Abstract
Steel slag, which contains basic oxides capable of generating high levels of alkalinity, may be used in leaching beds or leaching channels to neutralize acid mine drainage (AMD). In the present study, electric arc furnace (EAF) slag from the Pasargad Steel Complex was used to investigate the influence of slag particle size on AMD treatment. Three slag samples with different particle size distributions (coarse, mixed, fine) were prepared. Slag composition, pH variation, level of alkalinity, and specific surface area were measured and the surfaces of the slag particles was examined by scanning electron microscopy. In both dynamic and static leaching tests, there proved to be three stages: an initial high pH, alkalinity digestion-exhaustion, and low pH. Depletion of fine particles and precipitation of Fe, Al, and Mn hydroxides on particle surfaces were critical factors in how the alkalinity in the slag was exhausted during the second stage. SEM images suggest that metal precipitates prevented the AMD from reaching the slag surface and hence its neutralization potential was reduced. We also found that finely-ground slag produced a higher initial pH in the leachate but that after 60 leaching cycles, the pH value dropped below that of the coarse slag. A mixed sample demonstrated an initial high pH and a moderate pH after the 60th cycle, which verified greater potential for long-term alkalinity generation than the well-sorted slag.
Resumen
La escoria de acero que contiene óxidos básicos capaces de generar altos niveles de alcalinidad, puede ser usada en camas o canales de lixiviación para neutralizar drenajes ácidos de mina (AMD). En el presente estudio, la escoria de horno de arco eléctrico (EAF) proveniente de Pasargad Steel Complex fue usado para investigar la influencia del tamaño de partícula sobre el tratamiento de AMD. Se prepararon tres muestras de escoria con diferentes distribución de tamaño de partícula (grueso, mezcla y fino). Se analizaron la composición de la escoria, la variación del pH, el nivel de alcalinidad, el área superficial específica y la superficie de las partículas por microscopía electrónica de barrido. En los ensayos estáticos y dinámicos de lixiviación, se encontraron tres estados: al pH inicial, durante la digestión alcalina y a bajo pH. El consumo de las partículas finas y la precipitación de hidróxidos de Fe, Al y Mn sobre la superficie de las partículas resultaron factores críticos que agotaron la alcalinidad de la escoria en el segundo estado. Las imágenes SEM sugieren que los precipitados impidieron el AMD al cubrir la superficie de la escoria y de ese modo se redujo el potencial de neutralización. También encontramos que las partículas de escoria finamente divididas produjeron un mayor pH inicial en el lixiviado pero luego de 60 ciclos de lixiviación el pH cayó aún más que para las partículas gruesas. La mezcla de ambos tamaños mostró un alto pH inicial y un moderado valor de pH luego de 60 ciclos lo cual indica un gran potencial para generación de alcalinidad a más largo plazo que las partículas con tamaño semejante entre sí.
Zusammenfassung
Durch den Anteil an basischen Oxiden hat Stahlschlacke die Fähigkeit, hohe Basenkapazitäten zu erzeugen. Stahlschlacke kann bei der Verwendung in Laugungsbetten bzw. –kanälen somit saure Grubenabwässer (acid mine drainage, AMD) neutralisieren. In dieser Arbeit wird (gemahlene) Stahlschlacke aus elektrischen Lichtbogenöfen (EAF) des Pasargad Steel Complex verwendet, um den Einfluss der Partikelgröße auf die AMD-Aufbereitung zu untersuchen. Drei Proben mit unterschiedlichen Korngrößenverteilungen (Grob, Gemischt, Fein) wurden verwendet. Die Zusammensetzung der Stahlschlacke, pH-Wert Änderungen, der Alkalinitätsgrad und die spezifische Oberfläche wurden einbezogen, die Oberfläche der Schlackenpartikel mittels Raster-Elektronenmikroskopie (REM) untersucht. Sowohl in statischen als auch in dynamischen Laugungsversuchen wurde jeweils ein dreiphasiger Verlauf beobachtet. Nach hohen pH-Werten am Anfang (1) erschöpfte sich die Basenkapazität (2). Dies führte am Ende zu geringen pH-Werten (3). Der Rückgang des Feinpartikelanteils und die Fällung von Fe-, Al- und Mn-Hydroxiden auf Partikeloberflächen waren die hauptsächlichen Einflussfaktoren für den Rückgang der Alkalinität der Schlacken während Phase (2). Anhand von REM-Aufnahmen war zu erkennen, dass die Fällungsprodukte der Metalle die AMD-Lösungen daran hinderten, die Schlackenoberfläche zu erreichen, was zur Reduktion des Neutralisationspotenzials führte. Ein weiteres Ergebnis war, dass fein gemahlene Partikel zwar einen höheren Ausgangs-pH-Wert erzeugen, dieser jedoch nach 60 Laugungszyklen unter den pH-Wert gröber gemahlener Schlacke fällt. Die ungleichförmige Mischprobe zeigt ebenfalls einen hohen Ausgangs-pH-Wert, welcher jedoch nach 60 Laugungszyklen immer noch höher ist als bei den anderen Versuchsreihen. Dies zeigt im Ergebnis ein höheres, bzw. ein über längere Zeiträume wirksameres Alkalinitätspotenzial der ungleichförmigen gegenüber den gut sortierten Schlackenproben.
摘要
钢渣含有大量碱性氧化物,能够用作酸性废水(AMD)处理系统的底衬和渗透材料。本文利用伊朗帕萨嘎德钢铁联合企(Pasargad Steel Complex)的电弧炉钢渣研究了钢渣粒径对酸性废水(AMD)处理效果的影响。试验用粗粒、粗-细混合、细粒三种粒径配比样品。测试了钢渣的成分、pH值、碱度和比表面积,用电子显微镜(SEM)观测了钢渣颗粒表面特征。动态与静态淋滤试验都经历了初始高pH值、中间碱度消耗和最后pH值降低三个阶段。细颗粒的消耗和颗粒表面铁(Fe)、铝(Al)、锰(Mn)氢氧化物沉淀的覆盖是影响第二阶段钢渣碱度消耗的关键因素。电子显微镜图像分析表明,金属沉淀阻止了酸性废水与钢渣颗粒表面的充分接触,使钢渣的中和能力降低。同时,研究发现,钢渣颗粒越细,淋滤液初始pH值越高;在经过60个淋滤周期后,淋滤液pH值已经降致粗颗粒钢渣作用水平。粗-细混合钢渣处理既可使AMD淋滤液具有较高的初始pH值又可在60个淋滤周期后仍保持相对中等的pH值,粗-细混合钢渣比单一级配统样品具有更长时间产碱能力。
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Slag, a mixture of silica, calcium oxide, magnesium oxide, alumina, and iron oxides, is a by-product of steel and iron production. It contains the impurities of the iron ore and additives such as limestone and ferroalloys (Fruehan 1998; Toulouevski and Zinurov 2010). During the smelting and purifying process, these impurities separate from the molten metal and rise to the surface of the liquid metal due to their lower density, eventually forming slag. The molten slag is easily collected and removed; on cooling, it solidifies into a nonmetallic glass-like structure. For many decades, slag was considered an industrial waste but, in recent years, the necessity for preserving our natural resources has motivated scientists to find new applications for slag (Márkus and Grega 2006), though before it can be used, further processes such as crushing, iron separation, and weathering may be required (Brandt and Warner 2009; Ghosh and Chatterjee 2008; Verhoeven 2007). Uses for slag include cement production, road construction, fertilizers, and hydraulic engineering. Use of slag in road construction is well-developed and numerous research studies have been carried out (Barišic et al. 2010; Chaurand et al. 2006; Sofilic et al. 2012). It replaces traditional aggregates in cement production (Faraone et al. 2009; Lizarazo et al. 2011; Patel 2008; Tomasiello and Felitti 2010). Slag is an “environmentally sound material” that can be used to restore environmentally damaged areas such as acid mine drainage (AMD) contaminated sites (Simmons et al. 2002; Yildirim and Prezzi 2009; Ziemkiewicz 1998).
Using slag to treat AMD is not a widespread practice; however, a few attempts have been made during the last two decades to spell out the merits of this technique. Simmons et al. (2002) investigated steel slag leaching beds (SLBs) and proved that because the slag contains CaO, MgO, and other alkaline compounds, it can increase the pH of AMD up to 10 or 11 (Simmons et al. 2002). Ziemkiewicz reported that slag yields hundreds of times more alkalinity than limestone and the neutralization potential (NP) of steel slag (ranging from 45 to 70 %) exceeds either hydrated lime or quicklime. Column test studies by Ziemkiewicz (1998) revealed that slag’s alkalinity generation remained high (as high as 2,000 mg/L) for a long period of time. Kruse et al. reported an alkalinity concentration of 1,500 mg/L for steel slag, and calculated that the average volume of effluent water that could be effectively treated with slag was about 76 t/year, which is 5–8 times more than other AMD control options (Kruse et al. 2012).
Mg- and Ca-bearing constituents of slag contribute hugely to the short-term acid neutralization process, while Si- and Al-bearing constituents discharge alkaline materials over a longer period of time due to their lower dissolution rate. Thus, the presence of alumino-silicate minerals prolongs the performance of SLBs to tens of years (Yan et al. 2000). It is important to design SLBs very precisely, in order to make them maintenance-free systems. On this account, Simmons et al. (2002) recommended that the chemical composition of the slag be monitored during design and construction. Slag particle size is another important consideration in AMD control systems. Particles less than 3 mm were shown to be most effective in AMD treatment because of rapid dissolution due to their high surface area (Simmons et al. 2002).
Concerns that should be dealt with when using slag to treat AMD include the fact that the surface openings in slag particles can become clogged with metal precipitates. The main source of the metallic components is AMD, but it is also introduced into the leachate by dissolution of the EAF slag. This is a more severe problem with fine slag particles. In order to eliminate this, fresh water should be introduced into the SLB or slag leaching channel (SLC) to wash sediment and precipitation products from the slag surface (Simmons et al. 2002; Ziemkiewicz 1998). We investigated the effect of slag particle size on acid neutralization. Also, the relative effectiveness of two different types of leaching systems (dynamic and static) was compared.
Experimental Methods
Sample Preparation: EAF Slag
The electric arc furnace (EAF) slag used in this study was collected from stockpiles in the Pasargad Steel Complex (Shiraz, Iran). The liquid slag is routinely air-cooled, stockpiled, and subjected to weathering. A magnetic belt is used to separate metallic iron from the slag. The collected slag ranged in size from 3 mm to 5 cm. The slag sample was first crushed using an industrial crusher and then finely ground in the laboratory using a planetary ball mill crusher. Iron separation was repeated after each crushing and grinding phase (using a magnetic bar). To exclude moisture, the sample was heated for 20 min at 130 °C in an electric oven.
Three slag samples were prepared with different particle size distributions. Ground samples was sieved according to ASTM E11-95 and ASTM D422-63 standards and particles retained above sieve number 20 (with an opening size of 850 µm) were mixed together with particles that were coarser than that to make a coarse-particle slag (coarse slag).
To prepare a slag sample with fine particles (fine slag), the grinding time was extended for 15 more minutes, followed by sieving; particles retained on sieve number 20 were mixed together with finer particles. It should be noted that these sample sets were prepared during separate grinding and sieving steps.
In order to make a sample with a broader dimensional range (mix slag), a mixture containing 70 wt% of coarse slag and 30 wt% of fine slag was prepared and sieved to analyze its exact particle size distribution. Mixed particles sizes were used to control a parameter called the packing factor, which is the ratio of filled space to the total space in a volume of material. It was important to control the packing factor because the higher the packing, the more contact area is available between slag and AMD. If we consider all the slag particles as spherical and the same size, i.e. 850 μm, we can reach the maximum packing factor of 70 % (0.7), which means that 30 % of the space is empty (ideal situation). In our case (non-ideal), the packing factor is estimated to be less than 50 % (though this was not measured). We used smaller particles to fill in the empty spaces between larger particles and increase packing factor in order to increase the contact area between slag and AMD. The disadvantage was some decrease in hydraulic performance.
Please note that we still have distinct particle size distributions in the three slag samples; particle size distribution curves are presented in Figs. 1, 2, and 3. Chemical analysis of the slag was carried out with a Spectro optical emission spectrometer (Table 1).
Sample Preparation: AMD
To provide an AMD sample, effluent water from iron ore mine waste was collected using an all-plastic Kemmerer water sampler. Four different locations were randomly sampled at different times of the day and the samples were sent to the laboratory at the Dept of Environment at Zanjan University, where they were mixed together to produce 240 L of AMD. This representative sample was the only AMD used during this study. It was stored in environmentally sealed polyethylene containers, repeatedly agitated to keep it uniform, and filtered prior to use with a 25 μm paper filter. The pH of the AMD sample was measured with a Metrohm 781 pH/ion meter. Subsequently, the sample was analyzed using inductively coupled plasma—optical emission spectrometry (Perkin Elmer ICP-OES) per ASTM UOP714-07 (Table 2).
Test Design
To analyze the influence of particle size on AMD remediation, two laboratory leaching (namely dynamic and static) set-ups were designed. These configurations were intended to represent SLCs and SLBs, respectively. The dynamic set-up consisted of a glass cylindrical vessel (diameter: 5.08 cm, height: 15.2 cm) and a piping system to circulate AMD (Fig. 4). Both sides of the vessel were sealed with gaskets made of an inert material.
A 10 cm tall column of slag was placed inside the vessel. Down-flow circulation was applied, i.e. AMD was injected from the top of vessel and after passing through the slag layer, extracted from a prearranged drain hole at the bottom. Circulation was facilitated by a conventional pump, normally used in an evaporative cooler. The average flow rate was measured at 6.6 L/min. The pH of the system was measured after each 24 h cycle, and the AMD was replaced with “fresh” AMD. This procedure was repeated for 60 successive cycles. Alkalinity was analyzed at the end of 10th-, 20th, 30th and …60th cycles. To remove metal precipitates, fresh water was introduced after every ten cycles and circulated for 5 min.
The static test is exactly the same as the dynamic test except that in the static test procedure, there was no AMD circulation; instead, 3 cm of AMD covered the slag. AMD remained in the vessel for 24 h and renewal took place exactly as in the dynamic experiment. Alkalinity and pH were monitored as in the dynamic system.
Generally, the acid-neutralizing capacity (ANC) of rocks and minerals is determined by the Sobek method (Sobek et al. 1978). This method involves acid digestion followed by back titration. However, in minerals with a high Fe and Al content, this method is not precise enough because Al and Fe are hydrolyzed and precipitate during the back titration but do not contribute to acid neutralization. To resolve this situation, the Sobek method has been modified to include addition of H2O2 during titration and elimination of boiling stage (Weber et al. 2005). In this study, we used the modified Sobek method to measure ANC.
Measurement of BET specific surface area (SSA) was carried out in accordance with standard test method ASTM B 922-02. For this purpose, the multilayer method using nitrogen gas with 99.999 % purity was applied. Finally, scanning electron microscopy (SEM) was used to study the slag surface and precipitation of metals and metal hydroxides on the slag particles. Only fine slag was used for these SEM studies.
Results and Discussion
As presented in Table 1, CaO, SiO2, MnO, MgO, Al2O3, and ferrous oxides (Fe2Ox, where 2 < x < 3) were the main components of the EAF slag used in this research. These compounds generate high levels of alkalinity (Simmons et al. 2002; Yildirim and Prezzi 2009; Ziemkiewicz 1998). Among these components, CaO, MnO, and MgO improve the ANC of slag and are the most active in AMD remediation. On the other hand, Al2O3 and ferrous oxides hydrolyze during leaching, even when the pH is as low as 4–5, causing hydrolysis products to precipitate on the slag particles. Therefore, these compounds do not participate in AMD remediation processes at higher pH values. Hydrolyzation of manganese (Mn) begins only when pH is above 8 and so Mn compounds are more involved in acid neutralization (Brookings 1998; Weber et al. 2005).
The ANC of the slag sample was calculated to be 1,082 kg (CaCO3 equivalent)/t using the modified Sobek method. Ziemkiewicz reported ANC levels up to 770 kg (CaCO3 equivalent)/t for few different slag samples. The higher ANC of the slag sample in this study may be due to the high CaO content (more than one-third of the whole composition, Table 1). Also, the iron separation process improved ANC by removing metallic iron from the slag, thereby increasing the concentration of the CaO, MnO, and MgO.
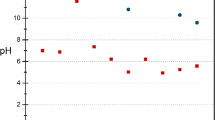
Figure 5 displays measured pH values in the leachate in dynamic experiments for three slag samples. In order to evaluate the effect of pH, 3 stages are introduced: an initial leaching stage, an alkalinity digestion stage, and a stable low pH stage.
In the first stage, the alkaline compounds of slag start to dissolve in AMD, leading to an increased pH. In this stage, a great amount of contact area between slag particles and AMD is available and the dissolution rate of alkali compounds in AMD is high. Therefore, the amount of alkaline materials that leaches out from the slag is considerable and the acid neutralization rate is high, resulting in high pH values. Due to exposure of new slag surfaces to AMD during leaching and the presence of abundant alkali in the leachate, some fluctuation in pH (particularly minor increases in pH during the initial cycles) are noticeable.
As it is obvious from the curves, the pH began to decline somewhere between the 10th and 20th cycles, in contrast with results presented by Simmons et al. (2002), who reported an instant pH decline. An explanation could be the higher ratio of slag to AMD in the present study. Simmons et al. used 2 L of AMD and 100 g of slag, while 1.2 L of AMD was treated with 180 g of slag in the present study.
Transition to the second stage began when alkalinity generation began to shrink. Two main factors control this transition. Initially, alkalinity generation was reduced after the finest particles were dissolved. This transition occurred earlier with the fine slag, where the average particle size was less than 250 µm. The other parameter is the initial pH of the AMD (it was 2.85). A lower initial pH means that more alkalinity was consumed before the pH reached alkaline levels, which caused the transition to occur at an earlier time (fewer leaching cycles). During the alkalinity digestion stage, most of the alkaline components of the slag were consumed, and the AMD-slag contact area decreased (due to precipitation), causing the pH to drop. The pH dramatically fell, from 10.85 (11th cycle) to 4.19 (40th cycle) with an average of −0.22 pH per cycle in the fine slag sample. In the coarse slag, the pH dropped from 9.22 (20th cycle) to 4.76 (49th cycle); the average pH change was −0.15 in each cycle. The lowest average pH decline was associated with the mixed slag sample, at 0.13 pH per cycle, from 8.56 (21st cycle) to 4.96 (49th cycle).
The low pH stage (on the right side of the curves) occurred when most of the soluble alkalinity of the slag had been consumed and generation of alkaline materials was extremely limited. Also, available surfaces were blocked by precipitated reaction products. During this stage, pH gradually declined at a very low rate. This was buffered, in part, by Al and Fe carbonates and silicates for, at low pH, these Al and Fe compounds contribute to neutralization (Weber et al. 2005). In this area of the plot, the rate of pH change was between −0.025 and −0.046 pH units per cycle for the different particle sizes.
Trends of pH in the static leaching experiment (Fig. 6) are quite similar to that of the dynamic leaching system. However, two main differences are recognizable. Initially, for each sample, the pH was clearly lower in the static experiment. This indicates that flowing AMD can effectively leach more alkalinity. When the interaction between slag particles and AMD was static, an alkaline-rich area developed near each particle-AMD interface, which reduced further alkalinity generation. This was true regardless of particle size distribution.
The second difference was the lower rate of pH drop. The pH changed at a rate of −0.16 units per cycle in fine slag, −0.10 units per cycle in coarse slag, and −0.09 units per cycle in mixed slag. This is explained by the immobility of the AMD in the system, preventing further decreases in pH.
The net alkalinity change during the dynamic and static leaching experiments for the fine slag samples confirmed these suggestions (Fig. 7). In our study, alkalinity was leached out by the AMD, while the high alkalinity concentrations reported by Ziemkiewicz and Kruse were based on leaching with neutral water. In our study, some alkalinity was consumed neutralizing the AMD before the pH rose to neutral.
Precipitation of metals and metallic compounds, which is influenced by leachate pH, is a key issue in slag-based AMD control systems. The main source of the metallic components is the AMD, but it is also introduced into the leachate by dissolution of the EAF slag. Particles precipitate on the slag particles and clog surface openings, thus reducing available AMD-slag contact area, and consequently decreasing the generation of alkalinity. SEM images (Figs. 8, 9) show precipitates on the surface and pores of the fine slag. Precipitates may include hydroxides such as Fe(OH)3(S), Al(OH)3, and Mn(OH)2 or metallic elements (Al, Cd, Ca, Cr, Mo, Ni, and Sr) as well as some sulfate minerals. Dimension of precipitates is between 0.2 and 2 μm, as can be seen in the SEM images. Results given by Simmons et al. (2002) and Weber et al. (2005) confirm this model.
In this research, to reduce the effect of metal precipitation, fresh water was introduced into the leaching system. This procedure detaches metal-based precipitation products from the slag particles and provides more AMD-slag contact area, which in turn improves alkalinity generation and acid neutralization. As observed in the plots, there was a small increase in pH (measured after the 10th, 20th, 30th, 40th, and 50th cycles). A similar procedure was recommended by Ziemkiewicz (1998), who suggested applying slag particles along with running water to extend the service quality of a SLB or SLC.
As stated before, the initial pH has its maximum value for fine slag compared to a lower value for coarse slag (9.4 and 7.7 in the static experiment and 10.3 and 8.8 in the dynamic experiment). The total SSA of sample particles and dissolution rate of alkaline content of slag samples are simultaneously responsible for the pH behavior. As analyzed by gas adsorption, the SSA ratio for different slag samples was 1/2.52/7.83 respectively for coarse, mixed, and fine slag samples. Table 3 gives actual values of BET SSA in m2/g. BET SSA rose from 0.52 m2/g in coarse slag to 4.07 m2/g in fine slag. Higher BET SSA simply means that more surface area was in contact with AMD during leaching, which meant that higher amounts of alkaline materials could leach out from the slag particles, generating more alkalinity and more acid neutralization. After the 60th cycle, the situation was reversed in the dynamic experiment and the maximum pH value of the leachate belonged to the coarse slag sample, with a pH of 4.6. This is explained by the high rate of dissolution and depletion of very fine slag particles after prolonged reaction with AMD, which gradually reduced the NP of the system.
In conclusion, to determine the best solution for AMD control, several parameters have to be considered. Static and dynamic leaching test methods were evaluated in this study and our results show that the dynamic leaching system, in which AMD flowed continuously through slag column, was a more effective system for AMD neutralization than the static system where the AMD was replaced daily.
It is advised to use a broad particle size range of the steel slag, which enables one to take advantage of the high initial pH associated with the rapid dissolution of fine particles and the longer performance life of the larger particles, even though this decreases hydraulic performance. The rate of dissolution and alkalinity generation may be enhanced and maintained by increasing slag surface area exposed to the leaching solution, initially by grinding and sieving the slag and later by avoiding the accumulation of precipitates on the particle surfaces. To flush out precipitated solids and increase the slag surface available for leaching, purging fresh water into the leaching system at frequent intervals is recommended.
Evaluating the AMD-slag reaction from a chemical perspective, i.e. identification of precipitates, determination of the reactions that accompany neutralization, and monitoring the composition of slag and leachate over time are potential areas for further study.
References
Barišic I, Dimter S, Netinger I (2010) Possibilities of application of slag in road construction. Tech Gazette 17: 523–528, ISSN: 1330-3651
Brandt DA, Warner JC (2009) Metallurgy Fundamentals: Ferrous and Nonferrous. 5th ed, Goodheart-Willcox, Tinley Park, IL, USA, ISBN-10:1605250791
Brookings DG (1998) EH - pH Diagrams for Geochemistry. Springer-Verlag, Berlin, Germany, ISBN-13:978-3-642-73095-5
Chaurand P, Rose J, Briois V, Olivi L, Hazemann JL, Proux O, Domas J, Bottero JY (2006) Environmental impacts of steel slag reused in road construction: a crystallographic and molecular (XANES) approach. J Hazard Mater 139(3):537–542
Faraone N, Tonello G, Furlani E, Maschio S (2009) Technical note: steelmaking slag as aggregate for mortars: effects of particle dimension on compression strength. Chemosphere 77:1152–1156. doi:10.1016/j.chemosphere.2009.08.00
Fruehan RJ (1998) The Making, Shaping and Treating of Steel: Steelmaking and Refining Volume. 11th ed, AISE Steel Foundation, Pittsburgh, PA, USA, ISBN: 0930767020
Ghosh A, Chatterjee A (2008) Ironmaking and Steelmaking: Theory and Practice. PHI Pvt Ltd, Delhi, India, ISBN-13:978-8120332898
Kruse NA, Bowman JR, Riefler RJ, Mackey AL, Brewster K (2012) Alkalinity production as an indicator of failure in steel slag leach beds treating acid mine drainage. Environ Earth Sci 67(5):1389–1395. doi:10.1007/s12665-012-1583-5
Lizarazo MJ, Claisse P, Ganjian E (2011) Effect of steel slag and portland cement in the rate of hydration and strength of blast furnace slag pastes. J Mater Civ Eng 23:153–160. doi:10.1061/(ASCE)MT.1943-5533.0000149
Márkus R, Grega O (2006) EAF-slag treatment technology. Acta Metall Slovaca 12:269–276
Patel JP (2008) Broader use of steel slag aggregates in concrete. MS thesis, Cleveland State Univ, OH, USA
Simmons J, Ziemkiewicz P, Black PC (2002) Use of slag leach bed for the treatment of acid mine drainage. Mine Water Environ 21:91–99
Sobek A, Schuller, Freeman WJ, Smith R (1978) Field and laboratory methods applicable to overburdens and minesoil. EPA-600/2-78-054, US Environmental Protection Agency, Washington DC, USA, p 47–50
Sofilic T, Sofilic U, Brnardic I (2012) The significance of iron and steel slag as by-product for utilization in road construction. In: Proc, 12th Int’l Foundrymen Conf, Opatija, Croatia, p 419–436
Tomasiello S, Felitti M (2010) EAF slag in self-compacting concretes. Facta Univ Ser Archit Civil Eng 8(1):13–21. doi:10.2298/FUACE1001013T
Toulouevski YN, Zinurov IY (2010) Innovation in Electric Arc Furnaces. Springer, Heidelberg, Germany, ISBN 978-3-642-36272-9
Verhoeven JD (2007) Steel Metallurgy for the Non-metallurgist. ASM International, Materials Park, OH, USA, ISBN-10:0871708582
Weber PA, Thomas JE, Skinner WA, Smart R (2005) A methodology to determine the acid-neutralization capacity of rock samples. Can Mineral 43:1183–1192. doi:10.2113/gscanmin.43.4.1183
Yan J, Baverman C, Moreno L, Neretnieks I (2000) The long-term acid neutralizing capacity of steel slag. Waste Manage 20:217–223. doi:10.1016/S0956-053X(99)00318-9
Yildirim IZ, Prezzi M (2009) Use of steel slag in subgrade applications. Joint transportation research program: Indiana Dept of Transportation and Purdue Univ, West Lafayette, IN, USA, doi: 10.5703/1288284314275
Ziemkiewicz P (1998) Steel Slag: applications for AMD control. In: Proc, Conf on Hazardous Waste Research, Snowbird, UT, USA, p 44–62
Acknowledgments
The authors gratefully acknowledge the financial support received from Pasargad Steel Complex as well as their full assistance, especially in providing test materials and laboratory back-up on completion of this study. Great thanks are also extended to Mr. Mehrdad Motahari for his cooperation in all aspects of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alizadeh, A., Naseri, P.S.Z. An Investigation on the Influence of EAF Slag Particle Size on AMD Neutralization Behavior in Static and Dynamic Slag Leaching Systems. Mine Water Environ 34, 204–212 (2015). https://doi.org/10.1007/s10230-014-0302-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-014-0302-8