Abstract
In the present study, early morphological development of Erromyzon kalotaenia, a species of the Gastromyzontidae distributed in Guangxi, China, through the pre-incubation embryos to the juvenile stage was investigated. The eggs of E. kalotaenia were adhesive and demersal with egg membrane diameter 2.03 ± 0.04 mm. The yolk was white. At 24 °C, the embryo developed into a larva outside of the membrane at 45 hrs 28 min. The newly hatched larvae were 4.90 ± 0.14 mm in TL with 37 pairs of myomere. At the 30th day post-hatching, developments of fins were completed and then the juvenile stage was entered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Gastromyzontidae are a family of fish of the Cypriniformes formerly treated as a subfamily of the Balitoridae (Kottelat 2012a; Nelson et al. 2016). These two families can be easily distinguished based on the number of unbranched rays of the paired fins; the Gastromyzontidae have only one unbranched ray, whereas the Balitoridae exhibit more than one (Fang 1930). The gastromyzontids are small, highly diverse and widely distributed in streams in southern and southeastern Asia (Tan 2006; Dudgeon 2000; Yang and Dudgeon 2009). Their pectoral and pelvic fins have been modified into sucker organs for clinging to objects in fast-flowing streams (Hora 1932; Chen 1980). They are important bio-indicator species and are also popular in the aquarium trade (Tan 2006). However, no report has discussed gastromyzontid ontogeny, and the species currently sold in the aquarium market are mainly based on wild collection. (Huckstorf and Freyhof 2011a; Kong et al. 2008; Shao 2018).
Erromyzon kalotaenia Yang et al. 2012 is an ornamental fish endemic to the Liuding and Dishui Streams of the Guijiang River drainage in the Dayaoshan Mountain, Guangxi, China (Yang et al. 2012). Fish in the Dayaoshan Mountain, like freshwater fish elsewhere, are threatened by declining population and habitat degradation due to frequent desiccation, agricultural pollution, construction of hydropower dams and aquarium trade (Huckstorf and Freyhof 2011a, b; Huckstorf and Freyhof 2012; Kottelat 2012b; Kong et al. 2008; Shao 2018). Therefore, artificial propagation and habitat protection are needed to facilitate the conservation of E. kalotaenia.
The development of reproductive technology is crucial for artificial propagation, which can not only restore endangered species (Seo et al. 2010; Park et al. 2014), but also provide important references for research on early morphological development (Cheng et al. 2012). In addition, the distribution of melanocytes and the skeletal morphology of larva are an important basis for species classification and identification (Liu 2011; Cheng et al. 2012). However, previous studies on the early development and reproduction of gastromyzontids were limited to the Balitoridae (Xiong et al. 2008; Wu et al. 2011), and no research has been conducted on the Gastromyzontidae. To fill this gap, the present study is aimed to observe and record the development of pre-incubation embryos and the morphology from the post-hatching stage to the juvenile stage of E. kalotaenia. The results were then compared with species of the sister group Balitoridae (i.e., Lepturichthys fimbriata, Jinshaia sinensis and Sinogastromyzon szechuanensis) as well as with species of the Botiidae, Cobitidae and Nemacheilidae of the superfamily Cobitoidea.
Materials and methods
Parental fish and egg collection. Two glass aquariums with a volume of 54-L (60 × 30 × 30 cm) were used for reproduction. Each aquarium with water temperature at 25 ± 1 °C, comprised seven females (93–96 mm SL) (Fig. 1a) and eight male loaches (93–96 mm SL) (Fig. 1b) purchased from aquarium shops. In the aquariums, some round stones (up to 10 cm in diameter) and sand (about 5 cm thick) were placed in the bottom to mimic the habitat. The parental fish were fed once a day with artificial feed. Through July 15 to October 17, 2017, breeding behaviors of pairs of Erromyzon kalotaenia were observed in the aquariums, and 30 eggs were collected immediately by a plastic pipette after being laid.
Rearing. Eggs were transferred into a 15-L (30 × 20 × 25 cm) glass aquarium containing weakly aerated water and half of the water was changed every two days. Artificial feed and Artemia larvae were provided once a day to the larvae and juveniles since day 4 to 60 post-hatching. The incubation and rearing temperature was maintained at 25 ± 1 °C.
Observation and measurements. Observations and measurements were made on 30 eggs, 22 larvae, and four juveniles. All material, except for eggs, was preserved. Larvae and juveniles were sampled periodically from the rearing aquarium, being preserved in 10% buffered formalin solution. Some specimens were cleared and stained using alcian blue and alizarin red to observe fin ray formation. All eggs and larvae were observed using a compound microscope (Olympus CX31), and the morphology of each stage was recorded by a digital camera (Canon PowerShot G10). Observations and photographs were made on five eggs randomly taken from 30 eggs every 20 minutes, and the morphology of embryo development was drawn on a tracing pad with printed photos of eggs.
The sampling frequency of larvae followed Chen (2010). On the 1st to 7th days post-hatching (DPH), two individuals were collected every day; through the 8th to 15th DPH, two individuals were collected every other day; through the 16th to 30th DPH, two individuals were collected every five days; on the 60th DPH, two individuals were collected. Identification of the developmental stages followed Wu et al. (2011) and Xiong et al. (2008). Terminology of morphology followed Wu et al. (2011), Xiong et al. (2008), Yang et al. (2012) and Shao (2018). Measurement methods followed Sado and Kimura (2002) and Okiyama (1988).
Results
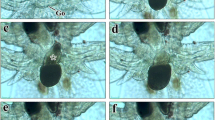
Spawning and eggs. Erromyzon kalotaenia spawned at the bottom of the water and had multiple spawning locations. During mating, the female and male breathe rapidly. The female stopped on a relatively round and flat stone, with the tail bent close to the stone. The male was side by side with the female, and the tail shook quickly to stimulate the female to lay eggs. When the eggs were laid, the male released semen at the same time. No parental care of eggs was observed. The eggs of E. kalotaenia weakly adhesive for a short period just after fertilization; demersal, almost spherical in shape. Egg membrane diameter was 2.03 ± 0.04 mm (n = 5). The yolk was white (Fig. 2). Embryonic development is shown in Table 1 and Fig. 3. At 24 °C, the embryo developed into larvae outside of the membrane at 45 hrs 28 min (n = 21) after fertilization.
Embryonic development of Erromyzon kalotaenia at 24 °C. (1) 16-cell; (2) morula; (3) early blastula; (4) middle blastula; (5) later blastula; (6) early gastrula; (7) middle gastrula; (8) later gastrula; (9) neurula; (10) closure of blastopore; (11) presence of myomere; (12) optic primordium; (13) optic vesicles; (14) olfactory lamellae; (15) tail bud; (16) auditory vesicle; (17) Kupffer’s vesicle; (18) presence of caudalfin; (19) presence of lentis; (20) beginning of motility; (21) heart pulsation; (22) newly hatched larva
Allometric growth. Growth of the larvae followed an exponential curve during the larval stages and was represented by the equation y = 0.0002x3 − 0.0121x2 + 0.4105x + 5.7175 (R2 = 0.9761, n = 28), where y is total length (TL) mm and x is DPH (Fig. 4). Four larval development stages were observed after hatching; yolk‐sac larvae, preflexion larvae, flexion larvae and postflexion larvae. The yolk sac has been completely consumed at the 7th DPH when TL was 8.65 ± 0.25 mm. Notochord has been flexed from the 9th to 11th DPH, between 8.73 ± 0.03 mm and 8.78 ± 0.17 mm TL. The finfold disappears completely and juvenile stage started at the 30th DPH with 12.02 ± 0.09 mm TL. All the meristic characters were completely developed at the 60th DPH with 21.03 ± 0.13 mm TL. Morphologies of four larval development stages were described as below.
Post-embryonic development. Body lengths of larvae and juveniles at each developmental stage are shown in Figs. 5 and 6.
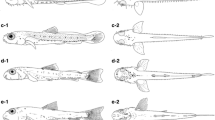
Larvae stage of Erromyzon kalotaenia. (a) Newly hatched larva, 4.90 ± 0.14 mm TL; (b-1) preflexion larva, 2nd DPH, 6.02 ± 0.12 mm TL; (b-2) preflexion larva, 3rd DPH, 7.45 ± 0.19 mm TL; (b-3) preflexion larva, 4th DPH, 7.58 ± 0.24 mm TL; (b-4) preflexion larva, 5th DPH, 8.1 ± 0.24 mm TL; (c-1) flexion larva, 6th DPH, 8.3 ± 0.22 mm TL; (c-2) flexion larva, 7th DPH, 8.65 ± 0.25 mm TL; (c-3) flexion larva, 9th DPH, 8.73 ± 0.03 mm TL; (c-4) flexion larva, 11th DPH, 8.78 ± 0.17 mm TL; (d-1) postflexion larva, 13th DPH, 8.98 ± 0.02 mm TL; (d-2) postflexion larva, 15th DPH, 9.06 ± 0.03 mm TL; (d-3) postflexion larva, 20th DPH, 9.83 ± 0.24 mm TL; (d-4) postflexion larva, 25th DPH, 10.79 ± 0.02 mm TL
Yolk sac larva.At 45 hrs 28 min after fertilization, entering the larval stage. Newly hatched larvae [4.90 ± 0.14 mm TL (n = 2)] with a transparent body without melanophores; eyes small (eye diameter 0.15 mm); 37 myomere; finfold around the tail, tail end round blunt; yolk sac very large, pear-shaped, front relatively large; pericardium cavity at the front end of the yolk sac, heart in the upper part of pericardium cavity, transparent, oval; auditory vesicle length slightly larger than eye diameter (Fig. 5a); at this stage, larvae without swimming ability and lying on the bottom of tank.
Preflexion larva. At the 2nd DPH (6.02 ± 0.12 mm TL, eye diameter 0.18 mm, n = 2) gape forming, but mouth not yet opened; gill filaments, operculum and pectoral-fin buds present, no external gill filaments observed through entire development; yolk sac becoming smaller, slightly concave in the middle; larvae swimming upward from time to time (Fig. 5b-1). At the 3rd DPH (7.45 ± 0.19 mm TL, eye diameter 0.25 mm, n = 2), melanocytes spreading widely throughout vertebrae junction, pericardium cavity, above the head kidney, auditory vesicle, top of head and front and rear edge of eye; inner edge of eye darkened; snout transforming from sub-prognathous to hypognathous; anus forming; 2 pairs of rostral barbels present; pectoral fins small, oval, extending behind the body (Fig. 5b-2). At the 4th DPH [7.58 ± 0.24 mm TL, eye diameter 0.25 mm (n = 2)], melanocytes on entire eye and upper edge of the yolk sac; dorsal finfold wide, slightly concave; rostral barbels becoming longer; yolk sac not exhausted, but start to feed on artificial feed (Fig. 5b-3); at this stage, larvae swimming for food and sheltering in the bottom gravel. At the 5th DPH [8.10 ± 0.24 mm TL, eye diameter 0.25 mm (n = 2)], pectoral fins larger, leaf- haped; dorsal finfold beginning to develop; melanocytes depositing in large amounts on head; head becoming longer; maxillary barbels present (Fig. 5b-4).
Flexion larva. At the 6th DPH [8.30 ± 0.22 mm TL, eye diameter 0.29 mm (n = 2)], melanocytes on front of yolk sac, a light-colored longitudinal band between nose and auditory vesicle; obvious depressions in dorsal finfold; caudal finfold forming, few melanocytes on fin rays (Fig. 5c-1); at this stage, larvae swimming around in the aquarium and swimming ability stronger. At the 7th DPH [8.65 ± 0.25 mm TL, eye diameter 0.29 mm (n = 2)], many cone papillae on maxillary barbels; pectoral fin rays and dorsal fin rays present, dorsal fin with 6 rays; a large number of melanocytes on caudal fin; yolk exhausted and completely feeding on exogenous nutrients (Fig. 5c-2). At the 9th DPH [8.73 ± 0.03 mm TL, eye diameter 0.29 mm (n = 2)], many scattered melanocyte clusters under eyes; pectoral fins wide; dorsal fin with 6 rays; anal finfold sagging slightly, anal fin with 5 rays; pelvic-fin buds present (Fig. 5c-3). At the 11th DPH [8.78 ± 0.17 mm, eye diameter 0.29 mm (n = 2)], dorsal finfold narrowed, dorsal fin with 8 rays; anal finfold obviously concave, anal fin with 5 rays; caudal fin with 15 rays; pelvic fins with 2 rays (Fig. 5c-4).
Postflexion larva. At the 13th DPH [8.98 ± 0.02 mm TL, eye diameter 0.29 mm (n = 2)], pectoral fins beginning to extend below the body; dorsal fin with 8 rays; anal finfold narrowed, anal fin with 5 rays; pelvic fins separated from the finfold, with 5 rays; caudal fin with 16 rays; starting to prey (Fig. 5d-1); at this stage, larvae sucking ability and adaptation to water velocity poor. At the 15th DPH [9.06 ± 0.03 mm TL, eye diameter 0.29 mm (n = 2)], dorsal fin separated from the finfold, with 8 rays; anal finfold narrowed, anal fin with 5 rays; pelvic fins with 5 rays; caudal fin with 16 rays (Fig. 5d-2). At the 20th DPH [9.83 ± 0.24 mm TL, eye diameter 0.29 mm (n = 2)], larvae reddish-brown, a light-colored longitudinal band at caudal peduncle, a reddish-brown pattern on the longitudinal band behind the eyes; base of caudal peduncle to caudal fin yellowish, a large black round spot on center of caudal peduncle base; a small black spot on front edge of dorsal fin base; longest rostral barbels not exceeding posterior edge of eye; belly flat; pectoral and pelvic fins laterally expanded; dorsal finfold shrinking, dorsal fin with 8 rays; anal fin separated from the finfold, with 6 rays; pelvic fins with 6 rays; caudal fin still connecting to dorsal and caudal finfold, fan shaped, with 16 rays; pectoral fins downward (Fig. 5d-3); at this stage, larvae with strong sucking ability to adapt to the torrent environment. At the 25th DPH [10.79 ± 0.02 mm TL, eye diameter 0.29 mm (n = 2)], dorsal fin with 8 rays; anal finfold narrowed, anal fin separated from finfold, with 6 rays; pelvic fins with 7 rays; caudal fin still connecting to finfold, with 16 rays; pectoral fins with 9 rays (Fig. 5d-4).
Juvenile stage. At the 30th DPH [12.02 ± 0.09 mm TL (n = 2)], finfold completely disappeared, fin rays begin to branch; pectoral fins with 13 rays; dorsal fin with i-8 rays; anal fin with 6 rays; pelvic fins with i-6 rays; caudal fin with 16 rays (Fig. 6a). At the 60th DPH [21.03 ± 0.13 mm (n = 2)], morphology almost the same as that of adult fish; pectoral fins with i-17 rays; dorsal fin with ii-8 rays; anal fin with ii-5 rays; pelvic fins with i-8 rays; caudal fin concave, forked into upper and lower leaves, with 16 rays (Fig. 6b).
Discussion
Some gastromyzontids have been evaluated as threatened species by IUCN (Huckstorf and Freyhof 2011a, b). This is the first study to present breeding and developmental records of the Gastromyzontidae. The results may serve as an important reference for future studies of related organisms. In the present study, the egg types of 18 species of five families of the Cobitoidea are summarized (Table 2). The demersal eggs of Erromyzon kalotaenia are weakly viscous and similar to those of most species of the Cobitoidea, except for the Botiidae and two species of the Balitoridae (i.e., Jinshaia sinensis and Lepturichthys fimbriata) that produce drifting and non-viscous eggs (Yang 2004; Liang et al. 1999; Xiong et al. 2008; Tang et al. 2010; Yue et al. 2011; Liu et al. 2013; He et al. 2014).
Xiong et al. (2008) indicated that demersal and drifting eggs may be associated with high and low ability to resist water flow, respectively. Our summary also showed that most demersal egg membranes were relatively small (0.97-2.03 mm), while drifting egg membranes were relatively large (1.60-5.34 mm; Table 2). The difference in size is because drifting eggs absorb water and swell, and Xiong et al. (2008) considered that the swollen membrane may facilitate the drift of eggs.
Newly hatched larvae with small eye diameters are common in species with well-developed rostral barbels, indicating that the newly hatched larvae are not highly dependent on vision in their early developmental stages. Other sensory organs may be adopted by some species to perceive changes in the external environment (Wikramanayake 1990), such as Triplophysa orientalis (Wang et al. 2017), Lepidocephalus thermalis, and Garra lamta (Wikramanayake 1990). In our comparison (Table 3), eye diameter was correlated with the DPH of barbel appearance; the newly hatched larvae of L. fimbriata had the smallest eye diameter (0.09 mm, 1.44% of TL) and the earliest presence of barbels (2nd DPH); barbels appeared at the 3rd DPH for Sinogastromyzon szechuanensis and E. kalotaenia, which exhibited eye diameters of 0.11 mm (2.51% of TL) and 0.15 mm (3.06% of TL), respectively. Smaller eyes and early-presence barbels may support the hypothesis of Wikramanayake (1990). However, the information of eye diameter at hatching and the DPH of barbel presence are not provided in the references cited herein except for Xiong et al. (2008) and Wu et al. (2011). More studies on these two factors are needed to substantiate the hypothesis.
References
Chen S, Song Y, Niu Y, Liu J, Xie C, Ren D, Fan Z (2015a) Embryonic and post-embryonic development of Triplophysa (Hedinichthys) yarkandensis (Day). J Fish Sci China 22:597–607
Chen XS, Chen J, Deng SH, Li C, Li XM, Wan YF, Tan ZL, Mao M, Yao L, He XF (2015b) Research on artificial breeding and fry cultivation of Triplophysa xichangensis. Mod Agric Sci Technol 3:287–288
Chen YT (2010) Studies on early skeletal development in larvae and juvenile of Epinephelus coioides and E. lanceolatus. Master thesis, Department of Aquaculture, National Taiwan Ocean University, Keelung, Taiwan
Chen YY (1980) Systematic studies on the fishes of the family Homalopteridae of China III. Phyletic studies of the Homalopterid fishes. Acta Zootax Sin 5:200–211
Cheng MJ, Jiang YY, Ho YS, Chang WB, Perng JJ, Chen WY (2012) Early osteological development of the spine-cheek anemonefish (Premnas biaculeatus) in Taiwan. J Taiwan Fish Res 20:37–48
Dudgeon D (2000) The ecology of tropical Asian rivers and streams in relation to biodiversity conservation. Annu Rev Ecol Syst 31:239–269
Fang PW (1930) New and inadequately known homalopterin loaches of China: with a rearrangement and revision of the generic characters of Gastromyzon, Sinogastromyzon and their related genera. Contr Biol Lab Sci Soc China 6:25–43
Geng LW, Jiang HF, Tong GX, Wu PF, Xu W, Li CY (2015) Study on the artificial propagation of Triplophysa dalaica. China J Fish 28:15–18
He B, Chen XJ, Wen T, Long ZH, Li MJ (2014) Embryonic development of Sinibotia superciliaris. Southwest China J Agri Sci 27:1332–1337
Hora SL (1932) Classification, bionomics and evolution of homalopterid fishes. Mem Ind Mus 12:263–330
Hu T, Liu S, Lian Q, Wang Y, Li Q, Wu J (2012) Study on developmental and morphological characters in Misgurnus anguillicaudatus larvae. China Agri Sci Bull 28:132–138
Huckstorf V, Freyhof J (2011a) Sewellia albisuera. The IUCN Red List of Threatened Species 2011: e.T187929A8638768. http://dx.doi.org/10.2305/IUCN.UK.2011-1.RLTS.T187929A8638768.en. Accessed 23 June 2018
Huckstorf V, Freyhof J (2011b) Sewellia marmorata. The IUCN Red List of Threatened Species 2011: e.T188036A8641699. https://dx.doi.org/10.2305/IUCN.UK.2011-1.RLTS.T188036A8641699.en. Accessed 23 June 2018
Huckstorf V, Freyhof J (2012) Beaufortia leveretti. The IUCN Red List of Threatened Species 2012: e.T166907A1150567. https://dx.doi.org/10.2305/IUCN.UK.2012-1.RLTS.T166907A1150567.en. Accessed 07 February 2020
Kong DP, Chan BPL, Yang JX (2008) Threatened fishes of the world: Protomyzon pachychilus Chen, 1980 (Balitoridae). Environ Biol Fishes 83:243–244
Kottelat M (2012a) Conspectus Cobitidum: an inventory of the loaches of the world (Teleostei: Cypriniformes: Cobitoidei). Raffles Bull Zool 91:1–199
Kottelat M (2012b) Annamia normani. The IUCN Red List of Threatened Species 2012: e.T180927A1678901. http://dx.doi.org/10.2305/IUCN.UK.2012-1.RLTS.T180927A1678901.en. Accessed 23 April 2018
Li ZL, Yan TM (2009) Morphology development of embryos and larvae of Trilophysa bleekeri. Acta Hydrobiol Sin 33:636–642
Liang YQ, Hu XJ, Huang DM, Lin ZY, Xie CG (1999) Observations on the embryonic development of Leptobotia elorvae. Acta Hydrobiol Sin 23:631–635
Liang ZS, Lian JY, Chen C, Li ZJ, Lin JH, Zhang JY (1998) Embryo development and fish culture of Paramisgurnus dabryanus. Acta Hydrobiol Sin 12:27–42
Liu CH (2011) Early osteological development of the yellow tail Seriola dumerili (Pisces: Carangidae). Zool Stud 40:289–298
Liu SW, Yang JX, Chen XY (2013) Discovery and its significance of spawning grounds of Jinshaia sinensis from upper and middle Jinshajiang River. Zool Res 34:626–630
Lu ZM, Li XZ (2009) Artificial reproduction and embryo development of Misgurnus anguillicaudatus. J Huangshan Univ 11:62–64
Nelson JS, Grande TC, Wilson MVH (2016) Fishes of the world, 5th edn. John Wiley and Sons, Inc. Hoboken, New Jersey
Okiyama M (1988) An atlas of the early stage fishes in Japan. Tokai Univ Press, Tokyo
Park JM, Kim NR, Han KH, Han JH, Son MH, and Cho JK (2014) Spawning behavior, egg development, larvae and juvenile morphology of Hyphessobrycon eques (Pisces: Characidae) characidae fishes. Dev Reprod 18:241–249
Sado T, Kimura S (2002) Developmental morphology of the cyprinid fish, Candidia barbatus. Ichthyol Res 49:350–354
Seo WI, Yoo DJ, Byun SG, Kim YC, Lee SH, Yeon IH, Yim HS, Lee BI (2010) Spawning behavior and early life history of endangered Cottus hangiongensis. Korean J Fish Aquat Sci 43:46–53
Shao KT (2018) Taiwan Fish Database WWW Web electronic publication. http://fishdb.sinica.edu.tw. Accessed 23 April 2018
Tan H H (2006) The Borneo suckers: revision of the torrent loaches of Borneo (Balitoridae: Gastromyzon, Neogastromyzon). Natural History Publications (Borneo). Kota Kinabalu.
Tang YG, Xu DB, Wang YN, Hong B, Fang CY, Meng Y, Hu JH, Rong ZZ, Zu GZ (2010) Artificial breeding preliminary test of Leptobotia taeniops. China Fish Mon 1:41–43
Wang B, Yao Y, Wang Z (2008) Embryo development of Paracobitis potanini. Freshw Fish 38:70–73
Wang F, Yang R, Fan Q (2017) Embryo and post-embryonic development of Triplophysa orientalis (Herzenstein). J Huazhong Agric Univ 36:69–98
Wang H, Guo Y, Zuo L, Mi J, Xiong Y, AW (2009) Embryonic and larval of development of Triplophysa angeli. Fish Sci 28:721–725
Wikramanayake ED (1990) Ecomorphology and biogeography of a tropical stream fish assemblage: evolution of assemblage structure. Ecology 71:1756–1764
Wu JM, Wang QQ, Liu F, Liu CC, Wang JW (2011) Early development of Sinogastromyzon szechuanensis in the Chishui River. Sichuan J Zool 30:527–529
Xiong YY, Qiao W, Liu HZ, Tan DQ (2008) Early development of Lepturichthys fimbriata. Acta Hydrobiol Sin 32:424–433
Yang GY, Dudgeon D (2009) Seasonal and inter-stream variations in the population dynamics, growth and secondary production of an algivorous fish (Pseudogastromyzon myersi: Balitoridae) in Monsoonal Hong Kong. Freshw Biol 54:1960–1976
Yang J, Kottelat M, Yang JX, Chen XY (2012) Yaoshania and Erromyzon kalotaenia, a new genus and a new species of balitorid loaches from Guangxi, China (Teleostei: Cypriniformes). Zootaxa 3586:173–186
Yang MS (2004) Observation on the embryonic development of Parabotia fasciata. Freshw Fish 34:34–36
Yue XJ, Wang F, Xie BW, Zhai QM, Wang Y, He B, Chen XJ (2011) Embryonic development of Sinibotia reevesae in Tuojian River. Sichuan J Zool 30:390–393
Acknowledgments
Data on this paper is from the dissertation of the first author in the partial fulfillment of the requirements for a master's degree at the Department and Graduate Institute of Aquaculture, National Kaohsiung University of Science and Technology, Taiwan. The authors would like to thank Crazy Aqua Studio trader, AquaMonsters trader and Creative Way Aqua for their help in the collection of fish, and greatly appreciate Sun, X-Y (YuanShan ornamental fish farm) for assistance in the rearing experiment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Jhuang, WC., Chiu, KH. & Liao, TY. Early morphological development of Erromyzon kalotaenia (Teleostei: Gastromyzontidae). Ichthyol Res 68, 303–311 (2021). https://doi.org/10.1007/s10228-020-00791-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-020-00791-1