Abstract
Larvae and juveniles of three species in the family Gobiidae were described based on reared material; Priolepis borea survived to post-settlement, and Priolepis cincta and Priolepis latifascima to seven days after hatching. Newly hatched larvae of Priolepis are distinguishable from each other by the presence or absence of melanophores on the yolk, ventral part of the abdomen, posterior angle of the lower jaw, and dorsal region of the body. The presence of a row of melanophores on the ventral part of the body and xanthophores may be useful to identify the genus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gobiid genus Priolepis currently comprises 37 species (Allen et al. 2018; Koeda et al. 2021) that are distributed in tropical to temperate waters of the Indo-Pacific and Atlantic oceans and inhabit coral or rocky reefs (Winterbottom and Burridge 1992; Senou et al. 2004; Nogawa and Endo 2007), except for Priolepis goldshmidtae, which is recorded from sandy bottoms in deep sea (Goren and Baranes 1995). The family Gobiidae includes about 1,950 species, though information on early-life history is limited to approximately 11% of the species (Borges et al. 2011), including larval descriptions of four species of Priolepis: Priolepis semidoliata (Sonoda and Imai 1971), Priolepis borea (Shiogaki and Dotsu 1974), Priolepis cincta (Sunobe and Nakazono 1989), and Priolepis nocturna (Wittenrich et al. 2007). In addition, Priolepis aureoviridis (see Baensch 2020) and P. cincta (see ORA 2014) have recently been reared through metamorphosis, but no detailed morphological changes have been described. Metamorphosis from the pelagic larva to benthic juvenile involves various morphological changes and is crucial in determining survival of the recruits and the community structure as the fish changes its ecological habits during this period (Caley et al. 1996; Searcy and Sponaugle 2001; Shima and Findlay 2002). Description of the early life history of each species is required as a basis for a variety of studies, such as ecology and phylogenetic relationships.
In the present study, we describe the early development of three species: P. borea, P. cincta and, Priolepis latifascima.
Materials and methods
A male and a female of Priolepis borea, P. cincta and P. latifascima were collected by hand nets from Tateyama Bay at Banda, Chiba Prefecture, Japan: Priolepis borea (male: 30.6 mm in standard length [SL] [captured on 7 July 2018]; female: 28.9 mm SL [7 July 2018]); P. cincta (male: 32.9 mm SL [26 May 2020]; female 33.4 mm SL [15 July 2020]); P. latifascima (male: 21.6 mm SL [21 May 2020]; female: 21.4 mm SL [15 July 2020]). The fish were brought to the laboratory and kept in 13-L seawater within a glass aquarium (30 × 18 × 24 cm). The seawater was circulated and filtered using an under-gravel airlift system. An opaque polyvinyl chloride pipe (inside diameter 2.5 cm, length 6 cm) was set on the bottom as a shelter or as a spawning ground. Waterproof paper (Elmer EK-300W; Somar Corp., Tokyo, Japan) was set up to cover the inside of the pipe to collect any eggs laid later. The adult fish were fed Artemia nauplii, twice daily. Water temperature was kept at ~ 24 °C. Salinity and photoperiod were about 29 ppt and 14 h light and 10 h dark, respectively.
Spawning of the P. borea, P. cincta and P. latifascima adult pairs took place on 19 June 2020, 5 August 2020, and 6 August 2020, respectively, and the period from collection to spawning was about two years for the former and about two months for the latter two species. Just after spawning, the eggs were transferred together with the waterproof paper into a cylindrical polycarbonate tank containing 30 liter of seawater equipped with an air stone and supplied with gentle aeration. The aeration was weakened just before hatching not to disturb the larvae. The larval culture was done using the same equipment.
After the larvae hatched, an appropriate dose of marine microalgae Nannochloropsis sp. (Marine fresh; Nisshin Marinetech Co. Ltd., Aichi, Japan) was added so that the water continually appeared light green. Feeding of the larvae began 1 day post-hatch (DPH). S-type rotifers Brachionus rotundiformis, fed with Chlorella (Super Fresh Chlorella V12; Chlorella Industry Co. Ltd, Tokyo, Japan), were added to the larval culture tank once per day; densities of rotifers in the tanks were maintained at 20 individuals/ml. For the P. borea larvae, Artemia nauplii were provided from 17 DPH. To increase the n-3 fatty acid concentration in the live foods, the rotifers and Artemia nauplii were incubated with Hyper Gloss (Nisshin Marinetech Co. Ltd., Aichi, Japan) before feeding to the larvae. Water temperature was kept at ~ 24 °C throughout the rearing.
The larvae and juveniles were observed under an optical microscope (Olympus BX51; Olympus Co. Ltd, Tokyo, Japan) or a stereomicroscope (Olympus SZ2-LGB; Olympus Co. Ltd, Tokyo, Japan), after euthanasia in clove oil. Priolepis borea larvae and juveniles were collected and observed immediately after hatching (n = 5), and at 2 (n = 5), 7 (n = 1), 12 (n = 1), 17 (n = 1), 22 (n = 1), 32 (n = 3), 42 (n = 3), and 73 (n = 3) DPH, and both P. cincta and P. latifascima after hatching (n = 5), and at 2 (n = 1) and 7 (n = 1) DPH. We selected larger specimens from the tank each day. Body length (BL = notochord length for preflexion and flexion larvae or SL after completion of notochord flexion), total length (TL), and yolk-sac diameter (YD) were measured to the nearest 0.01 mm under a microscope fitted with an ocular micrometer, or to the nearest 0.1 mm with calipers. Body depth (BD), head length (HL), preanal length (PAL), and eye diameter (ED) were also measured except for the newly hatched and 2 DPH individuals not shown in the sketches. The specimens were drawn in Photoshop (Adobe Inc., San Jose, CA, USA) using photographs taken with an optical microscope or stereomicroscope. Newly hatched larvae and 2 DPH larvae not shown in the sketch were used only for BL and YD measurements.
All observed specimens were preserved in 70% ethyl alcohol after fixation in 10% formalin for adults and 5% formalin diluted with seawater for larvae and juveniles and then deposited in the ichthyological collection of the Kanagawa Prefectural Museum of Natural History (KPM-NI 63257–63277) (note: the accession nos. for fishes in the museum are seven digits, with zeros added for convenience in the electronic ledger, but here they are expressed as significant digits). All pigments other than melanin disappeared after fixation. Size and age at settlement were defined by the first individual to settle on the bottom of the tank.
Results
Priolepis borea
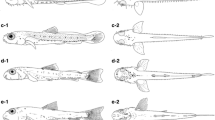
General morphology. Newly hatched larvae (Fig. 1a) measured 2.18–2.27 mm BL and 2.26–2.38 mm TL (n = 5). The larvae in flexion stage (Fig. 1e) measured 3.72 mm BL and 4.13 mm TL (17 DPH). The juveniles at 73 DPH (Fig. 2c) were 15.5–16.1 mm BL and 19.6–20.4 mm TL (n = 3). The body was elongated and compressed initially with 10 + 16 = 26 myomeres (adults: 10 + 16 = 26 myomeres). BD was about 16% BL initially, increased rapidly to reach about 29% BL during notochord flexion, and subsequently decreased to reach about 19% BL in juveniles at 15.5–16.1 mm BL (73 DPH) (Fig. 3). A nearly circular (slightly elliptical) yolk sac with a single oil globule was visible at hatching, with YD measured 0.20–0.26 mm (n = 5) (Fig. 1a), and which was completely absorbed at 2.16–2.39 mm BL and 2.23–2.50 mm TL (n = 5) (2 DPH) (Fig. 1b). Notochord flexion had begun at 3.72 mm BL (17 DPH) (Fig. 1e) and was complete at 5.31 mm BL (6.84 mm TL, 22 DPH) (Fig. 1f). Dorsal and ventral finfolds almost same depth just after hatching (Fig. 1a), with the caudal peduncle part of finfolds becoming lower at 3.72 mm BL (17 DPH) (Fig. 1e), and disappearing at 5.31 mm BL (22 DPH) (Fig. 1f). Anterior edge of the gas bladder was located at myomere 2 (Fig. 1a–f; not shown in Fig. 1f, but it exists) and moved to myomere 3 (Fig. 2a), with the posterior edge moving from myomere 4–5 (Fig.1a–d), to myomere 6 (Fig. 1e–f) and finally to myomere 7 (Fig. 2a). The gas bladder became invisible at 8.6–10.0 mm BL and 11.4–13.8 mm TL (n = 3) (42 DPH) (Fig. 2b). The mouth and anus opened by hatching (Fig. 1a). The premaxilla, maxilla and dentary are clearly visible at 2.16–2.39 mm BL (2 DPH) (Fig. 1b). Fine teeth appeared on both jaws at 3.72 mm BL (17 DPH) (Fig. 1e). Posterior of the two otoliths has an ontogenetic increase in size relative to the anterior otolith (Fig. 1d–f), the diameter of this otolith is more than half the diameter of the pupil at 5.31 mm BL (17 DPH) (Fig. 1f). Scales were present on the whole body and dorsal side of the head at 15.6 mm BL (73 DPH) (Fig. 2c). HL was about 21% BL initially, reaching a maximum of about 39% BL during flexion stage (Fig. 3). PAL was about 51% BL initially, reaching a maximum of about 60% BL during flexion stage. ED was about 9% BL in all specimens.
Reared larvae and juveniles of Priolepis borea. a Newly hatched larva, KPM-NI 63263, 2.26 mm BL; b at 2 days post-hatch (DPH), KPM-NI 63264, 2.39 mm BL; c at 7 DPH, KPM-NI 63265, 2.32 mm BL; d at 12 DPH, KPM-NI 63266, 2.94 mm BL; e at 17 DPH, KPM-NI 63267, 3.72 mm BL; f at 22 DPH, KPM-NI 63268, 5.31 mm BL
Fin development. Newly hatched larvae had a pair of pectoral fin buds (Fig. 1a). Pectoral fin rays appeared and all rays (16) were visible and already segmented at 5.31 mm BL (22 DPH) (Fig. 1f). Pelvic fin buds appeared at 3.72 mm BL (17 DPH) (Fig. 1e; the specimen in Fig. 1e had pelvic fin buds, but they were too small to observe in a lateral view). Pelvic fin rays appeared and all elements (I, 5) were visible at 5.31 mm BL (22 DPH) (Fig. 1f) and were segmented at 6.4–7.4 mm BL and 8.0–9.0 mm TL (n = 3) (32 DPH). Incipient caudal fin rays appeared at 3.72 mm BL (17 DPH) (Fig. 1e). Caudal fin rays segmented at 5.31 mm BL (22 DPH) (Fig. 1f) and branched at 6.4–7.4 mm BL (32 DPH). Incipient second dorsal and anal fins appeared at 3.72 mm BL (17 DPH) (Fig. 1e). First dorsal fin appeared at 5.31 mm BL (22 DPH) (Fig. 1f). Second dorsal and anal fin rays were segmented, and both fin rays had reached the number specific to this species: I, 9 and I, 7 for second dorsal fin and anal fin, respectively, at 5.31 mm BL (22 DPH) (Fig. 1f). First dorsal fin had all spines (VI) at 6.4–7.4 mm BL (32 DPH) (Fig. 2a).
Pigmentation. Melanophore deposition on eyes already completed by hatching (Fig. 1a). Newly hatched larva had 2 large dendritic melanophores on the yolk sac (Fig. 1a). There was a small melanophore at the posterior angle of the lower jaw until at 6.4–7.4 mm BL (32 DPH) (Figs. 1, 2a). Some melanophores on the ventral part of the abdomen were present until at 6.4–7.4 mm BL (32 DPH) (Figs. 1, 2a). Internal melanophores which lined the dorsal surface of the abdominal cavity around the gas bladder were present until they became invisible due to the development of pigment on the body surface (Figs. 1, 2a). Newly hatched larvae had a single row of melanophores on the dorsal region of the body between the 10th and 22nd myomeres (Fig. 1a); the dorsal melanophore series extended anteriorly and posteriorly at 2.16–2.39 mm BL (2 DPH) (Fig. 1b), then became intermittent at 5.31 mm BL (22 DPH) (Fig. 1f), and faded at 8.6–10.0 mm BL (42 DPH) (Fig. 2b). A single row of melanophores on the ventral region of the body was also present between the posterior edge of the gas bladder and 22nd myomere initially (Fig. 1a); the ventral melanophore series became intermittent and faded at the same time as the dorsal melanophore series. Numerous melanophores appeared on the laterally on the body between the dorsal and ventral series of melanophores at 2.16–2.39 mm BL (2 DPH) (Fig. 1b); the melanophores appeared denser at 2.32 mm BL and 2.45 mm TL (7 DPH) (Fig. 1c), then the extent of the melanophores reduced at 2.94 mm BL and 3.19 mm TL (12 DPH) (Fig. 1d), and disappeared at 3.72 mm BL (17 DPH) (Fig. 1e). Some dendritic melanophores appeared on the anal fin base at 3.72 mm BL (17 DPH) (Fig. 1e) and were present until at 6.4–7.4 mm BL (32 DPH) (Figs. 1f, 2a).
Xanthophores were present on the tip of the lower jaw in all larvae and juveniles (Figs. 1, 2a, b). Some xanthophores appeared laterally between the dorsal and ventral series of melanophores just after hatching (Fig. 1a); the xanthophores got denser at 2.16–2.39 mm BL (2 DPH) (Fig. 1b) and expanded at 5.31 mm BL (22 DPH) (Fig. 1c), then the extent of the xanthophores reduced in the vicinity of the lateral midline at 6.4–7.4 mm BL (32 DPH) (Fig. 2a). Vertical, yellow bands, as seen in adult fish, have begun to form on the head at 6.4–7.4 mm BL (32 DPH) (Fig. 2a). The vertical yellow bands bordered by black lines appeared on the body at 8.6–10.0 mm BL (42 DPH) (Fig. 2b); the vertical bands on the body faded at 15.5–16.1 mm BL (72 DPH) (Fig. 2c).
Some erythrophores appeared on the abdomen and ventrally on the tip of the tail at 2.94 mm BL (Fig. 1d), and in addition to these, on the ventral part of the body posterior to the anus at 5.31 mm BL (22 DPH) (Fig. 1f) and around the base of the dorsal fin at 6.4–7.4 mm BL (32 DPH) (Fig. 2a).
Ecological note. Larvae hatched at four and a half days after fertilization. The newly hatched larvae showed positive phototaxis. Fish started settling on the bottom at 22 DPH.
Priolepis cincta
General morphology. Newly hatched larvae (Fig. 4a) measured 1.80–1.97 mm BL and 1.91–2.05 mm TL (n = 5) with 10 + 16 = 26 myomeres (adults: 10 + 16 = 26 myomeres). The body was elongated and compressed in all specimens (Fig. 4). BD was about 15% BL (Fig. 3). A nearly circular (slightly elliptical) yolk sac with a single oil globule was visible at hatching, with YD measured 0.20–0.21 mm (n = 5) (Fig. 4a), and which was completely absorbed at 2.16 mm BL and 2.24 mm TL (2 DPH) (Fig. 4b). Dorsal and ventral finfolds almost same depth just after hatching (Fig. 4a). The gas bladder was located just posterior to the pectoral fin base in all specimens (Fig. 4). The mouth and anus opened by hatching (Fig. 4a). The premaxilla, maxilla and dentary are clearly visible at 2.16 mm BL (2 DPH) (Fig. 4b). HL, PAL, and ED were about 20% BL, 47% BL, and 8% BL, respectively (Fig. 3).
Fin development. Newly hatched larvae had a pair of pectoral fin buds (Fig. 4a).
Pigmentation. Melanophore deposition on eyes already completed by hatching (Fig. 4a). There were melanophores on the posterior angle of the lower jaw and ventral part of the abdomen; additionally, internal melanophores which lined the dorsal surface of the abdominal cavity around the gas bladder were present in all specimens (Fig. 4). Newly hatched larvae had a single row of melanophores on the dorsal region of the body between the 10th and 23rd myomeres (Fig. 4a); the dorsal melanophore series extended anteriorly at 2.20 mm BL and 2.27 mm TL (7 DPH) (Fig. 4c). A single row of melanophores on the ventral region of the body was also present between the posterior edge of the gas bladder and 24th myomere in all specimens (Fig. 4); the ventral melanophores increased in size and number and appeared slightly medial to the body at 2.20 mm BL (7 DPH) (Fig. 4c).
Xanthophores were present on the dorsal and ventral regions of the body; additionally, punctate xanthophores were scattered over the body in all specimens (Fig. 4). Xanthophores on the tip of the lower jaw were apparent at 2.16 mm BL (Fig. 4b).
Ecological note. Larvae hatched four and a half days after fertilization. The newly hatched larvae showed positive phototaxis. All larvae died at 9 DPH.
Priolepis latifascima
General morphology. Newly hatched larvae (Fig. 5a) measured 1.98–2.16 mm BL and 2.06–2.27 mm TL (n = 5) with 11 + 17 = 28 myomeres (adults: 10 + 16 = 26 myomeres). The myomeres decrease to 10 + 16 = 26 at 2.71 mm BL and 2.92 mm TL (7 DPH) (Fig. 5c). The body was elongated and compressed in all specimens (Fig. 5). The body depth was about 15% BL (Fig. 3). A nearly circular (slightly elliptical) yolk sac with a single oil globule was just slightly visible at hatching, with YD measuring 0.07–0.09 mm (mean: 0.08 mm; n = 5) (Fig. 5a), and which was completely absorbed at 2.37 mm BL and 2.65 mm TL (2 DPH) (Fig. 5b). Dorsal and ventral finfolds almost same depth just after hatching (Fig. 5a). A gas bladder was located just posterior to the pectoral fin base in all specimens (Fig. 5). The anus opened by hatching (Fig. 5a). The premaxilla, maxilla and dentary are clearly visible by hatching (Fig. 5a). HL, PAL, and ED were about 22% BL, 49% BL, and 9% BL, respectively (Fig. 3).
Fin development. Newly hatched larva had a pair of pectoral fin buds (Fig. 5a).
Pigmentation. Melanophore deposition on eyes already completed by hatching (Fig. 5a). Internal melanophores which lined the dorsal surface of the abdominal cavity around the gas bladder were present in all specimens (Fig. 5). Newly hatched larvae had a single row of melanophores on the dorsal region of the body between the 13th and 22nd myomeres (Fig. 5a); the dorsal melanophore series extended anteriorly at 2.71 mm BL (7 DPH) (Fig. 5c). A single row of melanophores on the ventral region of the body was also present between the posterior edge of the gas bladder and 22nd myomere in all specimens (Fig. 5).
Xanthophores were present on the dorsal and ventral regions of the body at hatching (Fig. 5a); the xanthophores expanded broadly expanded midway along the body at 2.71 mm BL (7 DPH) (Fig. 5c). Xanthophores on the tip of the lower jaw were apparent at 2.37 mm BL (2 DPH) (Fig. 5b).
Some erythrophores appeared ventrally on the tip of the tail at 2.71 mm BL (7 DPH) (Fig. 5c).
Ecological note. Larvae hatched at five and a half days after fertilization. The newly hatched larvae showed positive phototaxis. All larvae died at 9 DPH.
Discussion
Newly hatched larvae of Priolepis borea (Shiogaki and Dotsu 1974; this study), P. cincta (Sunobe and Nakazono 1989; this study), P. latifascima (this study), P. nocturna (Wittenrich et al. 2007), and P. semidoliata (Sonoda and Imai 1971) share the following traits: a nearly circular (slightly elliptical) yolk sac with a single oil globule, pigments on the dorsal surface of the abdominal cavity around the gas bladder, a single row of melanophores on the ventral region of the body, and xanthophores on various parts of the body. These traits may be useful to identify the genus. Priolepis is closely related to Trimma and Trimmatom (Winterbottom and Burridge 1992; Sunobe et al. 2017). Newly hatched larvae of Trimma okinawae, Trimma grammistes, and Trimmatom sp. described in Sunobe (1995) share with Priolepis described so far the yolk sac with a single globule, but can be distinguished by the absence of both the melanophore row on the ventral region of the body and of xanthophores, also by the presence of melanophores on the dorsal part of the rectum (Sunobe 1995).
Newly hatched larvae of present three species and known two species of Priolepis [for P. nocturna, see Wittenrich et al. (2007) and for P. semidoliata, see Sonoda and Imai (1971)] can be distinguished from each other by the distribution of melanophores (Table 1). Priolepis borea most closely resembles P. cincta, and both species share presence of melanophores on ventral part of the abdomen, posterior angle of the lower jaw and dorsal region of the tail. However, P. borea can be distinguished from P. cincta by the presence of melanophores on the yolk. Priolepis latifascima is distinguished from the other four species by the presence of melanophores on the dorsal region of the tail only. However, Sunobe and Nakazono (1989) did not identify melanophores at the posterior angle of the lower jaw or on the ventral part of the abdomen in larvae of P. cincta, and Shiogaki and Dotsu (1974) stated in larvae of P. borea that the melanophores at the posterior angle of the lower jaw appeared two days after hatching, not at hatching. Although these mismatches may be due to oversight, further study would be necessary to examine intraspecies variation and regional differences.
Seven DPH larva of P. borea were clearly distinguished from larvae of P. cincta and P. latifascima by melanophores distributed broadly on the body. From 7 DPH, these three species have xanthophores on the lower jaw tip; this trait may be useful to identify the genus.
Body size of a post-settlement larva of P. borea (7.0 mm TL) from Nagasaki Prefecture described by Shiogaki and Dotsu (1974) similar to that of a larva observed at 22 DPH in the present study (5.31 mm BL), immediately after settling on the bottom. However, the post-settlement larvae’s color and other morphological characteristics, reported by Shiogaki and Dotsu (1974), are similar to those of the reared larva at 6.4–7.4 mm BL (32 DPH). In the present study, this coloration appeared several days after settling, so it can be inferred that the specimen from Nagasaki Prefecture settled at a size of < 7.0 mm TL. Variation in the size of settlement within species is caused by a variety of internal and external factors (Searcy and Sponaugle 2000; Bay et al. 2006); therefore, the observed differences could be attributable to different conditions encountered by larvae.
The development of P. borea was very different from that of P. aureoviridis and P. cincta. The pelagic duration of P. borea (22 days) is much shorter than P. aureoviridis (65 days; based on reared material) (Baensch 2020) and P. cincta (60 days; based on reared material) (ORA 2014). HL and PAL of P. borea increased during flexion and metamorphose (Figs. 1e, 3), and settles at 6.84 mm TL, whereas the body of P. aureoviridis remains elongated after flexion (from the photographs) and settles at 10 mm TL (Baensch 2020). These intragenus differences in early life history may be due to the taxonomic differences; P. borea belongs to Priolepis semidoliatus grade (sensu Winterbottom and Burridge 1993a) but P. aureoviridis and P. cincta belong to Priolepis cinctus grade (sensu Winterbottom and Burridge 1993b) based on adult morphology. Additionally, the distribution of each species may also be a factor in these differences. The distribution area of P. borea is narrower than P. aureoviridis or P. cincta (Froese and Pauly 2021). Given that fish recruiting to remote locations may display longer pelagic duration than those recruiting well-connected ones (Victor 1986; Bay et al. 2006), the short pelagic duration of P. borea may be reasonable.
References
Allen GR, Erdmann MV, Brooks WM (2018) A new species of Priolepis (Pisces: Gobiidae) from Papua New Guinea. J Ocean Sci Found 31:32–37
Baensch F (2020) Golden goby culture. updated 7 September 2020. https://www.frankbaensch.com/marine-aquarium-fish-culture/my-research/golden-goby-culture/. Accessed 7 August 2021
Bay LK, Buechler K, Gagliano M, Caley MJ (2006) Intraspecific variation in the pelagic larval duration of tropical reef fishes. J Fish Biol 68:1206–1214
Borges R, Faria C, Gil F, Gonçalves EJ (2011) Early development of gobies. In: Patzner RA, Tassel JLV, Kovačić M, Kapoor BG (eds) The biology of gobies. CRC Press, Florida, pp 403–462
Caley MJ, Carr MH, Hixon MA, Hughes TP, Jones GP, Menge BA (1996) Recruitment and the local dynamics of open marine populations. Annu Rev Ecol Syst 27:477–500
Froese R, Pauly D (ed) (2021) FishBase. World Wide Web electronic publication. Updated June 2021. http://www.fishbase.org. Accessed 23 October 2021
Goren M, Baranes A (1995) Priolepis goldshmidtae (Gobiidae), a new species from the deep water of the northern Gulf of Aqaba, Red Sea. Cybium 19:343–347
Koeda K, Koido T, Matsuno Y, Endo H (2021) A new reefgoby, Priolepis duostella (Perciformes: Gobiidae) collected from off Kashiwa-jima Island, Kochi, Japan. Ichthyol Res. https://doi.org/https://doi.org/10.1007/s10228-021-00833-2
Nogawa Y, Endo H (2007) A new species of the genus Priolepis (Perciformes: Gobiidae) from Tosa Bay, Japan. Bull Natl Mus Nat Sci Ser A Suppl 1:153–161
ORA (2014) Experimenting with Priolepis unlocks the mysteries of the genus. Updated 13 June 2014. https://www.orafarm.com/blog/2014/06/13/girdled-goby/. Accessed 7 August 2021
Searcy SP, Sponaugle S (2000) Variable larval growth in a coral reef fish. Mar Ecol Prog Ser 206:213–226
Searcy SP, Sponaugle S (2001) Selective mortality during the larval-juvenile transition in two coral reef fishes. Ecology 82:2452–2470
Senou H, Suzuki T, Shibukawa K, Yano K (2004) A photographic guide to the gobioid fishes of Japan. Heibonsha, Tokyo
Shima JS, Findlay AM (2002) Pelagic larval growth rate impacts benthic settlement and survival of a temperate reef fish. Mar Ecol Prog Ser 235:303–309
Shiogaki M, Dotsu Y (1974) The life history of the gobiid fish Zonogobius borea. Bull Fac Fish Nagasaki Univ 37:1–8
Sonoda T, Imai S (1971) On the spawning and embryonic development of a marine goby, Zonogobius semidoliatus (Valenciennes). Mem Fac Fish Kagoshima Univ 20:197–202
Sunobe T (1995) Embryonic development and larvae of three gobiid fish, Trimma okinawae, Trimma grammistes and Trimmatom sp. Jpn J Ichthyol 42:11–16
Sunobe T, Nakazono A (1989) Embryonic development and pre-larva of a gobiid fish Priolepis naraharae. Jpn J Ichthyol 35:484–487
Sunobe T, Sado T, Hagiwara K, Manabe H, Suzuki T, Kobayashi Y, Sakurai M, Dewa S, Matsuoka M, Shinomiya A, Fukuda K, Miya M (2017) Evolution of bidirectional sex change and gonochorism in fishes of the gobiid genera Trimma, Priolepis, and Trimmatom. Sci Nat 104:15
Victor BC (1986) Duration of the planktonic larval stage of one hundred species of Pacific and Atlantic wrasses (family Labridae). Mar Biol 90:317–326
Winterbottom R, Burridge M (1992) Revision of Egglestonichthys and of Priolepis species possessing a transverse pattern of cheek papillae (Teleostei; Gobiidae), with a discussion of relationships. Can J Zool 70:1934–1946
Winterbottom R, Burridge M (1993a) Revision of the species of Priolepis possessing a reduced transverse pattern of cheek papillae and no predorsal scales (Teleostei; Gobiidae). Can J Zool 71:494–514
Winterbottom R, Burridge M (1993b) Revision of Indo-Pacific Priolepis species possessing a reduced transverse pattern of cheek papillae, and predorsal scales (Teleostei; Gobiidae). Can J Zool 71:2056–2076
Wittenrich ML, Turingan RG, Creswell RL (2007) Spawning, early development and first feeding in the gobiid fish Priolepis nocturna. Aquaculture 270:132–141
Acknowledgments
We are grateful to S. Shimizu, S. Ishibashi and R. Yazawa (Tateyama Science Center, Tokyo University of Marine Science and Technology) for their assistance throughout this study. Thanks are also due to H. Senou (Kanagawa Prefectural Museum of Natural History) for acceptance of the deposited specimens. C. Kulongowski (MSc), with the Edanz Group (https://en-author-services.edanz.com/ac), edited a draft of this manuscript. This work was partly supported by JSPS KAKENHI Grants to T. Sunobe (no. 16K07507) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Tanaka, S., Takeda, Y. & Sunobe, T. Larvae and juveniles of three gobiid species of the genus Priolepis. Ichthyol Res 70, 177–184 (2023). https://doi.org/10.1007/s10228-021-00857-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-021-00857-8