Abstract
This study used histological techniques to investigate sexual patterns in the freckled hawkfish Paracirrhites forsteri, which has a mating system intermediate between harem and male-territory-visiting polygamy. Males on reefs in the area of Kuchierabu-jima Island tended to be larger than females, and one hermaphrodite with ovarian cavities was observed at a size intermediate between most males and females. These findings indicate a protogynous sexual pattern in the studied population. However, some males were smaller than females, with the smallest individual collected being a male. Furthermore, most individuals with a body size smaller than that typical of females exhibited bisexual gonads. These new findings suggest that prematurational sex change occurs in this species, whereby some males derive directly through sexual differentiation as juveniles. Our study represents the first report suggesting diandry in the Cirrhitidae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sequential hermaphroditism (sex change) is a life history strategy and is currently recognized in more than 400 teleost species (Kuwamura et al. 2020, 2023). Sequential hermaphroditism includes three types of sexual patterns: protogyny (female-to-male sex change), protandry (male-to-female sex change), and bidirectional sex change. The adaptive significance of these sex-change patterns in teleosts is explained by the size-advantage model (Ghiselin 1969; Warner 1975, 1988). For instance, the model predicts that protogyny will evolve in a polygynous mating system where male reproductive success exponentially increases with body size, while that of females increases linearly. Typical examples of polygynous mating systems include harem polygyny and male-territory-visiting (MTV) polygamy. Harem polygyny is characterized by the cohabitation of multiple females within the territory of a single male and the stable monopolization of mating opportunities with the females by the male (Thresher 1984; Kuwamura 1996, 1997). MTV polygamy differs from harem polygyny in that the females are usually located outside the male mating territories and choose their mating partners (Kuwamura 1996, 1997). In fact, protogyny has been widely observed in fish with either harem polygyny or MTV polygamy (Warner 1984; Nakazono and Kuwamura 1987; Kuwamura et al. 2020, 2023).
Protogyny has two types, monandry and diandry, evidenced by the number of pathways of male development (Reinboth 1970; Munday et al. 2006; Sakai 2023). In monandry, all males derive from functional females through sex change (i.e., secondary males). Diandric species have two male developmental pathways: some males derive through sexual differentiation directly as juveniles (i.e., primary males), while others derive as secondary males. In general, monandry tends to occur in harem polygyny, wherein small males have limited mating opportunities; in contrast, diandry tends to occur in MTV polygamy, wherein small males often have mating opportunities through alternative mating tactics such as sneaking and group spawning (Sakai 2023).
The family Cirrhitidae comprises 33 species in 12 genera, with most species distributed on rocky and coral reefs in tropical to subtropical waters of the Indo-West Pacific (Nelson et al. 2016). At least six species within this family (Amblycirrhitus pinos, Cirrhitichthys aprinus, Cirrhitichthys aureus, Cirrhitichthys falco, Cirrhitichthys oxycephalus, and Neocirrhites armatus) are confirmed to exhibit protogyny (Kuwamura et al. 2020, 2023). Moreover, at least four species (C. aprinus, C. falco, C. oxycephalus, and N. armatus) have been suggested to be monandric based on gonadal histological evaluations or field observations (Kobayashi and Suzuki 1992; Sadovy and Donaldson 1995; Kadota et al. 2011; Sakai 2023). In addition, two species (C. aureus and C. falco) have been found to exhibit the ability to change back to female (i.e., reversed sex change) (Kobayashi and Suzuki 1992; Kadota et al. 2012; Kadota 2023). Whereas harem polygyny has been determined in mating systems of four monandric protogynous hawkfishes (Donaldson 1987, 1990; Kadota et al. 2011), diandry has not yet been reported in any cirrhitid hawkfishes (Sakai 2023).
A unique mating system was recently reported in the freckled hawkfish Paracirrhites forsteri (Kadota and Sakai 2016). In this mating system, large males stably monopolize their mating opportunities with multiple females, which resembles harem polygyny; however, the majority of females leave the male’s territory outside the spawning period, which resembles MTV polygamy. Furthermore, small males with a body size similar to that of females coexist with large males and have mating opportunities with smaller females within the territory of the large male. This mating system differs from that of other hawkfishes by suggesting a possibility of diandry. To date, a histological study of gonads in P. forsteri examined only two individuals (Sadovy and Donaldson 1995); therefore, little is known about the sexual patterns in this species through histology. To elucidate the sexual patterns of P. forsteri, we collected specimens from reefs at Kuchierabu-jima Island and neighboring islands for a detailed histological examination of the gonads.
Materials and methods
Fifty-five specimens of Paracirrhites forsteri were collected from reefs at Kuchierabu-jima Island (30°28′ N, 130°10′ E) and neighboring islands, namely Kuchinoshima Island (29°59′ N, 129°54′ E), Nakanoshima Island (29°50′ N, 129°51′ E), Taira-jima Island (29°68′ N, 129°53′ E), and Kodakara-jima Island (29°22′ N, 129°32′ E), in southern Japan, from May to October in 2002 to 2007. Many tropical or subtropical fish species, including P. forsteri, are distributed on these islands (Sakai et al. 2009; Kadota et al. 2024). The spawning season of P. forsteri at Kuchierabu-jima Island is known to occur between early May and late October (Kadota et al. 2010). Small fish of <100 mm total length (TL) were captured using hand and screen nets and a diluted solution of quinaldine (1%). Larger individuals with higher swimming ability were captured using a spear. Each living specimen was euthanized in ice water immediately after collection.
All specimens were transported to the laboratory and fixed in 10% formalin. After fixation, the TL of each fish was measured with calipers to the nearest 0.1 mm. Later, the body weight and gonad weight of each specimen were measured to the nearest 0.01 g. The gonadosomatic index (GSI) was calculated as [gonad weight (GW)/body weight (BW) × 100 (%)]. If the gonads were too light to be weighed, they were recorded as 0.001 g for calculation of the GSI. The gonad samples were then dehydrated, embedded in paraffin, transversely sectioned at a thickness of 4–6 μm, and stained with hematoxylin and eosin. All histological slides were observed under an optical microscope. Individuals with occasional oocytes in primary growth stage in testis were defined as male in this study, because these oocytes are considered to be remnants of the prior ovarian or juvenile phase (Sadovy and Shapiro 1987). Hermaphrodites were defined as the other individuals with bisexual gonads. For observations of hermaphroditic gonads and the male gonads with a few oocytes, the oocyte area was measured using Image J software, and the proportions of the area occupied in the gonads were calculated and compared. A single histological section or a part of the gonad was used for this calculation. Statistical analysis was conducted using R version 4.3.0 (R Core Team 2019).
Results
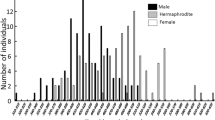
Body size composition. Of the 55 specimens examined histologically, 26 were males, 22 were females, and seven were hermaphrodites (Fig. 1). The body size of males (median 179.3 mm TL, range 51.4–199.8, n = 26) was significantly larger than that of females (median 137.3 mm TL, range 92.2–159.8 mm, n = 22; Mann–Whitney U-test, P < 0.001). However, four males (51.4, 96.4, 113.5, and 134.0 mm TL) were smaller than the median size of females (137.3 mm TL), and the smallest male (51.4 mm TL) was smaller than the smallest female (92.2 mm TL).
Gonads of females and males. The gonads of 21 females were filled with previtellogenic and vitellogenic oocytes. One relatively small-sized female (106.0 mm TL) contained only previtellogenic oocytes within the ovarian structure, indicating an immature reproductive status.
Of the 26 males, the gonads of 19 (73%), including the smallest male, comprised only spermatogenic tissue (Fig. 2a, b), while seven males (27%) had a few previtellogenic oocytes within the spermatogenic tissue (Fig. 2c). Ovarian cavities, remnants of ovarian structure, were observed in a male of the latter type (Fig. 2d). There was no significant difference in TL between the former type of males (median 179.6 mm TL, range 51.4–199.8, n = 19) and the latter type of males (median 164.5 mm TL, range 113.5–196.5, n = 7; Mann–Whitney U-test, P > 0.05). The GSI of the former type of males (median 0.098%, range 0.007–0.882, n = 19) also did not differ significantly from that of the latter type (median 0.125%, range 0.002–0.161, n = 7; Mann–Whitney U-test, P > 0.05).
Gonad structure in Paracirrhites forsteri males: a testis of a large male (179.0 mm TL); b testis of the smallest male (51.4 mm TL); c testis with previtellogenic oocytes (at 172.8 mm TL); and d testis with previtellogenic oocytes and ovarian cavities (at 146.0 mm TL). op oocytes in perinucleolus stage, oc ovarian cavity. Bar 100 μm
Hermaphroditic gonads. Among the seven hermaphrodites, five individuals (median 86.9 mm TL, range 77.5–93.2) had some previtellogenic oocytes in their spermatogenic tissues (Fig. 3a, b). These gonads were structurally similar to those of males containing a few previtellogenic oocytes. However, previtellogenic oocytes were relatively abundant in the hermaphroditic gonads, whereas spermatogenic tissues were relatively underdeveloped. Consequently, the percentage of oocytes in gonads was significantly higher in hermaphrodites (median 3.568%, range 2.121–10.650, n = 5) than in males (median 0.317%, range 0.024–0.666, n = 7; Mann–Whitney U-test, P < 0.01). Among those five hermaphrodites, three individuals (77.5–86.9 mm TL) were smaller than the smallest female (92.2 mm TL).
Another hermaphroditic individual (104.0 mm TL; Fig. 3c, d) had a gonad with two separate regions, each occupied by previtellogenic oocytes and spermatogenic tissue. The other hermaphroditic individual (146.0 mm TL) possessed a different type of intermediate gonad that contained both previtellogenic oocytes and spermatogenic tissue in the ovarian structure (Fig. 3e, f). Ovarian cavities were also observed in this largest hermaphrodite. The hermaphroditic individual surpassed the median size of the females and was in a size class intermediate between the males and females.
The GSI values of the hermaphrodites (median 0.094%, range 0.007–0.148, n = 7) were much lower than the GSI values of females (median 4.371%, range 0.526–7.568, n =21; Mann–Whitney U-test, P < 0.001), although the GSI of the hermaphrodites did not differ significantly from that of males (median 0.099%, range 0.002–0.882, n =26; Mann–Whitney U-test, P > 0.05).
Discussion
Protogyny within the family Cirrhitidae has been reported in the genera Amblycirrhitus, Cirrhitichthys, and Neocirrhites (Sadovy and Donaldson 1995; Kuwamura et al. 2020, 2023). We found that most males of Paracirrhites forsteri maintained a larger body size than females, and one hermaphrodite individual with bisexual gonads occurred in a size class intermediate between the males and females. Additionally, one male exhibited ovarian cavities and a few previtellogenic oocytes within its testis structure. These findings suggest that the sexual pattern of P. forsteri in the studied population is consistent with protogyny based on the criteria for functional hermaphroditism (Sadovy and Shapiro 1987).
However, we also found evidence suggesting diandric protogynous hermaphroditism in P. forsteri. Males occurred in a range of sizes, and, notably, the smallest male was smaller than the smallest female. The gonads of the smallest male were composed of spermatogenic tissue only, indicating that some males may directly derive through sexual differentiation as juveniles (i.e., primary males). Additionally, some hermaphrodites were smaller than the smallest mature female. This finding indicates that juveniles can flexibly determine their sex (i.e., prematurational sex change). However, it may not be necessary to distinguish between males in terms of direct maturation to primary males or by prematurational sex change (Munday et al. 2006; Sakai 2023). In some diandric fishes, juveniles are reported to determine their sex in response to social conditions (Liu and Sadovy 2004; Munday et al. 2006). Thus, it is suggested that the coexistence of the two types of males be considered as diandry, regardless of the process by which they are born (Sakai 2023). Further research is needed to determine under what social conditions juveniles become males or females. Diandry has been reported in at least five teleost families, including Labridae and Scaridae (Sakai 2023). Our study is the first to suggest that diandry also occurs in the Cirrhitidae.
In general, small-sized primary males have mating opportunities through alternative mating tactics, such as sneaking and group spawning (Nakazono and Kuwamura 1987; Warner 2001; Sakai 2023). As these mating tactics result in sperm competition, the GSI of a primary male is reported to be considerably high (Petersen and Warner 1998; Warner 2001). For example, the GSI of males reaches 3% on average in the bluehead wrasse Thalassoma bifasciatum (Warner 2001). In contrast, males of P. forsteri spawn in pairs regardless of their body size (Kadota and Sakai 2016). In addition, the GSI values of the P. forsteri were low (maximum 0.88%), irrespective of the males’ gonadal structure (i.e., with or without a few previtellogenic oocytes), although the presence of testicular oocytes is the weakest of all the criteria for protogyny (Sadovy and Shapiro 1987). This exception may be a feature of P. forsteri that distinguishes it from other diandric species.
What is the major male developmental pathway in the studied population of P. forsteri? Relatively few males were confirmed among the medium-sized individuals in our sample. This suggests that males largely derive from females through sex change; however, this observation could be attributed to collection bias. Large territorial males and females are known to appear on coral heads for courtship and spawning from late afternoon (Kadota and Sakai 2016). In contrast, males with a body size similar to that of females often hide among the corals when large territorial males are present. Thus, this behavioral difference may have contributed to female-biased sampling of medium-sized individuals in our study. Therefore, we posit that males are potentially more abundant as medium-sized individuals than indicated in the present study. Further research is needed to reveal the frequency of sex-change occurrences in P. forsteri.
In general, sexual patterns in fish are influenced by the mating system (Warner 1984; Nakazono and Kuwamura 1987; Kuwamura et al. 2020, 2023). Diandry is often observed in MTV polygamy, whereas monandry is frequently observed in harem polygyny (Sakai 2023). The mating system of P. forsteri is not typical of MTV polygamy in that small males can still have mating opportunities with females within the territories of large males (Kadota and Sakai 2016). Conversely, the mating system of monandric hawkfishes is typical harem polygyny (Donaldson 1990; Kadota et al. 2011, 2012). These studies highlight that the mating system in the Cirrhitidae can also influence the sexual patterns, as in other families such as Labridae. For instance, the mating system of P. forsteri has been classified as harem polygyny at low latitude study sites, such as Guam (Donaldson 1990), and monandry can also occur in P. forsteri in such populations.
References
Donaldson TJ (1987) Social organization and reproductive behavior of the hawkfish Cirrhitichthys falco (Cirrhitidae). Bull Mar Sci 41:531–540
Donaldson TJ (1990) Reproductive behavior and social organization of some Pacific hawkfishes (Cirrhitidae). Jpn J Ichthyol 36:439–458
Ghiselin MT (1969) The evolution of hermaphroditism among animals. Q Rev Biol 44:189–208
Kadota T (2023) Bidirectional sex change in fishes. In: Kuwamura T, Sawada K, Sunobe T, Sakai Y, Kadota T (eds) Hermaphroditism and mating systems in fish. Springer Nature, Singapore, pp 145–180
Kadota T, Osato J, Hashimoto H, Sakai Y (2011) Harem structure and female territoriality in the dwarf hawkfish Cirrhitichthys falco (Cirrhitidae). Environ Biol Fishes 92:79–88
Kadota T, Osato J, Nagata K, Sakai Y (2012) Reversed sex change in the haremic protogynous hawkfish Cirrhitichthys falco in natural conditions. Ethology 118:226–234
Kadota T, Sakai Y (2016) Mating system of the freckled hawkfish, Paracirrhites forsteri (Cirrhitidae) on Kuchierabu-jima Island reefs, southern Japan. Environ Biol Fishes 99:761–769
Kadota T, Sakai Y, Hashimoto H, Gushima K (2010) Diel and lunar spawning periodicity of the hawkfish Paracirrhites forsteri (Cirrhitidae) on the reefs of Kuchierabu-jima Island, southern Japan. Ichthyol Res 57:102–106
Kadota T, Shimizu N, Tsuboi M, Breno B, Sakai Y, Hashimoto H, Gushima K (2024) Change in the subtidal reef fish assemblage at Kuchierabu‑jima Island, southern Japan, between 1972 and 2005. Ichthyol Res.
Kobayashi K, Suzuki K (1992) Hermaphroditism and sexual function in Cirrhitichthys aureus and other Japanese hawkfishes (Cirrhitidae: Teleostei). Jpn J Ichthyol 38:397–410
Kuwamura T (1996) An introduction to reproductive strategies of fishes. In: Kuwamura T, Nagahama Y (eds) Reproductive strategies in fishes, vol. 1. Kaiyusha, Tokyo, pp 1–41
Kuwamura T (1997) The evolution of parental care and mating systems among Tanganyikan cichlids. In: Kawanabe H, Hori M, Nagoshi M (eds) Fish communities in Lake Tanganyika. Kyoto University Press, Kyoto, pp 57–86
Kuwamura T, Sawada K, Sunobe T, Sakai Y, Kadota T (2023) Hermaphroditism and mating systems in fish. Springer Nature, Singapore
Kuwamura T, Sunobe T, Sakai Y, Kadota T, Sawada K (2020) Hermaphroditism in fishes: an annotated list of species, phylogeny, and mating system. Ichthyol Res 67:341–360
Liu M, Sadovy Y (2004) The influence of social factors on adult sex change and juvenile sexual differentiation in a diandric, protogynous epinepheline, Cephalopholis boenak (Pisces, Serranidae). J Zool 264:239–248
Munday PL, Wilson White J, Warner RR (2006) A social basis for the development of primary males in a sex-changing fish. Proc R Soc B 273:2845–2851
Nakazono A, Kuwamura T (1987) Sex change in fishes. Tokai University Press, Tokyo
Nelson JS, Grande TC, Wilson MVH (2016) Fishes of the world, 5th edn. John Wiley & Sons, New Jersey
Petersen CW, Warner RR (1998) Sperm competition in fishes. In: Birkhead TR, Møller AP (eds) Sperm competition and sexual selection. Acadmic Press, San Diego, pp 435–463
R Core Team (2019) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria
Reinboth R (1970) Intersexuality in fishes. Mem Soc Endocrinol 18:515–543
Sadovy Y, Donaldson TJ (1995) Sexual pattern of Neocirrhites armatus (Cirrhitidae) with notes on other hawkfish species. Environ Biol Fishes 42:143–150
Sadovy Y, Shapiro DY (1987) Criteria for the diagnosis of hermaphroditism in fishes. Copeia 1987:136–156
Sakai Y (2023) Protogyny in fishes. In: Kuwamura T, Sawada K, Sunobe T, Sakai Y, Kadota T (eds) Hermaphroditism and mating system in fish. Springer Nature, Singapore, pp 87–143
Sakai Y, Kadota T, Shimizu N, Tsuboi M, Yamaguchi S, Nakaguchi K, Go A, Masui Y, Hashimoto H, Gushima K (2009) Fish fauna on reefs of Tokara Islands, southern Japan, surveyed by underwater census during 2002–2007. J Grad Sch Biosp Sci, Hiroshima Univ 48:19–35
Thresher RE (1984) Reproduction in reef fish. T.F.H. Publications, Neptune City
Warner RR (1975) The adaptive significance of sequential hermaphroditism in animals. Am Nat 109:61–82
Warner RR (1984) Mating behavior and hermaphroditism in coral reef fishes. Am Sci 72:128–136
Warner RR (1988) Sex change and the size-advantage model. Trends Ecol Evol 3:133–136
Warner RR (2001) Synthesis: environment, mating systems, and life history allocations in the bluehead wrasse. In: Dugatkin LA (ed) Model systems in behavioral ecology. Princeton University Press, Prinston, pp 227–244
Acknowledgment
We are grateful to the people of Kuchierabu-jima Island for allowing us to conduct the field work. We thank H. Hashimoto and colleagues at the Laboratory of the Biology of Aquatic Resources, Hiroshima University, for their advice. We also appreciate Y. Fukui, K. Kobayashi, and R. Murata for providing advice on conducting the histological examinations. We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. This work was partially supported by the Mikimoto Fund for Marine Ecology to T. Kadota.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study complied with the current laws of Japan and the guidelines of the Ichthyological Society of Japan.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kadota, T., Sakai, Y., Shimizu, N. et al. Histological notes on diandric protogyny in the freckled hawkfish Paracirrhites forsteri (Cirrhitidae) from Kuchierabu-jima Island, Japan. Ichthyol Res (2024). https://doi.org/10.1007/s10228-024-00988-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10228-024-00988-8