Abstract
In this study, the long-term leaching behaviors of Cd, Cr, Cu, Ni, Pb, and Zn in municipal solid waste incineration (MSWI) fly ash samples from grate-type (GT) and circulating fluidized bed (CFB) incinerators were investigated and compared under the simulated landfill leachate corrosion scenario, which was determined to be more severe than the acid rain corrosion scenario. The total heavy metal contents showed increasing hierarchies of Ni<Cr<Cd<Cu<Pb<Zn in the GT fly ash samples and Cd<Ni<Cr<Pb<Cu<Zn in the CFB fly ash samples. During the leaching processes, all heavy metals followed the two-stage leaching mode, including quick accumulation in stage 1 and then stable release in stage 2. The heavy metals with the highest accumulative leaching amounts were Cd, Pb, and Zn in GT fly ash and Cr, Cu, and Ni in CFB fly ash. In the landfill leachate corrosion scenario, Cd and Cr showed cationic patterns while Pb, Zn, and Cu showed amphoteric patterns. The leaching of Cd, Ni, and Cr arose from the dissolution of the salts they formed (solubility control), while the leaching of Cu, Pb, and Zn was controlled by the Ca-bearing compounds (sorption and precipitation control). A large difference in Pb leaching was observed: the cumulative leaching amount of GT fly ash (707.59–3072.36 mg/kg) was an order of magnitude higher than that of CFB fly ash (22.47–407.314 mg/kg), as a result of the higher primary content and larger proportion of the residual fraction in CFB fly ash. The acid-soluble and reducible fractions exhibited higher percentages than those of other fractions representing higher levels of environmental toxicity and risk. Therefore, more emphasis should be placed on the conversion of bioavailable fractions into stable fractions for the stabilization and utilization of MSWI fly ash.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among environmental pollutants, much research has been devoted to studying the heavy metal contamination in soil that can be digested by plants and animals, further endangering human health. Such a serious phenomenon is mainly caused by industrial emissions. Rapid industrialization has contributed to the increase in municipal solid waste (MSW). The mass of MSW in China in 2018 was 0.228 billion tons with an annual increase of 5.95% (Statistics, N.B.o. 2019). This amount of waste has promoted the development of municipal solid waste incineration (MSWI) technology. The advantages of MSWI are effective energy recovery, waste mass and volume reduction, and decomposition and immobilization of hazardous substances (Huai et al. 2008). Accordingly, the number of MSWI plants in China increased to 331 in 2018, approximately two-thirds of these waste-to-energy plants adopted grate-type (GT) incinerators and others applied circulating fluidized bed (CFB) incinerators. The application of these two main incinerators solved the garbage siege problem. However, the incineration by-product, fly ash, adsorbs and enriches soluble salts (Petrlik and Bell 2017), dioxins (Chen et al. 2020), and heavy metals (e.g. Cd, Cr, Ni, Cu, Pb, Zn) (Luo et al. 2019), which are toxic and harmful to the environment. Therefore, many studies have been performed to explore the appropriate treatments and utilization of MSWI fly ash. The main disposal methods can be divided into resource utilization and landfill categories as follows: (1) supplements for construction materials (Yakubu et al. 2018); (2) raw materials for synthetic zeolites (Qiu et al. 2016); and (3) landfill after stabilization/solidification (Zhu et al. 2020). The widespread promotion of the first management strategy is restricted by the toxicity of heavy metals along with the reduced strength of construction products when MSWI fly ash is added. The second highly valued strategy incurs a large financial burden for preinvestment and operation. In China, landfill disposal after solidification/stabilization is the most widely adopted management strategy for MSWI fly ash (Li et al. 2019; Li et al. 2020; Wang et al. 2019), because it incurs fewer economic burdens and has the advantage of convenient management.
Before landfilling, fly ash needs to meet the demands of the “Standard for pollution control on the landfill site of municipal solid waste” (GB 16889–2008) (China, M.o.E.a.E.o.t.P.s.R.o 2008), which imposes restrictions on the minimum single leaching toxicity of heavy metals. Nonetheless, this standard fails to guarantee long-term environmental safety. In actual landfill disposal, fly ash will inevitably encounter a more severe problem regarding heavy metal contamination under adverse and complex scenarios. When fly ash is exposed to these scenarios, the leaching behavior of heavy metals can be categorized into three situations: bioleaching, gas corrosion, and liquid corrosion. In the bioleaching process, over 80.7% of the Cd and 72.5% of the Zn are released (Zhang et al. 2020). Moreover, gas corrosion, including natural carbonation mainly occurs due to weathering from the atmosphere and secondary carbonation from landfill gas (Du et al. 2019; Du et al. 2018), can intensify the leaching of heavy metals by generating easily soluble carbonates from alkaline substances in fly ash. Due to the consumption of the alkaline components in fly ash, the potential risk of heavy metal environmental contamination significantly increases, because the heaviest metals are more likely to transfer to the environment under acidic conditions (Ni et al. 2017a; Zhang et al. 2016a). The acidity of the liquid corrosion creates favorable conditions where such leaching and liquid corrosion scenarios are mainly induced by acid rain and leachate in landfill sites. To better understand the leaching behavior of heavy metals (Wang et al. 2018), nitric acid (Zhao et al. 2017b) was used to simulate the acidic liquid corrosion, but this simulation was far from the acid rain and landfill leachate corrosion scenario. According to Standard GB 16889–2008, the acetic acid buffer solution method (HJ/T300–2007) is recommended to evaluate the treatment of hazardous waste exposed to landfill leachate corrosion scenarios. The acetic acid is also used in the toxicity characteristic leaching procedure (TCLP) recommended by the Environmental Protection Agency (EPA). However, compared to HJ/T300–2007, TCLP with a lower concentration of acetic acid was used to identify the toxicity of heavy metals in hazardous waste. These methods mentioned above can provide solely a preliminary assessment without long-term evaluation of leaching toxicity. To obtain a better understanding of the long-term leaching behavior of heavy metals in MSWI fly ash upon landfill disposal, research on long-term leaching based on landfill simulation is needed.
Apart from the external leaching environment, the properties of MSWI fly ash also play a vital role in heavy metal leaching. In general, the leaching behavior occurs based on multiple controls of physical (particle size and porosity) and chemical (alkaline substance content) properties. To determine the detailed effects of the factors mentioned above, many studies have been carried out to draw strong conclusions. Ni et al. proposed the smaller particle size and larger Brunauer–Emmett–Teller (BET) surface area significantly facilitated leaching (Ni et al. 2017a). A stronger fly ash alkalinity led to lower levels of heavy metal release (Zhang et al. 2016a). To further identify the intrinsic mechanism that controls the leaching behavior, previous studies (Qiu et al. 2018; Wang et al. 2015a) focused on the distribution of heavy metal speciation, which was directly related to the waste sources, seasons, and incinerator types. In our previous work (Long et al. 2020), the fly ash properties were proven to differ significantly between GT and CFB incinerators. Meanwhile, to our knowledge, the long-term leaching difference between fly ash produced by GT and CFB incinerators has not been explored in depth.
In previous studies (Chen et al. 2012b; Li et al. 2020), it was proven that the cumulative heavy metal leaching amount far exceeds the standard limit of the soil background value in a diversified corrosion scenario (including solely carbonation and acid rain corrosion scenarios). Therefore, exploration of the long-term leaching behavior would supplement the understanding of MSWI fly ash exposed to a simulated landfill leachate corrosion scenario, as proposed above. In addition to this, the long-term leaching characteristics can also provide valid guidance for MSWI fly ash management. Therefore, the primary objective of our study was to identify the long-term leaching behavior and speciation of heavy metals in MSWI fly ash. Different kinds of MSWI fly ash have been reported to differ considerably between the two main GT and CFB incinerators (Long et al. 2020). In our experiments, ten fly ash samples from GT and CFB incinerator plants were used to identify the long-term leaching differences between different incinerators. Furthermore, the detailed leaching mechanisms are also discussed for further utilization and management of MSWI fly ash.
Materials and methods
Preparation of the MSWI fly ash samples
The ten fly ash samples used in this study were collected from the baghouse filters of ten GT and CFB incinerators at MSWI plants in ten cities, to avoid regional restrictions. The basic information concerning the ten fly ash samples is presented in Table S1. The raw fly ash was preliminarily dried at 105 °C in an oven for 12 h and then analyzed.
Physicochemical characteristics
Before the leaching test, the elemental contents of fly ash samples were determined by energy dispersive spectroscopy (EDS), and the chemical compositions were examined through X-ray diffraction (XRD) with Cu Kα radiation set at 40 kV and 250 mA. Since the amounts of heavy metals were under the minimum EDS detection limit, inductively coupled plasma atomic emission spectrometry (ICP-AES) was used to determine the amounts of heavy metals in raw samples. The mixed acid digestion method (6 mL HNO3 + 4 mL HCl + 2 mL HF+ 4 mL H2O2) was used to extract the heavy metals from samples (0.1 g) (Qiu et al. 2018). The digestion method was carried out in a closed microwave system to achieve a higher efficiency. A filtration process using a 0.45-μm syringe filter was adopted to make the digestion solution in preparation for analysis.
Leaching test design
As mentioned above, liquid corrosion scenarios in landfill sites can be categorized into acid rain corrosion and leachate corrosion scenarios. The simulated acid rain (SAR) and simulated landfill leachate (SLL) were adopted to simulate the scenarios of acid rain corrosion and landfill leachate corrosion, respectively. Preparation of SAR and SLL was based on “Solid waste—Extraction procedure for leaching toxicity - Sulphuric acid & nitric acid method” (HJ/T 299–2007) and “Solid waste—Extraction procedure for leaching toxicity-acetic acid buffer solution method” (HJ/T 300–2007), respectively. “Standard for pollution control on the landfill site of municipal solid waste” (GB 16889–2008) was used for evaluating the suitability of fly ash for landfilling. The leaching of targeted heavy metals was investigated using these two methods.
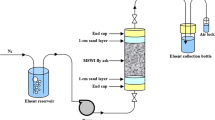
Generally, because acid rain is uncommon in most areas, its corresponding impact on heavy metals is not realistic and continuous. Therefore, the long-term effect of landfill leachate corrosion is more worthy of investigation. To thoroughly mimic a landfill leaching environment in laboratory conditions, the samples were mixed with an extraction buffer of acetic acid (pH=2.64±0.05) at a L/S ratio of 20:1 and a speed of 30 ± 2 rpm. Fifteen milliliters of the supernatant was decanted, filtered through a 0.45-μm syringe of filter liquid, and kept in a polyethylene container in a refrigerator at approximately 4 °C prior to heavy metal analysis. The residue was washed by adding 20 mL of distilled water, shaking manually for 5 min, and centrifuging for 10 min at 3000 rpm. The supernatant liquid was then decanted and discarded. The solid residue was used for the next leaching test. The frequency of long-term leaching was once a day. ICP-AES was used to analyze all solution samples obtained in the leaching experiment for heavy metal concentrations. Additionally, the end-point pH value of the extract was identified with a pH meter. After the long-term leaching experiment, residual solid samples were taken for the chemical composition analysis via XRD.
Sequential extraction procedure
To better understand the morphological mechanism controlling leaching, the heavy metal speciation distribution was measured based on the sequential extraction procedure (SEP) recommended by the European Community Bureau of Reference (BCR). Some modifications (Qiu et al. 2018; Rauret et al. 1999) were made to the procedure, and the detailed operating steps are presented in Table S2. The heavy metal concentration was detected through ICP-AES for the calculation of different fractions.
Results and discussion
Major elements and total heavy metal content
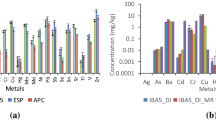
Before investigating the heavy metal characteristics, it is important to note that the elemental composition of raw fly ash, as listed in Table S3, is comparable between GT and CFB fly ash. The major elements (C, O, Si, Al, Cl, Na, S, K, and Ca) in MSWI fly ash accounted for approximately 95 wt%. The high content of Ca in fly ash resulted from the CaO spraying to treat sulfur dioxide, as observed in previous research (Liu et al. 2009). CaO is the main contributor to the MSWI fly ash alkalinity, which can hinder the leaching rate of heavy metals through neutralization (Zhang et al. 2016a). The contents of Al and Si in CFB1–5 were four times higher than those in GT1–5, indicating better treatment and application potential in utilization, such as zeolite synthesis (Qiu et al. 2019). The high content of Cl in the MSWI fly ash was mainly absorbed based on volatile chlorine in the flue gas. The presence of Cl was associated with heavy metal leaching behavior (Jiao et al. 2016; Weibel et al. 2018). For instance, Cl can facilitate leaching by complexing Pb (Assi et al. 2020). Apparently, the presence of some major elements was related with the presence of heavy metals.
As presented in Table 1, the contents of heavy metals showed increasing hierarchies of Ni<Cr<Cd<Cu<Pb<Zn in GT1–5 and Cd<Ni<Cr<Pb<Cu<Zn in CFB1–5. The Pb, Cd, and Zn contents far exceeded the soil background values of 36, 0.134, and 57.69 mg/kg, respectively(Chen et al. 2012b; Zhong et al. 2013). Excessive levels of Pb and Cd can be potential risks to the environment due to their ability to easily migrate into water or soil (Zhu et al. 2020). Evident differences in the total heavy metal contents can be observed among the different fly ashes. The contents of Cd and Pb in GT1–5 were higher than those in CFB1–5. Conversely, the contents of Cr, Cu, and Ni were much higher in the fly ash samples from CFB incinerators. Statistically, the contents of Cd in GT1–5 (106.93–276.00 mg/kg) were approximately twice as high as those in CFB1–5 (20.00–89.5 mg/kg). The same difference was also observed for Pb in GT1-5. However, the contents of Cr, Cu, and Ni in GT1–5 were over three times lower than those in CFB1–5. This phenomenon can be explained by the fact that circulating fluidized bed incineration involves more serious particle entrainment (Chen 2019; Barton et al. 1990; Brunner and Mönch 1986), which means that a large amount of bottom slag containing more Cr, Cu, and Ni compounds was incorporated into fly ash during incineration, thus causing the corresponding attenuation in proportion.
The contents of the major elements and heavy metals in fly ash samples from GT and CFB incinerators exhibited slight differences, which were deemed to correlate to the heavy metal leaching behavior under different scenarios. The specific leaching differences in liquid corrosion scenarios were investigated in detail with the methods mentioned above.
Comparative leaching behaviors under different scenarios
The leaching ratios and concentrations for these different methods are presented in Tables 2 and S4, respectively. In general, the concentrations of Cd, Cu, Ni, and Zn obtained from SAR were much lower than those from SLL. The higher overall heavy metal leaching percentage in Table 2 shows that SLL was more conducive to the release of heavy metals, implying a higher risk of environmental pollution. According to the requirements for these two methods, the initial pH values of the leaching solution are different. As it has been proven that the leaching potential of heavy metals is impacted by various pH conditions (Zhang et al. 2016a), this discrepancy of leaching concentration was caused by the difference between the end-point pH values of the extracts, where those from SAR were higher than those from SLL, as shown in Fig. 1. The more acidic leaching environment resulted in more heavy metal pollution (Ni et al. 2017a). This could be proven based on the higher leaching percentages of Cd, Ni, Cu, and Zn under SLL, because the nearly neutral extracts from SLL were not conducive to leaching. It is worth noting that for GT1–4, the leaching ratio of Pb obtained from SAR was higher than that from SLL while the opposite phenomenon occurred for CFB1–5. Since this difference cannot be explained solely based on pH conditions, more attention should be given to other factors in Pb leaching, such as chemical speciation distribution.
Compared to SLL, the lower level of heavy metal release FROM SAR suggested that MSWI fly ash has a certain resistance to acid rain corrosion. When the fly ash was exposed to landfill leachate corrosion scenarios, the leaching risk was considerably higher due to the higher leaching ratios, as listed in Table 2. Moreover, the landfill leachate corrosion situation is more realistic and common due to the low frequency and short duration of acid rain in most areas. However, the single static batch tests failed to provide reliable long-term information. Therefore, it is more meaningful to conduct long-term experiments of heavy metal leaching behavior under the landfill leachate corrosion scenario.
Long-term leaching behavior and mechanism
Figure 2 shows that generally the long-term leaching process was divided into two stages. In stage 1 (days 1–5), the initial cumulative rate was clearly higher and decreased to a flat trend in stage 2 (days 6–23). However, the results for leaching of Pb in CFB1–5 failed to completely match this two-stage pattern. To identify the detailed differences between the fly ash samples during the whole leaching process, the daily leaching concentrations are presented in Fig. S1. The L-shaped curves of leaching concentrations in Fig. S1 proved that the leaching trends for all heavy metals followed a two-stage pattern. The Pb cumulative leaching curves for CFB1–5 resembled diagonal lines because the decline in the daily Pb leaching concentration was flatter compared to that of other heavy metals.
In stage 1, the growth rates of the cumulative leaching amount (Fig. 2) showed a discrepancy in the same element among fly ash generated from different incinerators, which was also observed in the daily leaching concentrations (Fig. S1). Generally, the larger cumulative leaching amounts for the targeted heavy metals were related to the higher primary heavy metal contents as shown in Table 1. However, upon comparing the data in Table 1 and Fig. 2, it is clear that the cumulative leaching did not increase linearly with the primary content. This result was attributed to the differences in the quantities and varieties of alkaline substances in the different MSWI fly ash samples. These substances can easily dissolve into the leaching solution, thus increasing the pH condition, which is regarded as one of the most vital external contributing factors that alter heavy metal leaching (Zhang et al. 2016b). Figure 3 shows the leaching end-point pH trends under the landfill leachate corrosion simulation process. The large drop in the solution pH stemmed from the neutralization reaction of the alkaline substances in MSWI fly ash with H+ ions. Moreover, the difference in the cumulative leaching amount was associated with the heavy metal leaching performance, which follows three distinct patterns: cationic, oxyanionic, and amphoteric patterns (Komonweeraket et al. 2015b). Cd and Cr follow the cationic pattern where the level of release increases as the pH decreases. For example, the concentrations of Cd and Cr in GTs 3 and 4 (Fig. S1(a) and (b)) increased on day 2 because the extracts changed from alkaline to acidic. Pb, Zn, and Cu follow the amphoteric pattern where the leaching reaches a minimum in neutral conditions (pH=7) and increases under acidic or alkaline conditions (Zhang et al. 2016a). As shown in Table 2 and Fig. 1, the levels of release of Pb and Zn under near-neutral conditions (HJ/T 300) were much lower than those under alkaline conditions (HJ/T 299); this result was a consequence of their amphoteric leaching patterns. As shown in Fig. 3(a), the leaching end-point pH of GT1–5 was lower than that of the original extract after day 12, which might be associated with the dissolution of abundant chloride. The leaching end-point pH values of CFB1–5 were slightly higher than the set value (Fig.3(b)); this resulted from the existence of some particular solid phases that are capable of resisting a pH decrease (Isenburg and Moore 1992).
The rapid release times of heavy metals in stage 1 were 4 (Cd and Zn) and 6 days (Cu and Ni). Considering the Cr release time, it took 7 days for GT1–5 to descend to a flat leaching trend and this time doubled for CFB1–5. Although the Pb daily leaching curve of GT5 exhibited a peak on day 3, the cumulative leaching amount reached a stable period after 7 days. Moreover, it is remarkable that the Pb leaching amounts in GT1–5 were an order of magnitude higher than those of CFB1–5 and the Cu and Cd leaching amounts in GT1–5 were two times higher. Higher leaching leads to higher contamination potentials, requiring more attention regarding the investigation of Pb, Cu, and Cd in GT fly ash when exposed to landfill leachate corrosion.
To thoroughly comprehend the differences in leaching behaviors among different heavy metals, it is also important to consider the leaching control mechanism. Generally, potential mechanisms can be categorized into two controls—solubility control (Cd, Ni, and Cr) and sorption and precipitation control (Cu, Pb, and Zn). Solubility control is highly related to the dissolution of metal oxides while the sorption process controls the release of heavy metals through the active sites on the solid surface (Luo et al. 2019), such as those in (hydr)oxides (Zhao et al. 2017a).
The dissolution of Ni(OH)2 metal hydroxide is considered the cause of Ni leaching (Wang et al. 2018). as proposed by Komonweeraket et al.; the mineral that controls the Cr leaching is likely BaCrO4(s), which was further proven in their recent research (Komonweeraket et al. 2015a; Mudd et al. 2004). Another mineral in MSWI fly ash that potentially controls the leaching of Cr is CaCrO4, which is formed by oxidization with CaO at approximately 900 °C (Chen et al. 2012a). This mineral might be the reason for higher cumulative leaching amounts in CFB fly ash, for which the content of CaO was higher than that in GT fly ash. The leaching of Cd (days 3–23) resulted from the dissolution of metal-bearing minerals (carbonate and sulfate) due to the abundant presence of H+ (Ni et al. 2017b) when the pH value decreased below 4. The leaching of Cd and Cr was controlled by the solubility process, which can be verified based on the rapid accumulation of the leaching amount in stage 1. The research of Hyks et al. suggested that the leaching behavior of Cr did not conform to the sorption control mechanism (Hyks et al. 2009), accounting for extremely low concentrations (under 0.05 mg/L) in stage 2. In contrast, the higher concentrations of Cu, Pb, and Zn in stage 2 (Fig. S1) can be attributed to the Ca-bearing compounds, which are associated with the sorption and precipitation control. This conclusion was further testified by the presence of rankinite, gismondine, vuagatite, hillebrandite, and calcium iron oxide in the residue after leaching, as shown in Fig. 4. After long-term leaching, there were no significant peaks of soluble salts (i.e., KCl, NaCl, carbonates, and sulfates) in Fig. 4, although such salts were the main components in raw fly ash according to our previous work (Long et al. 2020). As reported in a previous study (Wang et al. 2018), the considerable loss of soluble salts is related to the leaching of heavy metals, as controlled by the solubility mechanism. The compounds in the residue were mainly crystalline aluminosilicate and silicon dioxide. This indicates that acid washing can be a beneficial treatment for separating the recyclable and valuable aluminosilicates and silicon dioxides from MSWI fly ash.
By comparing the long-term leaching curves, it was concluded that the targeted heavy metals (except Pb in CFB1–5) reached nearly saturated leaching amounts. However, the leached amounts were far below the total amounts, as presented in Table 2. Therefore, we hoped to develop a specific quantitative interpretation through the heavy metal speciation distribution.
Distribution pattern of heavy metals
Heavy metal speciation can be categorized into four fractions, namely, acid-soluble (F1), reducible (F2), oxidizable (F3), and residual (F4) fractions. The chemical speciation distribution was presented in Fig. 5, and the BCR data are listed in Table S5.
For all fly ash samples, the minimal fraction for these six heavy metals was the F3 fraction. The dominant fractions of Cd were the F1 and F2 fractions (approximately 90%). This distribution trend was consistent with those of Cu and Zn. The residual fraction exhibited the largest proportion of Cr in all fly ash samples except GT1 and of Ni in all samples except GT1-3. Considering Pb, almost 80% was the F2 fraction. The dominance of fractions F1 and F2 yielded environmental toxicity. Previous studies (Wang et al. 2018; Zhang et al. 2016a; Zhu et al. 2020) have indicated that the acid-soluble fraction exhibits high leachability under most corrosion scenarios. Wang et al. reported that Fe and Mn oxides belonging to F2 have an effective ability to retain some heavy metals (Wang et al. 2015b). The speciation characteristics show that the great mass of Cd, Cu, Pb, and Zn could easily transfer to the environment under acidic conditions, as proven by their high cumulative leaching percentages.
As shown in Fig. 5(e), GT1–5 had a higher proportion of the F2 fraction of Cu (76.14–93.54%) than that of CFB1–5, while the F4 fraction in CFB1–5 (20.96–50.08%) was ten times higher. Moreover, the F4 fractions of Cr (49.81-83.56%) and Ni (33.33–74.86%) were also predominant in all fly ash samples except GT1. Only a small amount of Cr and Ni appeared in the F1 fraction, which is the easiest fraction to leach out. This kind of distribution made the environmental risks of these two metals lower than others, because the residual fraction is the most stable and difficult to leach. The small leaching amount of Cr can be attributed to the formation of some insoluble chromates (Xiong et al. 2014) in the F4 fraction. The Ni in the residual fraction is mostly contained the silicate lattice and aluminate matrix within the MSWI fly ash, the significant peaks of which can be observed in Fig. 4.
In summary, the residual fraction accounted for the main fraction of Cr and Ni, and the large proportion of the bioavailable F1 and F2 fractions contributed to environmental toxicity. Heavy metals in the F1 and F2 fractions can become soluble under favorable pH and redox conditions (Li et al. 2019), thus contaminating the soil and groundwater and leading to ingestion by plants and animals. The potential environmental risk was tested based on the long-term leaching behavior in the landfill leachate corrosion scenario.
Conclusions
This study has provided a comprehensive understanding of heavy metal leaching behavior from the perspective of different MSWI fly ash samples obtained from GT and CFB incinerators. Moreover, we also compared the long-term leaching characteristics and speciation distributions of heavy metals. The experimental results and analysis can be condensed as follows:
(1) The total contents of heavy metals showed increasing hierarchies of Ni<Cr<Cd<Cu<Pb<Zn in GT1–5, and Cd<Ni<Cr<Pb<Cu<Zn in CFB1–5. The contents of Cd and Pb in GT1–5 were higher than those in CFB1–5 while the amounts of Ni and Cr in CFB1–5 incinerators were larger than those in GT1–5.
(2) Based on the comparison of MSWI fly ash samples exposed to different simulative liquid corrosion scenarios, the potential for heavy metal contamination under landfill leachate corrosion was much more severe than that under acid rain corrosion.
(3) During the simulation process in which MSWI fly ash was exposed to the landfill leachate corrosion scenario, all heavy metals followed the two-stage leaching mode, which included quick accumulation in stage 1 and then stable release in stage 2.
(4) It is worth noting that the leaching of Pb differed considerably between the two types of fly ash. The total amount of Pb in GT1–5 was approximately twice that of CFB1–5, and the cumulative leaching amount was an order of magnitude higher in GT1–5 than that in CFB1–5. According to the speciation distribution, these differences resulted from the larger proportion of residual fraction, the most stable fraction, in CFB1–5.
(5) Considering the six heavy metals, the ratio of the F1 and F2 fractions was extremely high, leading to environmental toxicity. This result indicated that MSWI fly ash requires effective treatment before landfilling. More importantly, the differences in heavy metal leaching behavior require the application of different treatments to GT and CFB fly ash. Specifically, more attention should be given to the stabilization or removal of Pb and Cd in GT fly ash, while the Cr and Cu in CFB fly ash necessitate high-efficiency treatments. More exploration and experiments are needed to verify these treatment methods.
Data availability
Most data analyzed during our research are included in this article and its supplementary materials files. The rest of the included data are available from the authors on reasonable request.
References
Assi A, Bilo F, Zanoletti A, Ponti J, Valsesia A, La Spina R, Zacco A, Bontempi E (2020) Zero-waste approach in municipal solid waste incineration: reuse of bottom ash to stabilize fly ash. J Clean Prod 245:118779. https://doi.org/10.1016/j.jclepro.2019.118779
Chen J, Jiao F, Zhang L, Yao H, Ninomiya Y (2012a) Use of synchrotron XANES and Cr-doped coal to further confirm the vaporization of organically bound Cr and the formation of chromium(VI) during coal oxy-fuel combustion. Environ Sci Technol 46(6):3567–3573. https://doi.org/10.1021/es204255h
Chen J, Wang PY, Yang YM (2012b) Pollution and assessment of heavy mental status in sediments in Chongqing urban section of three Gorges project after impoundment. Adv Mater Res 518-523:2670–2676. https://doi.org/10.4028/www.scientific.net/AMR.518-523.2670
Chen Z (2019) Mechanism study of mechanochemistry on PCDD/Fs degradation and on heavy metals stabilization in MSWI fly ash. Zhejiang University, Hangzhou http://cdmd.cnki.com.cn/article/cdmd-10335-1019028701.htm
Barton RG, Clark W, Seeker W (1990) Fate of metals in waste combustion systems. Combust Sci Technol 74:327–342. https://doi.org/10.1080/00102209008951696
Brunner PH, Mönch H (1986) The flux of metals through municipal solid waste incinerators. Waste Manag Res 4:105–119
Chen Z, Yu G, Zou X, Wang Y (2020) Co-disposal of incineration fly ash and sewage sludge via hydrothermal treatment combined with pyrolysis: Cl removal and PCDD/F detoxification. Chemosphere 260:127632. https://doi.org/10.1016/j.chemosphere.2020.127632
China, M.o.E.a.E.o.t.P.s.R.o (2008) Standard for pollution control on the landfill site of municipal solid waste (GB 16889-2008). China Environmental Science Press, Beijing
Du B, Li J, Fang W, Liu J (2019) Comparison of long-term stability under natural ageing between cement solidified and chelator-stabilised MSWI fly ash. Environ Pollut 250:68–78. https://doi.org/10.1016/j.envpol.2019.03.124
Du B, Li JT, Fang W, Liu YL, Yu SY, Li Y, Liu JG (2018) Characterization of naturally aged cement-solidified MSWI fly ash. Waste Manag 80:101–111. https://doi.org/10.1016/j.wasman.2018.08.053
Huai XL, Xu WL, Qu ZY, Li ZG, Zhang FP, Xiang GM, Zhu SY, Chen G (2008) Numerical simulation of municipal solid waste combustion in a novel two-stage reciprocating incinerator. Waste Manag 28:15–29. https://doi.org/10.1016/j.wasman.2006.11.010
Hyks J, Astrup T, Christensen TH (2009) Long-term leaching from MSWI air-pollution-control residues: leaching characterization and modeling. J Hazard Mater 162:80–91. https://doi.org/10.1016/j.jhazmat.2008.05.011
Isenburg J, Moore M (1992) Generalized acid neutralization capacity test. ASTM Special Techn Public 1123:361–377. https://doi.org/10.1002/pssb.2221960120
Jiao FC, Zhang L, Dong ZB, Namioka T, Yamada N, Ninomiya Y (2016) Study on the species of heavy metals in MSW incineration fly ash and their leaching behavior. Fuel Process Technol 152:108–115. https://doi.org/10.1016/j.fuproc.2016.06.013
Komonweeraket K, Cetin B, Aydilek AH, Benson CH, Edil TB (2015a) Effects of pH on the leaching mechanisms of elements from fly ash mixed soils. Fuel 140:788–802
Komonweeraket K, Cetin B, Benson CH, Aydilek AH, Edil TB (2015b) Leaching characteristics of toxic constituents from coal fly ash mixed soils under the influence of pH. Waste Manag 38:174–184. https://doi.org/10.1016/j.fuel.2014.09.068
Li W, Sun Y, Huang Y, Shimaoka T, Wang H, Wang YN, Ma L, Zhang D (2019) Evaluation of chemical speciation and environmental risk levels of heavy metals during varied acid corrosion conditions for raw and solidified/stabilized MSWI fly ash. Waste Manag 87:407–416. https://doi.org/10.1016/j.wasman.2019.02.033
Li W, Sun Y, Xin M, Bian R, Wang H, Wang YN, Hu Z, Linh HN, Zhang D (2020) Municipal solid waste incineration fly ash exposed to carbonation and acid rain corrosion scenarios: release behavior, environmental risk, and dissolution mechanism of toxic metals. Sci Total Environ 744:140857. https://doi.org/10.1016/j.scitotenv.2020.140857
Liu W, Hou H, Zhang C, Zhang D (2009) Feasibility study on solidification of municipal solid waste incinerator fly ash with circulating fluidized bed combustion coal fly ash. Waste Manag Res 27:258–266. https://doi.org/10.1177/0734242X08095017
Long L, Jiang X, Lv G, Chen Q, Liu X, Chi Y, Yan J, Zhao X, Kong L (2020) Characteristics of fly ash from waste-to-energy plants adopting grate-type or circulating fluidized bed incinerators: a comparative study. Energy Sour A Recov Utiliz Environ Effect 2020:1–17. https://doi.org/10.1080/15567036.2020.1796851
Luo H, Cheng Y, He D, Yang E-H (2019) Review of leaching behavior of municipal solid waste incineration (MSWI) ash. Sci Total Environ 668:90–103. https://doi.org/10.1016/j.scitotenv.2019.03.004
Mudd GM, Weaver TR, Kodikara J (2004) Environmental geochemistry of leachate from leached brown coal ash. J Environ Eng 130(12):1514–1526. https://doi.org/10.1061/(ASCE)0733-9372(2004)130:12(1514)
Ni P, Li HL, Zhao YC, Zhang JY, Zheng CG (2017a) Relation between leaching characteristics of heavy metals and physical properties of fly ashes from typical municipal solid waste incinerators. Environ Technol 38(17):2105–2118. https://doi.org/10.1080/09593330.2016.1246612
Ni P, Xiong Z, Tian C, Li H, Zhao Y, Zhang J, Zheng C (2017b) Influence of carbonation under oxy-fuel combustion flue gas on the leachability of heavy metals in MSWI fly ash. Waste Manag 67:171–180. https://doi.org/10.1080/09593330.2016.1246612
Petrlik J, Bell L (2017) Toxic ash poisons our food chain. https://doi.org/10.13140/RG.2.2.34619.21280
Qiu Q, Chen Q, Jiang X, Lv G, Chen Z, Lu S, Ni M, Yan J, Lin X, Song H, Cao J (2019) Improving microwave-assisted hydrothermal degradation of PCDD/Fs in fly ash with added Na2HPO4 and water-washing pretreatment. Chemosphere 220:1118–1125. https://doi.org/10.1016/j.chemosphere.2018.12.166
Qiu Q, Jiang X, Lv G, Chen Z, Lu S, Ni M, Yan J, Deng X (2018) Evolution of heavy metal speciation in MSWI fly ash after microwave-assisted hydrothermal treatment. Chem Lett 47:960–963. https://doi.org/10.1246/cl.180339
Qiu QL, Jiang XG, Lu SY, Ni MJ (2016) Effects of microwave-assisted hydrothermal treatment on the major heavy metals of municipal solid waste incineration fly ash in a circulating fluidized bed. Energy Fuel 30:5945–5952. https://doi.org/10.1021/acs.energyfuels.6b00547
Rauret G, López-Sánchez JF, Sahuquillo A, Rubio R, Davidson C, Ure A, Quevauviller P (1999) Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monit 1:57–61. https://doi.org/10.1039/a807854h
Statistics, N.B.o (2019) China statistical yearbook. China Statistics Press, Beijing http://www.stats.gov.cn/tjsj/ndsj/2019/indexch.htm
Wang F-H, Zhang F, Chen Y-J, Gao J, Zhao B (2015a) A comparative study on the heavy metal solidification/stabilization performance of four chemical solidifying agents in municipal solid waste incineration fly ash. J Hazard Mater 300:451–458. https://doi.org/10.1016/j.jhazmat.2015.07.037
Wang H, Fan X, Wang YN, Li W, Sun Y, Zhan M, Wu G (2018) Comparative leaching of six toxic metals from raw and chemically stabilized MSWI fly ash using citric acid. J Environ Manag 208:15–23. https://doi.org/10.1016/j.jenvman.2017.11.071
Wang H, Zhang D, Mou S, Song W, Al-Misned FA, Golam Mortuza M, Pan X (2015b) Simultaneous removal of tetracycline hydrochloride and As(III) using poorly-crystalline manganese dioxide. Chemosphere 136:102–110. https://doi.org/10.1016/j.chemosphere.2015.04.070
Wang P, Hu Y, Cheng H (2019) Municipal solid waste (MSW) incineration fly ash as an important source of heavy metal pollution in China. Environ Pollut 252:461–475. https://doi.org/10.1016/j.envpol.2019.04.082
Weibel G, Eggenberger U, Kulik DA, Hummel W, Schlumberger S, Klink W, Fisch M, Mäder UK (2018) Extraction of heavy metals from MSWI fly ash using hydrochloric acid and sodium chloride solution. Waste Manag 76:457–471. https://doi.org/10.1016/j.wasman.2018.03.022
Xiong Y, Zhu F, Zhao L, Jiang H, Zhang Z (2014) Heavy metal speciation in various types of fly ash from municipal solid waste incinerator. J Mater Cycle Waste Manag 16(4):608–615. https://doi.org/10.1007/s10163-014-0274-6
Yakubu Y, Zhou J, Shu Z, Zhang Y, Wang WB, Mbululo Y (2018) Potential application of pre-treated municipal solid waste incineration fly ash as cement supplement. Environ Sci Pollut Res 25:16167–16176. https://doi.org/10.1007/s11356-018-1851-3
Zhang R, Wei X, Hao Q, Si R (2020) Bioleaching of heavy metals from municipal solid waste incineration fly ash: availability of recoverable sulfur prills and form transformation of heavy metals. Metals 10:6. https://doi.org/10.3390/met10060815
Zhang Y, Cetin B, Likos WJ, Edil TB (2016a) Impacts of pH on leaching potential of elements from MSW incineration fly ash. Fuel 184:815–825. https://doi.org/10.1016/j.fuel.2016.07.089
Zhang Y, Chen J, Likos WJ, Edil TB (2016b) Leaching characteristics of trace elements from municipal solid waste incineration fly ash. Geotechn Special Public 273:168–178. https://doi.org/10.1061/9780784480168.018
Zhao S, Chen Z, Shen J, Kang J, Zhang J, Shen Y (2017a) Leaching mechanisms of constituents from fly ash under the influence of humic acid. J Hazard Mater 321:647–660. https://doi.org/10.1016/j.jhazmat.2016.09.054
Zhao X, Wang LA, Wang L, Zhang W (2017b) Distribution of remaining Cd in MSWI fly ash washed with nitric acid. J Mater Cycle Waste Manag 19:1415–1422. https://doi.org/10.1007/s10163-016-0535-7
Zhong S, Feng JK, Kuang W, Pan J (2013) Leaching kinetics of Zn in MSWI fly ash under acidic condition. Beijing Gongye Daxue Xuebao/J Beijing Univ Technol 39:1704–1709 http://en.cnki.com.cn/Article_en/CJFDTOTAL-BJGD201311018.htm
Zhu J, Hao Q, Chen J, Hu M, Tu T, Jiang C (2020) Distribution characteristics and comparison of chemical stabilization ways of heavy metals from MSW incineration fly ashes. Waste Manag 113:488–496. https://doi.org/10.1016/j.wasman.2020.06.032
Funding
This study is supported by the National Key Research and Development Program of China (Grant No. 2018YFC1901302, 2018YFF0215001, 2017YFC0703100), the Innovative Research Groups of the National Natural Science Foundation of China (Grant No. 51621005), Jiangsu Provincial Natural Science Foundation of China (BK20201032).
Author information
Authors and Affiliations
Contributions
Ling Long and Xuguang Jiang conceived the research idea. Ling Long conducted the experiments and wrote the manuscript. Guojun Lv, Qian Chen, and Xiaobo Liu have done the data calculations. Qili Qiu drew all the graphs. Yong Chi, Jianhua Yan, Xiaoli Zhao, and Litan Kong provided constructive comments on the experiments and manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 928 kb)
Rights and permissions
About this article
Cite this article
Long, L., Jiang, X., Lv, G. et al. Comparison of MSWI fly ash from grate-type and circulating fluidized bed incinerators under landfill leachate corrosion scenarios: the long-term leaching behavior and speciation of heavy metals. Environ Sci Pollut Res 29, 15057–15067 (2022). https://doi.org/10.1007/s11356-021-16618-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16618-z