Abstract
We describe the larval and juvenile development of Pike Gudgeon, Pseudogobio esocinus, using laboratory-reared specimens. Newly hatched larvae with a 4.2–4.6 mm body length (BL) and 22–23 + 16 = 38–39 myomeres had melanophores on their head and body. The yolk sac was completely absorbed at 5.0 mm BL. Notochord flexion was initiated at 5.0–6.0 mm BL and completed at 7.3 mm BL. The aggregate number of all fin rays was completed at 11 mm BL. Several rod-like cupulae were observed on the head and lateral side of the body at 4.2–11.0 mm BL and were completely distinguished at 11.0–14.0 mm BL. Squamation was initiated on the caudal body at approximately 11.0 mm BL and completed at 11.0–14.0 mm BL. The newly hatched larvae had well-developed eyes and large pectoral fins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gobioninae is a group of morphologically and ecologically diverse cyprinid fishes (Bănărescu and Nalbant 1973; Nelson 2006), and the subfamily consists of four monophyletic groups: Hemibarubus, Sarcocheilichthys, Gobio, and Pseudogobio (see Yang et al. 2006). The genus Pseudogobio includes three species, Pseudogobio esocinus, Pseudogobio vaillanti, and Pseudogobio guilinensis, and all of them are distributed in East Asia (Bănărescu 1992; Yue 1998; Eschmeyer 2014). This genus is characterized by the following features: no mental barbels; slender second unbranched dorsal ray; no more than 44 scales in the lateral line; mouth, which is relatively large, inferior, horse-shoe shaped; lips papillose, papillae on the upper lip in several rows; snout elongate, longer than postorbital distance; and pharyngeal teeth in two rows (Bănărescu and Nalbant 1965, 1973).
Pseudogobio esocinus is a common freshwater fish that is distributed in northeast China, the Korean Peninsula, and western Japan (Uchida 1939; Nakamura 1969; Bănărescu 1992). This species can be distinguished from the other two species of Pseudogobio by the feature of pharyngeal teeth, the relationships between the caudal depth and length, and the length of the pectoral fin (Bănărescu 1992; Yue 1998). Bănărescu and Nalbant (1965) treated P. vaillanti, which is distributed in southeastern China, as a subspecies of P. esocinus, whence the Japanese Pike Gudgeon has been treated as P. esocinus esocinus in some Japanese literature (e.g., Hosoya 2013, 2014). However, P. vaillanti has been elevated from subspecies to species level by Yue (1998); therefore, P. esocinus and P. vaillanti are now treated as taxonomically separate species (Eschmeyer 2014).
Nakamura (1969) described the newly hatched larva, preflexion larva, postflexion larva, and juvenile of P. esocinus on the basis of specimens that were collected from the Tonegawa River system, Saitama Prefecture, eastern Honshu, Japan. In addition, Hosoya (2014; as P. e. esocinus) has briefly reported the morphological features of the pre-larva, post-larva, and juvenile. However, these previous descriptions of the continuous morphological changes are insufficient. In the present study, we aimed to describe the morphology of the larvae and juveniles of P. esocinus in detail using a series of laboratory-reared specimens and report previously unknown unique morphological characteristics.
Materials and Methods
Parental fish and egg collection. The parental fish were collected by a cast net from Nakagawa River (33°32′07″N, 130°25′45″E), Fukuoka Prefecture, northern Kyushu, Japan. Three females [140–160 mm in standard length (SL)] and six males (90–170 mm SL) were maintained at the Fisheries Research Laboratory, Kyushu University, in a 500 L round polycarbonate aquarium. Naturally spawned eggs were collected from April to June 2005 and transferred into a 1 L glass aquarium containing aerated water, which was changed at the rate of 1 L/day. Water temperature during the incubation period was 20–24 °C. Tominaga et al. (2009) reported that Pseudogobio esocinus includes two highly differentiated mitochondrial DNA lineages called Group 1 and Group 2, and the population in Nakagawa River has been confirmed to be of only Group 1 (Tominaga and Nakajima, unpublished data).
Rearing of larvae and juveniles. Hatched larvae were transferred into a 1 L polyethylene aquarium containing aerated water, which was changed at a rate of 1 L/day. Artificial food (Ranchu Baby Gold: Kyorin Food Industries, Ltd., Hyogo, Japan) was provided two times a day from day 3 after hatching until the end of the experiment. Water temperature during the rearing period was 20–24 °C.
Observations and measurements. Observations and measurements were performed for 74 larvae and eight juveniles. The larvae and juveniles were sampled periodically from the rearing aquarium, and some of them were sketched while alive, following sedation using a narcotic (MS-222). After the observations, the specimens were preserved in 5 % buffered formalin solution. Some specimens were stained with methylene blue to observe the fin ray formation and squamation. Identification of the developmental stages and measurement methods were according to the procedures of Sado and Kimura (2005a, b, c, 2006). The fertilized and fixed larvae and juveniles were registered in the Kyushu University Museum (KYUM-PI 4528–4537).
Results
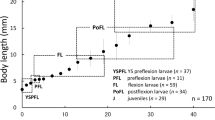
General morphology. Body length (BL) of larvae and juveniles at each developmental stage are shown in Table 1. Newly hatched larvae measured 4.2–4.5 (mean ± SD = 4.38 ± 0.10, n = 7) mm BL; yolk sac large (48–50 % BL), conical, located along venter of head (Fig. 1a), becoming smaller with growth; yolk completely absorbed > 5.0 mm BL (Fig. 1b). Fin fold of newly hatched larvae relatively low, dorsal finfold depth greater than ventral finfold (Fig. 1a). Dorsal and anal finfolds separating from caudal fin in postflexion larvae > 9.0 mm BL (Fig. 1e). Mouth, gill openings and nostrils forming in yolk sac larvae > 4.6 mm BL; nostrils dividing into anterior and posterior portions in postflexion larvae > 9.0 mm (Fig. 1e). Myomeres “V” shaped in newly hatched larvae (Fig. 1a), becoming “W” shaped in postflexion larvae (Fig. 1d). Larvae and juveniles with 22–23 + 16 = 38–39 myomeres (Fig. 1a); notochord flexion initiated and completed at 5.0 mm BL and 7.0 mm BL, respectively.
Reared larvae and juvenile of Pseudogobio esocinus. a Newly hatched larva, KYUM-PI 4528, 4.2 mm in body length (BL). b Preflexion larva, KYUM-PI 4529, 5.0 mm BL. c Flexion larva, KYUM-PI 4530, 7.4 mm BL. d Postflexion larva, KYUM-PI 4531, 9.2 mm BL. e Postflexion larva, KYUM-PI 4532, 11.2 mm BL. f Juvenile, KYUM-PI 4533, 15.9 mm BL. 1 Lateral view, 2 dorsal view
Proportions. Body depth ca. 12–15 % BL initially, rapidly decreasing to ca. 9–12 % BL in preflexion larval stage (ca. 5.0–6.0 mm BL), subsequently increasing in flexion and postflexion larval stages, reaching to ca. 14–17 % BL in juveniles > 11.0 mm BL (Fig. 2a). Head length (HL), ca. 14–20 % BL in yolk sac larvae, proportion increasing with growth, reaching to ca. 28–31 % BL in juveniles > 11.0 mm BL (Fig. 2b). Preanal length ca. 63–76 % BL initially, proportion decreasing to ca. 58–66 % BL in preflexion larval stage; subsequently increasing in flexion and postflexion larval stages, reaching to ca. 61–69 % BL in juveniles > 11.0 mm BL (Fig. 2c). Eye large, ca. 35–50 % of HL in yolk sac larvae, gradually decreasing with growth to ca. 26–29 % HL in juveniles > 11.0 mm BL (Fig. 2d).
Pigmentation. Melanophores deposition on eyes already initiated before hatching (Fig. 1a), completed in preflexion larvae (Fig. 1b). Two stellate melanophores existing on the head in newly hatched larvae (Fig. 1a), increasing in number in preflexion larvae (Fig. 1b); melanophores increasing more, covering widely on head during flexion and postflexion larval stages (Fig. 1c, d). Some stellate melanophores on the yolk in newly hatched larvae (Fig. 1a), becoming branched and large (Fig. 1b, c, d). Chained amorphous melanophores existing along caudal bottom in newly hatched larvae (Fig. 1a), becoming large and obvious during preflexion and flexion larval stages (Fig. 1b, c). Some stellate melanophores appearing on lateral side of the head in preflexion larvae (Fig. 1b), increasing in number during flexion and postflexion larval stages (Fig. 1c, d). Embedded melanophores appearing along vertebrae in flexion larvae (Fig. 1c). Melanophores appearing base of caudal fin in flexion larvae (Fig. 1c); melanophores appearing on snout, dorsal body, and base of dorsal fin in postflexion larvae (Fig. 1d).
Cupulae. No cupula existing in newly hatching larva (Fig. 1a); cupulae developing on body side and under head two days after hatching. Rod-like cupulae appearing in preflexion larvae, two paired on snout, 4–5 paired under head, and 38–40 paired on lateral side of body (Fig. 1b). Number of cupulae under head increasing during flexion and postflexion larvae (Fig. 1c, d, e); length of cupulae becoming short during flexion and postflexion larvae (Fig. 1c, d, e). All cupulae diminishing in juveniles (Fig. 1f). These rod-like cupulae shriveled up after being fixed by formalin.
Fin development. Dorsal fin anlage appearing at ca. 6.0 mm BL in preflexion larval stage, fin ray formation at ca. 5.0–7.0 mm BL in flexion larval stage (Fig. 1c), attaining full complement (iii + 7) in postflexion larval stage (Fig. 1d); ray branching completed until 11.0 mm BL (Fig. 1e). Anal fin anlage appearing at ca. 6.0–8.0 mm BL in postflexion larval stage (Fig. 1d), fin ray formation at ca. 7.0–9.0 mm in postflexion larval stage, attaining full complement (iii + 6) at ca. 8.0–10.0 mm BL in postflexion larval stage (Fig. 1e); ray branching completed at ca. 10.0–11.0 mm BL. Caudal fin rounded already after hatching, becoming emarginated in postflexion larval stage (Fig. 1d). Pectoral fin well developing in newly hatched larvae (Fig. 1a), fin ray formation initiated at ca. 8.0–9.0 mm BL in postflexion larvae (Fig. 1d); shape of pectoral fin changing from simple oval to comma like during preflexion and flexion larval stages (Fig. 1b, c). Pelvic fin anlage appearing at ca. 8.0–9.0 mm BL in postflexion larvae (Fig. 1d); fin ray formation initiated at ca. 9.0–10.0 mm BL in postflexion larvae (Fig. 1d, e).
Scales. A scale appears laterally on central portion of caudal peduncle in postflexion larvae >11.0 mm BL (Fig. 3a), extending anteriorly along lateral midline. Some scales appear on shoulder at 11.0–13.0 mm BL (Fig. 3b). Body except dorsal and abdominal part covered with scales at approximately 12.0–14.0 mm BL (Fig. 3e). Squamation completed in juveniles at 15.0 mm BL (Fig. 3d).
Ecological notes. Yolk sac larvae, preflexion larvae, and flexion larvae usually remain motionless at the bottom. Larvae moil when they feel vibration or irradiated with strong light. After postflexion larval stages, the sensitivity to bright light was weakened.
Discussion
In this study, the larval rod-like cupulae on the lateral body, continuous morphological changes, and squamation of Pseudogobio esocinus were described for the first time. Our results confirm previous descriptions of the larval morphology of P. esocinus given by Nakamura (1969) and Hosoya (2014). Nakamura (1969) has described the larval morphologies of 13 Japanese species of Gobioninae, including Abbottina rivularis, Biwia zezera, Gnathopogon elongatus, Hemibarbus barbus, Hemibarbus longirostris, Pseudogobio esocinus, Pseudorasbora parva, Pseudorasbora pumila, Pungtungia herzi, Sarcocheilichthys variegatus, Squalidus biwae, Squalidus gracilis, and Squalidus japonicus. Newly hatched larvae of five species, G. elongatus, Pseudogobio esocinus, Pseudorasbora parva, P. pumila, and S. variegatus, have pectoral fins and pigmented eyes. However, the pectoral fin of P. esocinus is large and well developed compared to that of the other four species. Although the flexion and postflexion larvae of P. esocinus resemble those of A. rivularis, B. zezera, and H. barbus, the myomere pattern is different: P. esocinus has 22–23 + 16 myomeres, A. rivularis has 24 + 12, B. zezera has 27 + 12, and H. barbus has 30 + 12 (Nakamura, 1969). Therefore, the larvae of P. esocinus can be easily distinguished from those of the above-mentioned Japanese species of Gobioninae.
Nakamura (1969) has sketched some rod-like cupulae under and on the front of the head of pre- and postflexion larvae, but no studies have described the several rod-like cupulae growing on the head and lateral body. These cupulae are difficult to see, and they shrivel up after fixation by formalin. Consequently, they may have been overlooked in the past. Similar larval rod-like cupulae have been described in various families of Teleostei, including Cypriniformes (Iwai 1967, 1972). Nakamura (1969) sketched a similar organ on the heads of three species of Gobioninae, A. rivularis, B. zezera, and S. gracilis.
No reports on the continuous proportional changes that occur in the Japanese cyprinids are available. Sado and Kimura (2005a, b, c, 2006) described in detail four species of Danioninae, Chela dadiburjori, Horadandia atukorali, Inlecypris auropurpureus, and Tanichthys albonubes and the continuous morphological changes in body depth to body length % (BD/BL), preanal length to body length % (PAL/BL), and eye diameter to head length % (ED/HL) are similar among the four species of Danioninae and P. esocinus. However, the change in head length to body length % (HL/BL) of P. esocinus differs from that of the other four species. The HL/BL decreases once to a lower value and then increases after the preflexion larval stage in the four species of Danioninae. In contrast, the HL/BL of P. esocinus increases only in newly hatched larva. These features suggest that newly hatched larvae of P. esocinus are relatively developed.
The squamation of Japanese Gobionine has only been described for H. barbus by Takeshita and Kimura (1998), and the pattern is different from that of P. esocinus. The scales of P. esocinus appear first on the tail and then on the pre-dorsal body. However, the scales of H. barbus appear first on the anterior dorsolateral trunk and then along the lateral body.
Newly hatched and preflexion larvae of P. esocinus are characterized by developed eyes, large pectoral fins, and several rod-like cupulae. These features are peculiar compared to those of other benthic species of Gobioninae (Table 2). The larvae of P. esocinus have a strong positive phototactic responses during the period from day 1 after hatching to the flexion larval stage (Nakajima 2006), and the larvae tend to gather in the shallow, slow-flowing water near the shores in natural rivers (Nakajima et al. 2008). The larval-developed cupulae contribute to foraging activities or habitat selection (Iwai 1972; Mukai and Kobayashi 1991). Therefore, the developed eyes, large pectoral fins, and rod-like cupulae of P. esocinus may contribute to habitat selection during the early life stages.
References
Bănărescu PM (1992) A critical updated checklist of Gobioninae (Pisces, Cyprinidae). Trav Mus d’Hist Nat “Grigore Antipa” 32:303–330
Bănărescu PM, Nalbant TT (1965) Revision of Pseudogobio (Pisces, Cyprinidae) with notes on related genera. Rev Roum Biol Zool 10:301–308
Bănărescu PM, Nalbant TT (1973) Das tierreich, lieferung 93 Pisces, Teleostei, Cyprinidae (Gobioninae). Walter de Gruyter, Berlin
Eschmeyer WN (2014) Catalogue of fishes. California Academy of Sciences. http://research.calacademy.org/ichthyology/catalog. Accessed 13 September 2014
Hosoya K (2013) Cyprinidae. In: Nakabo T (ed) Fishes of Japan with pictorial keys to the species, third edition. Tokai Univ Press, Hadano, pp 308–327, 1813–1819
Hosoya K (2014) Pseudogobio esocinus esocinus (Temminck and Schlegel). In: Okiyama M (ed) An atlas of the early stage fishes in Japan, second edition. Tokai Univ Press, Hadano, pp 125–126
Iwai T (1967) Structure and development of lateral line cupulae in teleost larvae. In: Cahn P (ed) Lateral line detectors. Indiana University Press, Bloomington, pp 27–44
Iwai T (1972) On the free neuromasts of some Teleost larva. Japan J Ichthyol 19: 307–311
Mukai Y, Kobayashi H (1991) Morphological studies on the cupulae of free neuromasts along the growth of larvae in Cyprinid fish. Nippon Suisan Gakkaishi 57:1339–1346
Nakajima J (2006) Larval phototaxis of the pike gudgeon, Pseudogobio esocinus esocinus (Cyprinidae). Bull Hoshizaki Green Found 9:244
Nakajima J, Onikura N, Oikawa S (2008) Habitat of the pike gudgeon Pseudogobio esocinus esocinus in the Nakagawa River, northern Kyushu, Japan. Fish Sci 74:842–845
Nelson JS (2006) Fishes of the World, forth edition. John Wiley and Sons, New York
Nakamura M (1969) Cyprinid fishes of Japan. Spec Publ Res Inst Nat Resour no 4. Shigen Kagaku Kenkyusyo, Tokyo
Sado T, Kimura S (2005a) Developmental morphology of the cyprinid fish Chela dadiburjori. Ichthyol Res 52:20–26
Sado T, Kimura S (2005b) Developmental morphology of the cyprinid fish Horadandia atukorali. Ichthyol Res 52:152–157
Sado T, Kimura S (2005c) Developmental morphology of the cyprinid fish Tanichthys albonubes. Ichthyol Res 52: 386–391
Sado T, Kimura S (2006) Developmental morphology of the cyprinid fish Inlecypris auropurpureus. Ichthyol Res 53:34–40
Takeshita N, Kimura S (1998) Habitat change with growth of the yearlings of the cyprinid fish Hemibarbus barbus in the Chikugo River. J Natl Fish Univ 47:15–21
Tominaga K, Watanabe K, Kakioka R, Mori S, Jeon SR (2009) Two highly divergent mitochondrial DNA lineages within Pseudogobio esocinus populations in central Honshu, Japan. Ichthyol Res 56:195–199
Uchida K (1939) The fishes of Tyōsen (Korea) part 1: Nematognathi, Eventognathi. Bull Fish Exp Sta Gov Gen Tyōsen 6:1–458
Yang J, He S, Freyhof J, Witte K, Liu H (2006) The phylogenic relationships of the Gobioninae (Teleostei: Cyprinidae) informed from mitochondrial cytochrome b gene sequences. Hydrobiologia 553:255–266
Yue PQ (1998) Gobioninae. In: Chen YY (ed) Fauna Sinica, Osteichthyes, Cypriniforms II. Science Press, Beijing, pp 232–389
Acknowledgments
We thank S. Oikawa, S. Matsui, and K. Tominaga for invaluable advice and suggestions. We are indebted to A. Tawa for his cooperation in the deposition of specimens.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as 461946A1-3271-463D-BAA0-BAE2B4766F42.
This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number.
About this article
Cite this article
Nakajima, J., Onikura, N. Larval and juvenile development of Pike Gudgeon, Pseudogobio esocinus (Cyprinidae: Gobioninae). Ichthyol Res 62, 268–273 (2015). https://doi.org/10.1007/s10228-014-0436-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-014-0436-5