homogenized with ice-cold 10 mM sodium phosphate buffer (pH 7.0). The homogenate was filtered through polypropylene mesh (32 μm) to obtain the cell wall fraction. This fraction was washed with the same buffer and then suspended in 10 mM sodium phosphate buffer (pH 6.0) containing 1 M NaCl. The suspension was kept for 24 h at 4°C and filtered through polypropylene mesh. The filtrate was used as enzyme extract for the measurement of the xyloglucan-degrading activity. The reaction mixture containing 20 μg of purified azuki xyloglucans (400–600 kDa) and ca. 5 μg cell wall proteins in 50 μl of 100 mM sodium phosphate buffer (pH 6.0) was incubated for 6 h at 37°C. After incubation, the reaction was terminated by boiling. The activity of xyloglucan-degrading enzymes was assayed by the iodine staining method (Kooiman 1960), and expressed in terms of the decrease in absorbance at 640 nm of the xyloglucan–iodine complex. Protein content was determined with a Protein Assay Kit (Bio-Rad, Hercules, CA, USA).
Measurement of the pH of apoplastic solution
The pH value of the apoplastic solution was measured as previously reported (Soga et al. 2000). The marked regions excised from azuki bean epicotyls were rinsed with water and placed on a stainless mesh in the barrel of a cut plastic syringe. After the excess water on the surface of the segments was removed by a flash centrifugation, the segments were centrifuged at 1,500 g for 20 min at 4°C to collect the apoplastic solution. Immediately after the centrifugation, the pH of the apoplastic solution was measured with a pH meter (B-112; Horiba, Tokyo, Japan) equipped with a flat-surface electrode.
Results
Growth and gravitropic responses
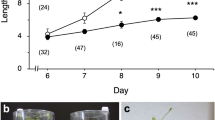
Figure 1 shows elongation growth of hypocotyls of wild-type Arabidopsis (Columbia) and gravitropic mutants (sgr1-1 and pgm1) grown at 1 g or under hypergravity conditions at 300 g produced by centrifugation. Elongation growth of hypocotyls of both sgr1-1 and pgm1 at 1 g was the almost same as that of Columbia (Fig. 1). Growth of hypocotyls of Columbia was suppressed by hypergravity at 300 g (Fig. 1). Hypergravity caused growth suppression in both sgr1-1 and pgm1 mutants as in wild-type plants. On the other hand, hypocotyls of sgr1-1 did not show any gravitropic curvature, and those of pgm1 showed reduced gravitropic responses (data not shown).
Lanthanum and gadolinium have been used as blockers of stretch-activated mechanosensitive ion channels (mechanoreceptors) in various organisms, including plants (Ding and Pickard 1993; for review, see Fasano et al. 2002). It is known that calcium ions function as second messengers in various signal responses. At high concentrations, lanthanum and gadolinium block calcium channels. Both blockers may cause abnormal calcium levels in cells at concentrations of 10 mM, leading to abnormal growth responses. In azuki bean epicotyls under 1-g conditions, elongation growth of marked regions was slightly inhibited at 10 mM concentrations of both blockers, but it was not affected at concentrations up to 1 mM (data not shown). In most plants, 0.1 mM lanthanum or gadolinium has been shown to be sufficient to inhibit the activity of mechanoreceptors (Sato et al. 2003). Thus, we used both blockers at 0.1 mM in the following experiments.
Hypergravity at 300 g had no effects on elongation growth of the marked regions of azuki bean epicotyls in the presence of 0.1 mM lanthanum or gadolinium (Fig. 2a). Also, in Arabidopsis hypocotyls, hypergravity-induced growth inhibition was not observed in the presence of these blockers of mechanoreceptors at 0.1 mM (data not shown). Figure 2b shows the gravitropic curvature of azuki bean epicotyls in the presence or absence of 0.1 mM lanthanum or gadolinium. In the absence of lanthanum or gadolinium, the gravitropic curvature of epicotyls started about 1 h after gravity stimulation and continued for following 4–5 h. Lanthanum and gadolinium at 0.1 mM had no effects on gravitropism of azuki bean epicotyls (Fig. 2b). Gravitropic curvature of Arabidopsis hypocotyls was also unaffected by treatment with lanthanum or gadolinium (data not shown).
Effects of lanthanum and gadolinium on elongation growth and the gravitropic curvature of azuki bean (Vigna angularis) epicotyls. Azuki bean seedlings whose epicotyls had been marked were grown at 1 g or 300 g in the presence or absence of 0.1 mM lanthanum or gadolinium for 5 h at 25°C. The initial length of the marked region was 10 mm. After the incubation, the lengths of the marked regions were measured using a scale (a). Azuki bean epicotyls treated with 0.1 mM lanthanum or gadolinium were placed horizontally and the gravitropic curvature was measured (b). Values are means ± SE (n=20). SEs for the gravitropic curvature were within the symbols
Cell wall extensibility
In the absence of lanthanum or gadolinium, the total cell wall extensibility of azuki bean epicotyls did not change during the incubation at 1 g (Fig. 3a). Hypergravity at 300 g, however, significantly decreased the total cell wall extensibility of epicotyls in the absence of lanthanum or gadolinium, indicating that hypergravity makes the cell wall mechanically rigid. Treatment with lanthanum or gadolinium at 0.1 mM did not affect the total cell wall extensibility of epicotyls at 1 g. A hypergravity-induced decrease in the total cell wall extensibility was not observed in the presence of 0.1 mM lanthanum or gadolinium (Fig. 3a). The total extensibility of segments was separated into the reversible and the apparent irreversible components by two successive extensions. No clear differences were found in the reversible extensibility between epicotyls grown under hypergravity conditions and those grown under 1-g conditions, independent of the presence of lanthanum or gadolinium (Fig. 3b). Hypergravity at 300 g, however, significantly decreased the apparent irreversible cell wall extensibility of epicotyls in the absence of lanthanum or gadolinium (Fig. 3c). Thus, the decrease in the total extensibility of epicotyls under hypergravity conditions was mainly due to the decrease in the apparent irreversible extensibility. In the presence of lanthanum or gadolinium, a hypergravity-induced decrease in the apparent irreversible extensibility was not observed.
Effects of lanthanum and gadolinium on the total, the reversible, and the apparent irreversible cell wall extensibility of azuki bean epicotyls grown at 1 g or 300 g. Azuki bean seedlings were grown as shown in Fig. 2a. The total extensibility (a) was calculated based on the slope of the load–extension curve. The materials were subjected to two successive extensions. The reversible extensibility (b) was measured from the slope of the second curve, and the apparent irreversible extensibility (c) was calculated as the difference in slopes between the first and the second extensions. Values are means ± SE (n=20)
Levels of cell wall polysaccharides
The cell walls of azuki bean epicotyls grown either at 1 g or 300 g were divided into four fractions (pectin, HC-I, HC-II and cellulose) and the amount of polysaccharides in each fraction was determined. The amount of cell wall polysaccharides per epicotyl increased during the incubation at both 1 g and 300 g, and hypergravity did not affect the amount, independent of the presence of lanthanum or gadolinium (data not shown). In the absence of lanthanum or gadolinium, hypergravity at 300 g increased the levels of cell wall polysaccharides per unit length, indicating that hypergravity induces the cell wall thickening in azuki bean epicotyls (Table 1). However, such a cell wall thickening due to hypergravity was not observed in the presence of lanthanum or gadolinium. No clear influences of hypergravity were detected on the proportions among the four cell wall polysaccharide fractions, independent of the presence of lanthanum or gadolinium (data not shown).
Molecular mass of xyloglucans
Figure 4 shows the molecular mass distribution of xyloglucans in the HC-II fraction of cell walls, as detected by the iodine method. In the absence of lanthanum or gadolinium, xyloglucans of the initial and 1-g-grown epicotyls eluted in similar molecular mass regions, while those obtained from hypergravity-treated epicotyls shifted to high-molecular-mass regions. Therefore, the calculated weight-average molecular mass of xyloglucans of hypergravity-treated epicotyls was significantly higher than that of the initial and 1-g-grown epicotyls (Table 1). This hypergravity-induced increase in the weight-average molecular mass of xyloglucans was not observed in the lanthanum- or gadolinium-treated epicotyls.
Elution profiles of xyloglucans in the HC-II fractions of the azuki bean epicotyls on a gel permeation column (TSK-GEL 5000 PW). The content of xyloglucan was determined by the iodine method. Vertical bars denote the elution positions of molecular mass standards (in kDa) and void volume (V 0 ). Each elution profile shows means of three replicates without SE
Activity of xyloglucan-degrading enzymes and apoplastic pH
The activity of xyloglucan-degrading enzymes was measured in a protein fraction extracted with 1 M NaCl from the cell walls. In the absence of lanthanum or gadolinium, the activity per epicotyl region increased during the incubation at 1 g, but hypergravity inhibited the increase (data not shown). Hypergravity at 300 g had no effects on the xyloglucan-degrading activity per epicotyl region in lanthanum- or gadolinium-treated epicotyls (data not shown). The specific activity was decreased by hypergravity in the absence of lanthanum or gadolinium (Table 2). A hypergravity-induced decrease in the specific activity was not observed in the lanthanum- or gadolinium-treated epicotyls.
In the absence of lanthanum or gadolinium, the pH of apoplastic fluid obtained from the 1-g-grown epicotyls did not change during the incubation, whereas that from the hypergravity-treated ones significantly increased (Table 2). The increase in the apoplastic pH due to hypergravity was not observed in the lanthanum- or gadolinium-treated epicotyls; moreover, the pH did not change during the incubation, irrespective of gravitational conditions.
Discussion
Hypergravity caused by centrifugation suppressed elongation growth of hypocotyls in both sgr1-1 and pgm1 mutants as in wild-type plants (Fig. 1). However, the sgr1-1 and pgm1 mutants show reduced or no gravitropic responses in inflorescence stems and hypocotyls, because of lack of sedimentable amyloplasts (Caspar et al. 1985; Fukaki et al. 1998). These results suggest that the gravity perception mechanism in hypergravity-induced growth inhibition of shoots is independent of that in gravitropism, which involves amyloplasts as statoliths. Inflorescence stems and hypocotyls of sgr1 lack an endodermal cell (statocytes) layer, which contains special cells for sensing of gravity in gravitropic responses (Fukaki et al. 1998). As described above, elongation growth of hypocotyls in sgr1-1 was inhibited by hypergravity as in wild-type plants (Fig. 1). This suggests that, in hypergravity-induced growth inhibition, all elongating cells, not just particular cell types, may be involved in the perception of gravity the signal.
Mechanoreceptors have been found in various organisms, including plants, in the plasma membrane (Kanzaki et al. 1999). In azuki bean epicotyls, hypergravity at 300 g had no effect on elongation growth in the presence of lanthanum or gadolinium, which are blockers of mechanoreceptors (Fig. 2a). Moreover, hypergravity-induced growth inhibition was not observed in Arabidopsis hypocotyls in the presence of either blocker (data not shown). These results suggest that mechanoreceptors are involved in the perception of the gravity signal in hypergravity-induced growth inhibition of shoots. In contrast, lanthanum and gadolinium at the same concentration did not influence gravitropism of azuki bean epicotyls (Fig. 2b) or Arabidopsis hypocotyls (data not shown), although the involvement of mechanoreceptors in gravitropism has been proposed (Sievers et al. 1991; Hemmersbach et al. 1999; Boonsirichai et al. 2002). Mechanoreceptors may not be responsible for the graviperception in gravitropism or may be less sensitive to the blockers, even if they are involved. Taken together, these findings show that the gravity perception mechanism in hypergravity-induced growth inhibition is independent of that of gravitropism.
The gravitational-pressure model for gravity sensing in gravitropism was reintroduced by Wayne and Staves (1997) and Staves (1997). In this model, the entire protoplast, not particular organelles, acts as the gravity sensor. Gravity may cause the protoplast to settle, resulting in differential tension and compression between the plasma membrane and the cell wall at the top and bottom of the cell, respectively. These differential pressures may activate the gravireceptors, which are located at the top and bottom of the cell. Although whether the gravitational-pressure model is applicable to gravitropism, instead of the amyloplast–statolith theory, is still debated, the model is compatible with the mechanisms of signal perception in hypergravity-induced growth inhibition of shoots. In hypergravity-induced growth inhibition, the gravity signal may be received by the plasma membrane mechanoreceptors.
In the presence of lanthanum or gadolinium, a hypergravity-induced decrease in the cell wall extensibility was not observed (Figs. 3a,c). The thickness of cell walls, xyloglucan metabolism, and the apoplastic pH were also unaffected by hypergravity in the presence of lanthanum or gadolinium (Fig. 4; Tables 1, 2). Thus, all changes induced by hypergravity disappeared as a result of treatment with lanthanum or gadolinium. In hypergravity-induced growth inhibition, the gravity signal may be received by the plasma membrane mechanoreceptors, transformed and transduced within the cell, and then may modify cell wall synthesis and xyloglucan breakdown, as well as the apoplastic pH, leading to the decrease in the cell wall extensibility.
Plants are highly resistant to gravity stimuli, and hypergravity at 30 g and above is required to induce significant growth suppression in shoot organs (Hoson et al. 1996; Soga et al. 1999a, 1999b). Thus, one possibility was that the observed effects of hypergravity could be caused by physiological damage to cells. However, when azuki bean and maize seedlings grown under hypergravity conditions at 300 g for several hours were transferred to 1-g conditions, the growth rate of shoot organs greatly increased within a few hours (Soga et al. 2003). In addition, the changes in cell wall properties, such as the cell wall extensibility and thickness, the molecular masses of xyloglucans and β-1,3:1,4-glucans, the activities of xyloglucan-degrading enzymes and β-1,3:1,4-glucanases, and the apoplastic pH, induced by hypergravity at 300 g for several hours were fully and promptly reversed by the removal of the hypergravity stimulus (Soga et al. 2003). In the present study, hypergravity at 300 g had no effects on growth of azuki bean epicotyls (Fig. 2a) and Arabidopsis hypocotyls (data not shown) in the presence of lanthanum or gadolinium. In addition, no cell wall properties measured in the present study were influenced by hypergravity in the presence of lanthanum or gadolinium. These results support the contention that hypergravity at 300 g is not an extraordinary stimulus for plants and that plant responses to this magnitude of gravity can be recognized as normal physiological responses.
The linear relationship between the logarithm of the magnitude of gravity and growth in Arabidopsis hypocotyls can be extrapolated into the microgravity range (Soga et al. 2001, 2002). The above-mentioned cell wall properties also varied in proportion to the logarithm of magnitude of gravity (Soga et al. 2001). The results of the present study suggest that hypergravity-induced changes in growth and the cell wall properties are mediated by the plasma membrane mechanoreceptors. These results suggest that gravity at 1 g, which regulates growth and the cell wall properties on earth, is also perceived by the plasma membrane mechanoreceptors. We are planning space experiment to clarify this possibility.
Abbreviations
- HC-I :
-
Hemicellulose-I
- HC-II :
-
Hemicellulose-II
References
Boonsirichai K, Guan C, Chen R, Masson PH (2002) Root gravitropism: an experimental tool to investigate basic cellular and molecular processes underlying mechanosensing and signal transmission in plants. Annu Rev Plant Biol 53:421–47
Caspar T, Huber SC, Somerville CR (1985) Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol 79:11–17
Chen R, Rosen E, Masson PH (1999) Gravitropism in higher plants. Plant Physiol 120:343–350
Ding JP, Pickard BG (1993) Mechanosensory calcium-selective cation channels in epidermal cells. Plant J 3:83–110
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Fasano JM, Massa GD, Bilroy S (2002) Ionic signaling in plant responses to gravity and touch. J Plant Growth Regul 21:71–88
Fukaki H, Wysocka-Diller J, Kato T, Fujisawa H, Benfey PN, Tasaka M (1998) Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J 14:425–430
Hemmersbach R, Volkmann D, Häder DP (1999) Graviorientation in protists and plants. Plant Physiol 154:1–15
Hoson T, Tabuchi A, Masuda Y (1995) Mechanism of xyloglucan breakdown in cell walls of azuki bean epicotyls. J Plant Physiol 147:219–224
Hoson T, Nishitani K, Miyamoto K, Ueda J, Kamisaka S, Yamamoto R, Masuda Y (1996) Effects of hypergravity on growth and cell wall properties of cress hypocotyls. J Exp Bot 47:513–517
Kanzaki M, Nagasawa M, Kojima I, Sato C, Naruse K, Sokabe M, Iida H (1999) Molecular identification of a eukaryotic, stretch-activated nonselective cation channel. Science 285:882–886
Kasahara H, Shiwa M, Takeuchi Y, Yamada M (1995) Effects of hypergravity on elongation growth in radish and cucumber hypocotyls. J Plant Res 108:59–64
Kiss JZ (2000) Mechanisms of the early phases of plant gravitropism. Crit Rev Plant Sci 19:551–573
Kooiman P (1960) A method for the determination of amyloid in plant seeds. Recl Trav Chim Pays-Bas 79:675–678
Nishitani K, Masuda Y (1981) Auxin-induced changes in the cell wall structure: changes in the sugar composition, intrinsic viscosity and molecular weight distributions of matrix polysaccharides of the epicotyl cell wall of Vigna angularis. Physiol Plant 52:482–494
Parvez MM, Wakabayashi K, Hoson T, Kamisaka S (1996) Changes in cellular osmotic potential and mechanical properties of cell walls during light-induced inhibition of cell elongation in maize coleoptiles. Physiol Plant 96:179–185
Sack FD (1997) Plastids and gravitropic sensing. Planta [Suppl] 203:63–68
Sato Y, Wada M, Kadota A (2003) Accumulation response of chloroplasts induced by mechanical stimulation in bryophyte cells. Planta 216:772–777
Sievers A, Buchen B, Volkmann D, Hejnowicz Z (1991) Role of the cytoskeleton in gravity perception. In: Lloyd CW (ed) The cytoskeletal basis of plant growth and form. Academic Press, London, pp 169–182
Soga K, Wakabayashi K, Hoson T, Kamisaka S (1999a) Hypergravity increases the molecular size of xyloglucans by decreasing xyloglucan-degrading activity in azuki bean epicotyls. Plant Cell Physiol 40:581–585
Soga K, Harada K, Wakabayashi K, Hoson T, Kamisaka, S (1999b) Increased molecular mass of hemicellulosic polysaccharides is involved in growth inhibition of maize coleoptiles and mesocotyls under hypergravity conditions. J Plant Res 112:273–278
Soga K, Wakabayashi K, Hoson T, Kamisaka S (2000) Changes in the apoplastic pH are involved in regulation of xyloglucan breakdown of azuki bean epicotyls under hypergravity conditions. Plant Cell Physiol 41:509–514
Soga K, Wakabayashi K, Hoson T, Kamisaka S (2001) Gravitational force regulates elongation growth of Arabidopsis hypocotyls by modifying xyloglucan metabolism. Adv Space Res 27:1011–1016
Soga K, Wakabayashi K, Kamisaka S, Hoson T (2002) Stimulation of elongation growth and xyloglucan breakdown in Arabidopsis hypocotyls under microgravity conditions in space. Planta 215:1040–1046
Soga K, Wakabayashi K, Kamisaka S, Hoson T (2003) Growth restoration in azuki bean and maize seedlings by removal of hypergravity stimuli. Adv Space Res 31:2269–2274
Staves MP (1997) Cytoplasmic streaming and gravity sensing in Chara internodal cells. Planta [Suppl] 203:79–84
Tabuchi T, Kamisaka S, Hoson T (1997) Purification of xyloglucan hydrolase/endotransferase from cell walls of azuki bean epicotyls. Plant Cell Physiol 38:653–658
Tasaka M, Kato T, Fukaki H (2001) Genetic regulation of gravitropism in higher plants. Int Rev Cytol 206:135–154
Waldron KW, Brett CT (1990) Effects of extreme acceleration on the germination, growth and cell wall composition of pea epicotyls. J Exp Bot 41:71–77
Wayne R, Staves MP (1997) A down-to-earth model of gravisensing. Gravi Space Biol Bull 10:57–64
Acknowledgements
We thank Professor Emeritus Yoshio Masuda of Osaka City University for invaluable suggestions and discussions, Professor John Z. Kiss of Miami University for providing the pgm1 mutant, and Professor Masao Tasaka and Dr. Hidehiro Fukaki of Nara Institute of Science and Technology for providing the sgr1-1 mutant. The present study was supported in part by a Grant for Ground Research for Space Utilization from the Japan Space Forum.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soga, K., Wakabayashi, K., Kamisaka, S. et al. Graviperception in growth inhibition of plant shoots under hypergravity conditions produced by centrifugation is independent of that in gravitropism and may involve mechanoreceptors. Planta 218, 1054–1061 (2004). https://doi.org/10.1007/s00425-003-1187-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-1187-0