Abstract
Sewage sludge can be a suitable, organic-rich substrate to promote vegetation of sulfide-mine tailings, but it may contain contaminants, that, when oxidized, can adversely affect underlying groundwater systems. The geochemical impact of a surface application of 12,000 metric tons of anaerobically-digested sewage sludge on the groundwater quality of a remediated sulfide-tailings impoundment in northern Sweden was evaluated to determine if sludge-borne metals and nitrate were released to the underlying groundwater system. Two years of data from a field-scale groundwater monitoring programme initiated just before the sludge application was compared to groundwater data from 1998 to 2006. Grass was successfully established within 2 years. However, until that occurred, elevated concentrations of sludge-borne metals (Cu, Ni, Pb, Zn) were released to the underlying groundwater. In addition, the release of nitrate likely exacerbated metal concentrations by providing an oxidant for pyrite in the underlying tailings. The release was periodic due to the establishment of the grass, which immobilized metals and nitrate in the sludge. Metals bound as organo-metallic complexes, due to dissolved organic carbon released from the sludge, migrated across the tailings impoundment. Model simulations indicate that the plume will take 6 years to exit the groundwater environment. Though the impacts are relatively short-term, this type of application should be reconsidered in the future.
Zusammenfassung
Klärschlamm kann ein passendes, an organischen Stoffen reiches Substrat sein, um die Vegetation auf Sulfid-reichen Tailings-Materialien zu fördern. Der Klärschlamm kann aber auch Kontaminationen enthalten, die im Falle einer Oxidation darunterliegende Grundwassersysteme negativ beeinflussen können. Der geochemische Einfluss einer Oberflächenaufbringung von 12 000 t anaerob ausgefaulten Klärschlamms auf die Grundwasserqualität eines sanierten Sulfid-Tailings-Beckens in Nordschweden wurde untersucht. Es wurde geprüft, ob aus dem Klärschlamm stammende Metalle oder Nitrat in das unterliegende Grundwassersystem abgegeben wurden. Daten aus einer zweijährigen Datenreihe aus Grundwasseruntersuchungen, die unmittelbar vor der Schlammausbringung begonnen wurden, wurden mit Grundwasserdaten aus den Jahren 1998–2006 verglichen. In den zwei Jahren konnte ein guter Aufwuchs von Gras erreicht werden. Seither wurden jedoch erhöhte Konzentrationen von Metallen aus dem Klärschlamm (Cu, Ni, Pb, Zn) in das unterliegende Grundwasser abgegeben. Außerdem erhöhte die Abgabe von Nitrat wahrscheinlich Metallkonzentrationen, indem das Nitrat als Oxidationsmittel für Pyrit in den Tailings-Materialien wirkte. Die Auswaschungen traten periodisch auf durch das Aufwachsen des Grases, das Metalle und Nitrat im Schlamm immobilisierte. Metalle migrierten durch die Tailings-Materialien, wenn sie durch aus dem Klärschlamm stammende organische Verbindungen als Metall-organische Komplexe gebunden waren. Modellsimulationen zeigten, dass die Kontaminationswolke sechs Jahre brauchen wird, um das Grundwasser zu verlassen. Trotz der relativ kleinen Zeit der negativen Beeinflussung des Grundwassers sollte für zukünftige Vorhaben die Anwendung von Klärschlamm wie im untersuchten Fall überdacht werden.
Resumen
El lodo de aguas residuales es un sustrato rico en compuestos orgánicos que es adecuado para promover la vegetación en colas de minas sulfuradas aunque puede contener contaminantes que, cuando son oxidados, pueden afectar negativamente las aguas subterráneas subyacentes. Se evaluó el impacto geoquímico de la aplicación superficial de 12.000 toneladas métricas de lodo de aguas residuales digerido anaeróbicamente sobre la calidad del agua subterránea de un dique de colas sulfuradas remediado en el norte de Suecia. Esta evaluación pretendía determinar si metales y nitratos habían sido liberados a las aguas subterráneas subyacentes. Los datos tomados durante dos años mediante un programa de monitoreo de agua subterránea a escala de campo que fue iniciado justo antes de la aplicación del lodo, fue comparado con datos obtenidos entre 1998 y 2006. La hierba fue exitosamente instalada dentro de los dos años. No obstante, hasta que eso ocurrió, elevadas concentraciones de metales (Cu, Ni, Pb, Zn) fueron liberados al agua subterránea subyacente. Además, la liberación de nitrato incrementó las concentraciones de metales al proveer un oxidante para la pirita presente en las colas subyacentes. La liberación fue periódica debido a la hierba que inmoviliza metales y nitratos en el lodo. Metales unidos como complejos órgano metálicos debido al carbono orgánico disuelto que se liberó del lodo, migraron a través del dique de colas. Las simulaciones a través de modelos indican que la pluma tomará 6 años para salir del ambiente de aguas subterráneas. Aunque los impactos son relativamente de corto tiempo, este tipo de aplicaciones debería ser reconsiderada en el futuro.
摘要
污水污泥是一种理想的富含有机质培养基,能够用以提高富含硫化物尾矿库的植被覆盖率。但是,污水污泥往往含有污染物;当其被氧化时,对地下水水质产生负面影响。文章研究了12000吨厌氧菌致分解污水污泥盖层对瑞典北部已修复硫化物尾矿库地下水水质的地球化学影响,识别了污水污泥是否将其携带的金属离子和硝酸盐释放到下伏地下水系统中。文章对比了污泥盖层铺设前已起动的地下水现场监测计划的前两年数据与1998-2006年地下水监测数据。在两年时间内,成功完成尾矿库草皮覆盖。然而,由污水污泥产生的高浓度重金属离子(Cu, Ni, Pb, n)已经释放到下伏地下水系统中。污水污泥释放的硝酸盐为下伏尾矿里的黄铁矿提供了氧化剂,使重金属浓度进一步提高。草皮可以固定污泥里的重金属和硝酸盐,草皮覆盖使金属离子释放呈现周期性特征。同时,由于污泥能够释放溶解有机炭,金属离子被束缚于有机金属络合物中而运移、穿过尾矿库。模拟结果表明,污染羽流将用六年时间流出地下水系统。虽然此类污染时间较短,但是应该慎重考虑污泥盖层的未来应用。
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Remedial measures for acid rock drainage (ARD) formation in sulfide-mine tailings repositories have been widely studied globally (INAP 2009; Lottermoser 2010). ARD is the result of exposure of pyrite (FeS2) and pyrrhotite (Fe(1−x)S) to oxygen and water. Unremediated tailings may produce elevated dissolved sulfate, iron, and acidity loadings, which may, in turn, further exacerbate sulfide weathering, contributing elevated concentrations of dissolved metals into peripheral surface and groundwater environments. Effective remediation of sulfide-bearing mine tailings is a fundamental aspect of mine plan development and is legislated through the European Parliament’s Management of Waste from Extractive Industries Directive (2006/21/EC).
In Sweden, 59 million metric tons (t) of mine waste is produced annually (Statistics-Sweden 2008). ARD mitigation can be directed at either covering the waste with water to restrict oxygen ingress or constructing engineered dry covers to cap the waste and reduce water ingress and oxygen diffusion to the underlying sulfide tailings (Carlsson 2002; Höglund et al. 2005; Holmström et al. 2001). Engineered composite dry covers (Lindvall et al. 1997) are designed to be a long-term, low maintenance solution to the abatement of ARD (INAP 2009). The cover materials must consist of durable, unreactive natural materials such as glacial overburden, natural soil, or clay. An overlying protective layer protects the integrity of an underlying sealing layer that is designed to prevent oxygen diffusion. A surface vegetation substrate layer is required to stabilize and reduce erosion over time. Sourcing and excavating large volumes of suitable natural soil is often problematic and of further environmental concern. Replacing natural soils with an alternative such as an organic waste generated from another industry has become an inexpensive and attractive solution, allowing the beneficial co-disposal of two separate wastes.
Alternative materials from the paper, pulp, water, and municipal waste industries have been thoroughly investigated in Sweden (Gotthardsson and Sundberg 2005; Hallberg et al. 2005; Hanæus and Mattsson 2009). One such organic waste is sewage sludge biosolids (SS), which is the solid by-product material generated during the treatment of domestic wastewater (Ahlberg et al. 2006). In 2005, the European Union’s Urban Waste Water Directive 91/271/EEC Article 14, banned SS disposal and declared that it should be re-used when appropriate (Fytili and Zabaniotou 2008). Approximately 210,000 t of SS are produced annually (Statistics-Sweden 2008) from more than 2100 wastewater treatment plants in Sweden (Marklund 1997). SS has been investigated as a vegetation substrate on the surface of tailings impoundments and waste rock dumps (Forsberg et al. 2008; Neuschütz et al. 2009; Porse 2002), and has proven to be a suitable substrate for supporting and sustaining long-term vegetation establishment (Pichtel et al. 1994). However, its geochemical influence on the tailings groundwater system has not previously been investigated.
SS is chemically unstable (Neuschütz 2009) and may contain readily-leachable elevated concentrations of contaminants such as Cd, Cu, Ni, Pb, and Zn (Eriksson 2001), which can migrate as soluble organo-metallic complexes (Andres and Francisco 2008). Surface agricultural SS applications have shown that such metals may accumulate in underlying soil horizons (Ahlberg et al. 2006), or be transported to peripheral ground or surface waters due to organo-metallic complexation formation with elevated dissolved organic carbon (DOC) (Ashworth and Alloway 2004; Christensen and Christensen 2000). Nitrate release also occurs (Evanylo 1994; Schroder et al. 2008) due to the oxidation of ammonium (NH4 +), which can be present in high concentrations in the original SS (Neuschütz 2009).
In the case of sulfide tailings, nitrate levels that exceed vegetation requirements can leach into the underlying tailings (Cravotta 1998), and facilitate the oxidation of pyrite where groundwater is devoid of oxygen (Appelo and Postma 2005). The use of SS may therefore be problematic. We evaluated the geochemical impact of a surface application of SS on the groundwater quality of a remediated sulfide-tailings impoundment in northern Sweden. The aim was to determine if sludge-borne metals and nitrate were released to the underlying groundwater system, and if so, to delineate their magnitude, duration, and fate.
Study Area
The Kristineberg Zn-Cu mine operated by New Boliden AB is located in the Skellefte ore district in northern Sweden, 175 km southwest of the city of Luleå (Fig. 1). The climate is classified as sub-Arctic with sub-zero temperatures between October and April, and a mean annual temperature and precipitation of 0.7 °C and 600 mm/year (Axelsson et al. 1991a), respectively. The dominant vegetation and soil type in the area is coniferous forest and podzol weathered till, respectively (Carlsson 2002).
Location of the Kristineberg mine site, northern Sweden, and a map of the impoundment 1 study site showing cover types and the location of the BAT® groundwater wells. Line A–B is shown in the detailed cross-section in Fig. 2
Field trials were conducted at a formally remediated sulfide-tailings facility, impoundment 1 (Fig. 1). Tailings were deposited into the Vormbäcken stream valley from 1946 until 1952, over a 0.11 km2 area with a tailings thickness ranging from 2 to 10 m deep (Alakangas 2006). The impoundment is underlain by glacial moraine to a depth of 1.5 m (Axelsson et al. 1986) and by underlying bedrock that has a hydraulic conductivity of 5 × 10−8–2 × 10−7 m/s (Axelsson et al. 1991a). The bedrock underlying the impoundment is relatively impermeable except for two fracture zones striking NW–SE through the center of the deposit and at the impoundment toe (Axelsson et al. 1991b), which are areas where groundwater is lost. Recharge to the impoundment groundwater occurs in the western till slope, though the impoundment groundwater is its own hydraulic unit, independent of the peripheral bedrock groundwater fluctuations (Axelsson et al. 1991a).
A 0.15–1.1 m thick oxidized tailings vadose zone formed after 50 years of natural weathering, sulfide oxidation, and ARD formation (Holmström et al. 2001). The original mineralogy of the unoxidized tailings is dominated by a high sulfide content, primarily comprised of pyrite (26 %), sphalerite (1.3 %), chalcopyrite (0.28 %), and galena (0.05 %), with the oxidized tailings containing 1–2 % total sulfides (Holmström et al. 2001). The gangue minerals consisted of quartz [SiO2], muscovite [KAl2(Si3AlO10)(OH)2], cordierite [Mg2Al4Si5O18], chlorite [(Mg, Fe)6(SiAl4O10)(OH)8], talc [Mg3Si4O10(OH)2], microcline [KAlSi3O8], diopside [Ca(Mg, Al)(Si, Al)2O6], K-feldspar [KAlSi3O8], and albite [NaAlSi3O8]. Calcite [CaCO3] and dolomite [CaMg(CO3)2] contents were 2.5 % each. The tailings have a vertical and horizontal hydraulic conductivity of 3–7 × 10−6 and 2–5 × 10−5 m/s respectively, and a porosity of 0.25 (Axelsson et al. 1986).
In 1996, the impoundment was remediated (Fig. 2) by applying an engineered composite dry cover (1.5 m protective layer of glacial till and 0.3 m of a clay-rich till sealing layer) to the formerly raised tailings dam area, and by applying a simple 1 m thick water-saturated till cover in the west of the impoundment by raising the groundwater table with sealing dykes. The vertical hydraulic conductivity (and porosity) of the protective till layer and clay-rich till sealing layer were 6 × 10−7 m/s (0.23) (Lindvall et al. 1999) and 1 × 10−9 m/s (0.18), respectively (Höglund et al. 2005). Infiltration into the impoundment groundwater beneath the composite dry covered area due to precipitation is minimal (0–5 % of annual precipitation of 600 mm/year) and only contributes a minor amount of recharge to the groundwater system in these areas (Werner et al. 2001). Almost 100 % of the precipitation infiltrates into the water-saturated areas of the impoundment. The water level in the water-saturated area varies between the ground surface and 1 m below it, and is governed by the potentiometric surface. The impoundment groundwater is directed to the southwest by a dominant fracture zone in the bedrock, and by lateral engineered drainage from the composite dry cover (Fig. 2).
Ten kg/m2 of crushed limestone were added before the covers were constructed (Lindvall et al. 1999). Dissolved metals (Al, Cd, Co, Cr, Cu, Ni) were significantly reduced and the pH increased (Alakangas 2006) in the underlying groundwater a few years after remediation due to reduced oxygen diffusion (Alakangas and Nason 2010). In August 2009, anaerobically-digested SS from the municipality of Stockholm was applied to areas of the impoundment that had had poor vegetation since remediation in 1996 to provide a vegetation substrate and nutrients. 10,800 t of SS were applied onto the dry covered area (DCA) of the impoundment, and 1200 t were applied onto the water-saturated cover areas (WSA), to a depth of 0.2–0.3 m (Fig. 1). The SS was seeded in 2010, and grass establishment had occurred by spring 2011.
Materials and Methods
Groundwater Sampling and Analysis
The groundwater was monitored during two sampling periods. The first period was initiated a few years after remediation in 1998–2006; a full methodology can be found in Alakangas (2006). The second period commenced just prior to the SS application from June 2009-October 2011. Samples were collected monthly from May to October from five installed BAT® groundwater wells, designed to allow sample collection with minimal exposure to atmospheric oxygen (Torstensson 1984). This maintained the groundwater redox potential, removing the possibility for Fe-(oxy) hydroxides to precipitate and adsorb or co-precipitate other metals out of solution. The wells were chosen because of their relative position in the impoundment groundwater system (Fig. 2). Well P represented the uncontaminated inflowing groundwater into the impoundment, located in the western till slope. Wells G and F are located in the WSA of the impoundment at different depths. Well Q is situated in the DCA of the impoundment. Well L represents the groundwater outflow at the impoundment toe.
The acid-washed 120 mL BAT® groundwater tubes were purged with argon and vacuumed prior to sampling. Groundwater samples were filtered in the field in an argon-filled chamber devoid of oxygen using 0.22 μm acid-washed Millipore nitrocellulose membrane filters and syringes. The dissolved fraction was transferred into 125 mL acid-rinsed bottles. Separate samples were collected unfiltered into non-acid washed bottles for the anions NO3 −, SO4 2−, and dissolved organic carbon (DOC). In the field, all samples were stored in a dark, refrigerated environment in a field-lab station prior to analysis. Measurements were conducted using a WTW GmbH Multi-340i meter with a combined ConOx electrode, which had been calibrated using an Oxi-Cal®-SL air calibration vessel for dissolved oxygen and a 0.01 mol/L KCl solution for electrical conductivity (EC). Temperature and pH were measured using a WTW SenTix 41 electrode with a two-point calibration at pH 4.01 and 7. All samples for analysis were sent to the accredited commercial laboratory, ALS, situated in Luleå, Sweden.
Determination of dissolved fractions of major (Fe) and minor (Cu, Ni, Pb, Zn) elements in the water were performed using ICP-AES and ICP-SFMS, respectively. Nitrate and sulfate were analyzed using ion chromatography. DOC samples in the water were calculated by filtration through 25 mm diameter glass micro fiber filters (0.47 μm) mounted in stainless steel filter holders; analysis was performed using a Shimadzu TOC-5000 high-temperature combustion instrument. The analyses were conducted according to the EPA-methods (modified) 200.7 (ICP-AES) and 200.8 (ICP-SFMS). Blank analysis using Milli-Q® water indicated a contribution of <2 % for all dissolved cations except for Pb, which occasionally contributed concentrations exceeding 2 %.
Sewage Sludge Sampling and Analysis
SS samples were collected on two occasions: 3 spot samples during application of the fresh SS in July 2009; and 1 and 2 spot samples from the WSA and DCA, respectively, in Oct. 2011. They were collected using a handheld non-metallic auger, placed into non-diffusive plastic containers, and frozen before sample analysis the following day. They were dried at 50 °C and leached in 7 M nitric acid in closed Teflon vessels in a microwave oven for As, Cd, Cu, Co, Hg, Ni, Pb, S, and Zn. The remaining elements were determined after fusion with lithium metaborate and dissolved in dilute nitric acid. These solutions were centrifuged and diluted before analysis. The determination of Al, Ca, Cr, Fe, K, P, S, and Ti were made using ICP-AES and As, Cd, Co, Cu, Ni, Pb, and Zn were analyzed using ICP-SFMS. Instrumental analysis was carried out according to modified USEPA methods. Precision in all methods was generally better than 5 %. Dry matter reported as total solids (TS %) was determined by drying the sample prior to analysis in an oven at 105 °C for 24 h according to Swedish standard SS 028113-1. Loss of ignition (LOI) was determined by placing an aliquot of the sample and heating it to 1,000 °C and subtracting the difference in weights.
MINTEQ Organic Complexation and Speciation Modeling
Speciation and organic complexation of dissolved metals in the groundwater of each well were modeled using the chemical equilibrium Visual MINTEQ modeling software (Version 3.0., Beta) to further understand the transport of metals in the impoundment groundwater system. The Stockholm Humic Model was used to model the dissolved groundwater geochemical data (Gustafsson 2001). Five time periods were chosen to compare the average impoundment groundwater data and the SO 24 -/Fe2+ ratios NO3 −, DOC, and EC over time: the post remediation flush (PRF) from 1998 to 2000; the stable period (SP) from 2001 until just before SS application in summer 2009; the first year after sludge application (SS1) during 2009; the second year after sludge application (SS2) during 2010; the third year after sludge application (SS3) during 2011.
Results and Discussion
Release of Constituents from Sewage Sludge
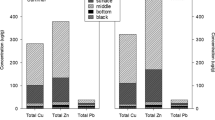
To quantify the mass change of constituents (Table 1) in the solid fraction of the SS 2 years after the application in June 2009, mass balance calculations were used by normalizing each constituent to a fixed element, Ti. This element is assumed to be relatively immobile in the sludge, as the minerals it normally resides in, such as rutile, ilmenite, or as an accessory element in pyroxene and amphiboles, resist weathering and dissolution. It is assumed that if oxidation and geochemical processes occurred within the surface SS layer, a total mass change (Eq. 1) between the original concentration (CO) and the final concentration (CF) of a particular element (e) (Eq. 2) would have occurred, identified by using a normalizing element (n). The results are presented in Fig. 3.
The SS mass volume decreased during the 2 years after application from both the WSA and DCA by 17.76 and 16.25 %, respectively (Fig. 3). The mass lost was dominantly attributed to organic matter (OM) depletion and the leaching of sulfate, calcium, and metals. Aerobic degradation of the OM fraction (LOI) due to surface oxidation (Eq. 3) resulted in a mass depletion of 22 % in both applications.
Copper, Ni, Pb, and Zn were removed from the SS (Fig. 3). It is likely these sludge-borne metals were mobilized due to surface weathering of the reduced metals (Ahlberg 2006). The loss of minor constituents was unequal between the two areas. Compared to the SS on the DCA, the Cu, Pb, and Zn were more readily removed from the WSA, whereas more Ni was lost from the DCA.
A major loss of CaO and S, which was elevated in the original solid geochemistry (Table 1), accounted for much of the mass lost from the SS. However, the high original concentrations of Fe and Al were retained (Fig. 3). The Fe and Al likely were added as soluble Fe/Al-sulfate flocculants during waste water processing (Neuschütz 2009). Though the types of sulfates are unknown, dissolution of melanterite (Eq. 4) and jurbanite (Eq. 5) may have occurred, releasing sulfur as sulfate into solution while the cations would have precipitated as hydroxides (Lottermoser 2010). This would account for the high S loss but the retention of Fe and Al (Fig. 3). Furthermore, the sulfate may have reacted with the Ca2+ released from the SS to precipitate gypsum (Eq. 6) (Lottermoser 2010), though speciation modeling using Visual MINTEQ suggests that the impoundment groundwater was undersaturated with CaSO 04 .
Inorganic Water Geochemistry in the Impoundment Groundwater System
Water-Saturated Areas of the Impoundment
Prior to the SS application, dissolved metals and sulfate (Fig. 4) concentrations had stabilized after the 1996 remediation (Alakangas 2006). After SS application in 2009, metal and sulfate concentrations increased independent of EC, except in well F (Table 2). This was observed 1 month after the application in the areas of the WSA where SS was applied. Wells F and G in the WSA responded differently to the SS application. Well F, the deeper of the two wells, received peak concentrations of the metals Fe (396 mg/L), Ni (182 μg/L), and Zn (9,680 μg/L) 1 month after the SS application, up from 2009 pre-SS concentrations of Fe (84 mg/L), Ni (0 μg/L), and Zn (104 μg/L). Concentrations in this well subsided to within ±10 % of pre-SS concentrations by 2010 and had stabilized by 2011.
Well G, the shallower well, received a concentration pulse of the elements Fe (154 mg/L), Ni (365 μg/L), and Zn (2,530 μg/L) 2 months after the well F peak, up from 2009 pre-SS concentrations of Fe (80 mg/L), Ni (0 μg/L), and Zn (1,770 μg/L) (Fig. 4). In 2010, the peak still remained moderately high in concentrations of Cu (28 μg/L), Fe (129 mg/L), Ni (208 μg/L), Pb (11 μg/L), and Zn (1,850 μg/L), but had returned to pre-SS concentrations by early 2011.
The rapid movement of the sludge-borne constituents into the shallow and deep tailings groundwater was likely because the potentiometric surface fluctuates from 0.5 m below- to ground-level within the water-saturated cover during the spring-melt and autumn rain events. This rise in the water surface due to increasing piezometric pressure enabled the mobilized metals that had been oxidized by atmospheric oxygen and meteoric rainfall to enter the underlying groundwater. As the groundwater in this part of the impoundment may travel both horizontally and vertically due to the heterogeneity of the tailings (Axelsson et al. 1991b), metals were able to migrate away from the SS. As metals in both the WSA wells returned to pre-SS concentrations within 2 years, it is likely the release of sludge-borne metals had ceased. Thereafter, it appears that the vegetation established on the SS in spring 2011 took up and immobilized the metals, as has been observed in similar studies conducted in Sweden (Forsberg 2008; Forsberg et al. 2008).
Dry-Covered Areas of the Impoundment
Well Q did not exhibit any changes in water geochemistry until the end of 2011 when a rise in Cu (188 μg/L), Ni (263 μg/L), Pb (95 μg/L), and Zn (2,060 μg/L) occurred (Fig. 4). Pre-SS concentrations of these constituents in 2009 were Cu (0 μg/L), Ni (3.8 μg/L), Pb (0.4 μg/L), and Zn (1,610 μg/L). Concentrations of Fe did not change. A decrease in pH was observed from pH 6 (pre-SS in 2009) to 5.5 (Fig. 4).
The source of the metal plume is not likely from the SS applied onto the DCA as the sealing layer in the composite dry cover is designed to allow only minor amounts of water infiltration: 4 × 10−3 m3/m2/year into the underlying tailings (Carlsson et al. 2003). Based on the mean annual precipitation of 600 mm/year, it would take 150 years for 1 year of rainfall to percolate through the SS and dry cover.
As lateral groundwater movement across the impoundment is 30–90 m3/m2/year (Corrège et al. 2001), the source of the metal plume that affected the groundwater underlying the DCA was likely derived from the SS on the WSA. The two areas where the SS was applied are separated by 150 m, so it should have taken 1.7–5 years for this plume to migrate. The data indicates that the plume took 2.2 years to affect the groundwater underlying the DCA, agreeing with this calculation. The Ni and Zn concentrations in well Q were less than the concentrations found at the WSA wells. This is attributed to dilution within the groundwater under the DCA due to inflow of uncontaminated groundwater from the western till slope, as data from well P illustrates (Fig. 4).
As the metal contamination plume was released 2 years after the SS applied onto the WSA, the peak in concentration in well Q should theoretically subside within 2 years as the groundwater plume migrates past, due to the dominant groundwater movement rates and direction (Corrège et al. 2001). Based on the distance to the impoundment toe, it is predicted that the contamination plume will take an additional 4 years to migrate out of the impoundment groundwater system. Further monitoring will quantify this rate.
The fate of the sludge-borne constituents derived from the DCA, hindered from infiltrating through the low hydraulic conductivity sealing layer (1 × 10−9 m/s), traveled laterally from west to east through the protective layer to the impoundment toe, on a designed 1:3 gradient (Lindvall et al. 1999). As the protective layer material has a hydraulic conductivity of 6 × 10−7 m/s, horizontal water movement (Lindvall et al. 1999) occurred. Elevated metal concentrations were measured in the groundwater at well L at the impoundment toe. This peak occurred within 2 months after the SS application, as the SS located on the DCA is less than 100 m from the toe (Fig. 2). The transport of dissolved metal solutes through the highly permeable protective cover was thus rapid. Peak concentrations of Cu (776 μg/L), Ni (493 μg/L), and Zn (20.4 mg/L) occurred as the leached constituents were funneled towards the toe. This large peak declined rapidly and had decreased to pre-SS concentrations within 1 year, as the pH increased (Fig. 4). Similarly to the SS on the WSA, prominent vegetation establishment on the SS in 2011 decreased the amount of sludge-borne metals released. The observed and predicted metal peaks in each well are illustrated (Fig. 5), based on the dominant hydrological regime. The peaks observed in wells F and G are observed data, whereas well Q is predicted based on the plume migration. Well L at the toe received peak concentrations from the SS on the DCA. The second peak is modeled to occur as the plume derived from the groundwater migrates to the toe within 6 years of the SS application, based on the dominant hydrological groundwater regime.
Nitrate: Formation, Dispersal, and Fate
Nitrate concentrations increased in well G and Q (Table 2) in 2010–2011. The increase was likely due to nitrification (Eq. 7) in the SS. This process is prevalent in surface applications of SS (Ahlberg 2006; Evanylo 1994; Schroder et al. 2008). As the nitrate was released from the SS to the tailings groundwater, it may have become a primary terminal electron acceptor for pyrite oxidation (Appelo and Postma 2005) as dissolved oxygen concentrations were relatively low (2–4 mg/L) in all wells (except well Q) over the study periods. Pyrite oxidation may be indicated by SO4 2−/Fe2+ molar ratios derived from the groundwater data (Karlsson et al. 2010). Oxidation of pyrite by oxygen or nitrate may release dissolved Fe2+ and SO4 2−, resulting in a ratio of 2 (Eqs. 8 or 9) (Appelo and Postma 2005). A ratio <2 may indicate the absence of oxidation.
All wells (except Q) in the impoundment displayed a declining SO4 2−/Fe2+ ratio (<2) from 1998 to 2009 (Table 2), due to successful remediation in 1996, which prevented oxygen diffusion to the tailings. The groundwater table was raised during these stages and may have created reductive dissolution of Fe-hydroxides within the oxidized vadose zone, causing an increase in dissolved Fe concentrations in the groundwater. Both of these processes may have contributed to lowering the SO4 2−/Fe2+ ratio to <2 prior to the SS application.
Water-Saturated Areas of the Impoundment
The molar SO4 2−/Fe2+ ratio increased marginally in well F and G during 2009 after the SS application (Table 2). However, from 2010 to 2011 in well G, the molar ratio increased >2, due to an increase in concentrations of 260 mg/L Fe and 4,100 mg/L SO4 2− (Fig. 4), yet nitrate remained relatively unchanged (Table 2). These concentrations may indicate pyrite oxidation by nitrate, as there was a rise in dissolved Fe, SO4 2−, and nitrate, though released, was consumed in the reaction. The pH also decreased by a mean pH of 0.5. The Fe is unlikely sludge-borne as it was retained in the SS (Fig. 3). The ratio and nitrate concentration in Well F, the deeper well, remained unchanged; it is surmised that because it is the deeper of the two wells, it did not respond as nitrate was consumed in the overlying tailings and oxidation of pyrite did not occur at this depth.
The slight decline of the molar ratio in well G in 2011 was likely due to two processes: the conversion of ammonium to nitrate has been shown to be very rapid when applied as a surface cover, and up to 26 % of the nitrogen content of an anaerobically-digested sludge can be released as nitrate in the first year, but only 3–5 % each year thereafter (Evanylo 1994); plant establishment on SS can take up both nitrate and ammonium (Forsberg 2008). A decline in sulfate concentrations was mirrored by a decline in dissolved Ca2+ (Fig. 4), which may have indicated gypsum formation, removing sulfate from solution and lowering the ratio.
Dry-Covered Areas of the Impoundment
At well Q, the SO4 2−/Fe2+ molar ratio stabilized to 4 before SS application, showing that some oxygen was likely still present in the tailings after the initial remediation (Table 2). Dissolved oxygen concentrations ranged from 2.00 to 5.76 mg/L from 2009 to 2011. After the SS application, the SO4 2−/Fe2+ ratio remained unaltered in well Q and was maintained at >2. Nitrate concentrations peaked to 100 mg/L (Table 2) and sulfate to 1,460 mg/L (Fig. 4). The data may indicate pyrite oxidation by nitrate may not have occurred as Fe concentrations and the pH remained stable, nitrate was not consumed, and the ratio remained unchanged. This is because oxygen present remained as the primary terminal electron acceptor for pyrite.
The results suggest that, similar to the sludge-borne metals that entered the groundwater beneath the DCA in 2011, nitrate was sourced from the episodic release from the WSA. A similar decline and migration of nitrate should hence occur.
The molar ratio (Table 2) in the groundwater at well L at the impoundment toe increased to >2 after SS application in 2009, and remained at this level throughout the monitoring. The nitrate concentration increased slightly (Table 2), but by 2011, nitrate concentrations fell to 0 mg/L. However, dissolved Fe and SO4 2− concentrations remained unchanged (Fig. 4). There was no evidence at the impoundment toe of an interaction of the sludge-borne nitrate with the tailings groundwater system.
Metal Speciation Related to Organic Geochemistry
Speciation and organic complexation modeling using Visual MINTEQ was conducted for the divalent cations Cu, Fe, Ni, Pb, and Zn from each well in the impoundment (Fig. 6) to understand the mechanisms for metal transport. Redox potential was estimated to be low and was calculated using the redox couple nitrate/ammonium. The model calculated that Fe2+ was prevented from oxidizing to Fe3+, and hence, metals did not co-precipitate or adsorb to Fe-(oxy) hydroxides. This explains why 100 % of the metals Cu, Fe, Ni, Pb and Zn were present in solution as free divalent ions, metal sulfates, or bound as mobile organo-metallic complexes in all wells. After SS application, the elevated DOC concentrations (Table 2) and pH, rather than redox potential, controlled the susceptibility of metals to form complexes. As the pH of the groundwater in wells F, G, and Q was pH 5 to near-neutral, the affinity of metals to form organo-metallic complexes increased when elevated DOC concentrations occurred simultaneously. This is because the attraction of cations to binding sites such as those of soluble carboxylic acids (R-COOH) in the fulvic acid fraction of the DOC peaks at pH 7, due to proton dissociation (H+) at higher pH (Eq. 10) (Gustafsson 2001). This creates the formation of strong electrostatically bound bidentate organo-metallic complexes with divalent cations (Fletcher and Beckett 1987; Garcia-Gil et al. 2007) (Eq. 11). Weaker, hydrostatic monodentate complexes may also form (Eq. 12).
The pH in well L was less than 3 after the sludge application (Fig. 4), and even though DOC concentrations peaked at 8 mg/L (Table 2), all metals failed to form a strong dependency to complex with the organic fraction, as carboxylic acids do not dissociate below pH 3 (Fig. 6). The two wells located in the WSA, G and F, exhibited peak metal concentrations in 2009, but organo-metallic complexes didn’t dominate the water speciation until higher DOC concentrations were released in 2010 (Fig. 6). In both wells, Cu, Ni, and Pb had a strong tendency to form organo-metallic complexes, whereas Fe and Zn did not.
In well Q, situated in the DCA, the peak metal concentrations (Fig. 6) coincided with an elevated DOC concentration of 38 mg/L (Table 2) in autumn 2011. This influenced a high percentage of the Cu and Pb, and to a lesser extent Ni, to form complexes. Iron however, remained as free ions in solution. This is most probably due to the reduced pH in 2011 to 5.5 (Fig. 4). The tendency of Cu to form a strong complex has been recognized in similar studies conducted during surface applications of SS and peaks at pH 6.5 (Forsberg et al. 2008), which was within the pH range of in Impoundment 1. Sludge-borne Ni and Cu have been found to similarly form complexes within the groundwater of tailings applied with SS (Andres and Francisco 2008).
The implications on the water chemistry from the results is that the metals were more likely to be transported through the impoundment groundwater system as these mobile complexes and not be immobilized or retained in the tailings by reactions such as precipitation or adsorption. This may cause readily-mobile metals to be transported and released to peripheral environments.
Conclusions
The surface application of 12,000 t of SS to a remediated tailings impoundment at the Kristineberg Mine was successful in that it provided and supported vegetation establishment within 2 years of application. However, the sludge adversely affected the tailings groundwater quality as weathering and surface oxidation of reduced metal compounds and ammonium in the SS led to the release of sludge-borne metals, nitrate, and DOC.
The results suggest that the effect of the SS application on the dry-composite covered areas was negligible, as the sludge-borne leachate, unable to enter through the cover, was directed laterally, and was concentrated at the impoundment toe, without reacting with the tailings groundwater system. However, the SS application on the water-saturated cover area contributed excessive metals (Cu, Ni, Pb, Zn), DOC, and nitrate into the impoundment groundwater system. These migrated laterally beneath the dry covered areas due to the dominant hydrogeological regime across the impoundment. The metals were readily-transported in solution or as organo-metallic complexes due to the elevated DOC released from the SS. Nitrate released from the SS likely caused pyrite oxidation in the unoxidized tailings of the water-saturated areas, further exacerbating metal concentrations and releasing Fe. However, the release of sludge-borne constituents was temporary as vegetation establishment after 2 years immobilized the constituents. The migrating contamination plume is expected to flush through the impoundment within 6 years after the SS application.
Fortunately, at this study site, all surface and groundwater flow derived from the impoundment is directed into three larger impoundments that are limed to remove metals from solution. Hence, the peripheral environment was not hindered in this case study. However, applications in similar situations should be avoided or treated, as necessary. Successful capture and treatment of the water may be necessary to prevent surface- and groundwater contamination.
References
Ahlberg G (2006) Ageing of sewage sludge—some physical and chemical properties in relation to landscaping. Doctoral Thesis, Göteborg University, Sweden
Ahlberg G, Gustafsson O, Wedel P (2006) Leaching of metals from sewage sludge during one year and their relationship to particle size. Environ Pollut 144:545–553
Alakangas L (2006) Sulphide oxidation, oxygen diffusion and metal mobility in sulphide-bearing mine tailings in Northern Sweden. PhD Thesis, Luleå University of Technology, Sweden
Alakangas L, Nason PA (2010) Declining element concentrations in groundwater after remediation in sulphide-rich tailings at Kristineberg, northern Sweden. In: Wolkersdorfer C, Freund A (Eds), Proceedings of Symposium International Mine Water Association, Water and Innovative Thinking, Sydney, pp 323–326
Andres NF, Francisco MS (2008) Effects of sewage sludge application on heavy metal leaching from mine tailings impoundments. Bioresour Technol 99:7521–7530
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution, 2nd edn. In: Balkema AA, Leiden
Ashworth DJ, Alloway BJ (2004) Soil mobility of sewage sludge-derived dissolved organic matter, copper, nickel and zinc. Environ Pollut 127:137–144
Axelsson C-L, Karlkvist L, Lintu Y, Olsson T (1986) Gruvindustrins restproduktupplag—fältundersökningar med vattenbalansstudie i Kristineberg. Uppsala Geosystem AB [in Swedish, with summary in English]
Axelsson C-L, Ekstav A, Holmén J, Jansson T (1991a) Efterbehandling av sandmagasin i Kristineberg, Hydrogeologiska förutsättningar för åtgärdsplan: Lakvattenbalanser och vittringsbegränsande åtgärder. Report [in Swedish]
Axelsson C-L, Ekstav A, Jansson T (1991b) Provtagning av sand och grundvatten i sand-magasin 1, 1B och 2 Kristineberg—Fältrapport. Golder Geosystems AB. Report 917–1687 [in Swedish]
Carlsson E (2002) Sulphide-Rich tailings remediated by soil cover—evaluation of cover efficiency and tailings geochemistry, Kristineberg, northern Sweden. PhD Thesis, Luleå University of Technology, Sweden
Carlsson E, Ohlander B, Holmstrom H (2003) Geochemistry of the infiltrating water in the vadose zone of a remediated tailings impoundment, Kristineberg mine, northern Sweden. Appl Geochem 18:659–674
Christensen JB, Christensen TH (2000) The effect of pH on the complexation of Cd, Ni and Zn by dissolved organic carbon from leachate-polluted groundwater. Water Res 34:3743–3754
Corrège O, Carlsson E, Öhlander B (2001) Geochemical investigations of the groundwater in sulphide-bearing tailings remediated by applying till cover. In: Proceedings of Symposium Securing the Future: International Conference on Mining and the Environment, Skellefteå, Sweden, pp 97–114
Cravotta CA (1998) Effect of sewage sludge on formation of acidic ground water at a reclaimed coal mine. Groundwater 36:9–19
Eriksson J (2001) Concentrations of 61 trace elements in sewage sludge, farmyard manure, mineral fertiliser, precipitation and in soil and crops. Report 5159:69, Swedish Environmental Protection Agency, Stockholm
Evanylo GK (ed) (1994) Mineralisation and availability of nitrogen in organic waste-amended mid-Atlantic soils. Chesapeake Research Consortium Inc, Edgewater
Fletcher P, Beckett PHT (1987) The chemistry of heavy-metals in digested sewage sludge. 2. Heavy-metal complexation with soluble organic matter. Water Res 21:1163–1172
Forsberg SL (2008) Reclamation of copper mine tailings using sewage sludge. PhD Thesis, Swedish University of Agricultural Sciences, Sweden
Forsberg LS, Gustafsson JP, Kleja DB, Ledin S (2008) Leaching of metals from oxidising sulphide mine tailings with and without sewage sludge application. Water Air Soil Pollut 194:331–341
Fytili D, Zabaniotou A (2008) Utilization of sewage sludge in EU application of old and new methods—a review. Renew Sust Energ Rev 12:116–140
Garcia-Gil JC, Plaza C, Senesl N, Brunetti G, Polo A (2007) Effects of long-term sewage sludge amendment on the composition, structure and proton binding activity of soil fulvic acids. Clean Soil Air Water 35:480–487
Gotthardsson J, Sundberg Å (2005) Mine site reclamation using products from the pulp industry. In: Proceedings of Symposium Securing the Future, International Conference on Mining and the Environment, Metals and Energy Recovery
Gustafsson JP (2001) Modeling the acid-base properties and metal complexation of humic substances with the stockholm humic model. J Colloid Interface Sci 244:102–112
Hallberg RO, Granhagen JR, Liljemark A (2005) A fly ash/biosludge dry cover for the mitigation of AMD at the falun mine. Chem Der Erde-Geochem 65:43–63
Hanæus Å, Mattsson E (2009) Remedial measures taken at the Falun Mine site, Sweden. In: Proceedings of Symposium Securing the Future, 8th International Conference on Acid Rock Drainage, Skellefteå, Sweden
Höglund LO, Herbert RB, Lövgren L, Öhlander B, Neretnieks I, Moreno L, Malmström M, Elander P, Linvall M, Lindström B (2005) MiMi—performance assessment–main report: MiMi report 2003:3. In: Höglund LO, Herbert RB (eds), MiMi Print, Luleå, Stockholm
Holmström H, Salmon UJ, Carlsson E, Petrov P, Öhlander B (2001) Geochemical investigations of sulfide-bearing tailings at Kristineberg, northern Sweden, a few years after remediation. Sci Total Environ 273:111–133
INAP (2009) Global Acid Rock Drainage (GARD) Guide. www.gardguide.com
Karlsson S, Allard B, Bäckström M (2010) Weathering mechanisms and composition of effluents from a sulphide mine waste deposit after covering—twenty years of field data. In: Wolkersdorfer C, Freund A (eds), Proceedings of Symposium International Mine Water Association, Water and Innovative Thinking, Sydney, pp 359–362
Lindvall M, Lindahl L, Eriksson N (1997) The reclamation project at the Saxberget mine, Sweden. In: Proceedings of 4th International Conference on Acid Rock Drainage, Vancouver, pp 1389–1400
Lindvall M, Eriksson N, Ljungberg J (1999) Decommissioning at Kristineberg mine, Sweden. In: Proceedings of 1999 Symposium Mining and the Environment, Sudbury, pp 855–862
Lottermoser B (2010) Mine wastes, characterization, treatment and environmental impacts. Springer, Berlin
Marklund S (1997) Dewatering of wastewater sludge by natural air drying. Licentiate Thesis, Luleå University of Technology, Sweden
Neuschütz C (2009) Phytostabilization of mine tailings covered with fly ash and sewage sludge. PhD Thesis, Stockholm University, Sweden
Neuschütz C, Isaksson K, Lundmark L, Gregor M (2009) Evaluation of a dry-cover treatment consisting of vegetation, sewage sludge and fly ash. In: Proceedings of Symposium Securing the Future, 8th International Conference on Acid Rock Drainage, Skellefteå, Sweden, pp 1–9
Pichtel JR, Dick WA, Sutton P (1994) Comparison of amendments and management-practices for long-term reclamation of abandoned mine lands. J Environ Qual 23:766–772
Porse E (2002) Jordartsgeologiska egenskaper hos avloppsslam. Project Work, University of Gothenberg, Report B340 [in Swedish]
Schroder JL, Zhang H, Zhou D, Basta N, Raun WR, Payton ME, Zazulak A (2008) The effect of long-term annual application of biosolids on soil properties, phosphorus, and metals. Soil Sci Soc of Am J 72:73–82
Statistics-Sweden (2008) Discharges to water and sewage sludge production in 2006. Publ in collaboration with the Swedish Environmental Protection Agency. http://www.scb.se/templates/Publikation__232141.asp
Torstensson BA (1984) A new system for groundwater monitoring. Groundwater Monit Rem 4:131–138
Werner K, Carlsson E, Berglund S (2001) Oxygen- and water fluxes into a soil-cover remediated mill tailings deposit: Evaluation of field data from the Kristineberg mine site, Northern Sweden. In: Proceedings of Symposium Securing the Future, International Conference Mining and the Environment, Skellefteå, Sweden, pp 896–905
Acknowledgments
This study was funded by Georange, which distributes EU-structural funds in Sweden and was conducted within the framework of the Centre of Advanced Mining and Metallurgy (CAMM) at the Luleå University of Technology. The authors express further gratitude to New Boliden AB for providing assistance and access to the site at Kristineberg Mine and to Bert-Sive of Bergteamet, who provided further field assistance. ALS Scandinavia is acknowledged for providing sample analyses. Finally, thanks goes to Milan Vnuk for help preparing the figures and Angela Lundberg for assistance with the groundwater intepretation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nason, P., Alakangas, L. & Öhlander, B. Impact of Sewage Sludge on Groundwater Quality at a Formerly Remediated Tailings Impoundment. Mine Water Environ 33, 66–78 (2014). https://doi.org/10.1007/s10230-013-0244-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-013-0244-6