Abstract
New and emerging advances in colorectal cancer (CRC) treatment combined with limited healthcare resources highlight the need for detailed decision-analytic models to evaluate costs, survival and quality-adjusted life years. The objectives of this article were to estimate the expected lifetime treatment cost of CRC for an average 70-year-old patient and to test the applicability and flexibility of a model in predicting survival and costs of changing treatment scenarios. The analyses were based on a validated semi-Markov model using data from a Norwegian observational study (2049 CRC patients) to estimate transition probabilities and the proportion resected. In addition, inputs from the Norwegian Patient Registry, guidelines, literature, and expert opinions were used to estimate resource use. We found that the expected lifetime treatment cost for a 70-year-old CRC patient was €47,300 (CRC stage I €26,630, II €38,130, III €56,800, and IV €69,890). Altered use of palliative chemotherapy would increase the costs by up to 29%. A 5% point reduction in recurrence rate for stages I–III would reduce the costs by 5.3% and increase overall survival by 8.2 months. Given the Norwegian willingness to pay threshold per QALY gained, society’s willingness to pay for interventions that could result in such a reduction was on average €28,540 per CRC patient. The life years gained by CRC treatment were 6.05 years. The overall CRC treatment costs appear to be low compared to the health gain, and the use of palliative chemotherapy can have a major impact on cost. The model was found to be flexible and applicable for estimating the cost and survival of several CRC treatment scenarios.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cancer is a major cause of morbidity and mortality in the Western world, with colorectal cancer (CRC) being the second most common cancer in women and the third most common cancer in men [1]. Norway is among the countries in the world with the highest incidence of CRC [2]. As the number of CRC cases increases with an ageing population, and new costly drugs are launched in the market, we expect a substantial increase in the cost of CRC treatment. For healthcare providers making decisions regarding reimbursement, it is important to consider the cost-effectiveness of preventive and treatment alternatives to optimise resource allocation.
Decision-analytic models are useful to achieve optimal allocation of resources, because these models can (1) provide information about the burden of diseases, (2) within a certain disease identify treatment strategies with potential health gains, and (3) evaluate the cost effectiveness of new treatment options (Table 1). Regarding (1), decision-analytic models can be used in comparative cost-of-illness studies, which compare the cost of CRC treatment with the cost of treating other diseases [3]. Regarding (2), for new ideas and innovations in surgery, chemotherapy, screening, and primary prevention, a decision-analytic model is useful for exploring the potential incremental cost and incremental health gain (reduced mortality, recurrence rate, and health-related quality of life). Based on these estimates and the willingness to pay (WTP) threshold (the value for an incremental health gain), we can identify the maximum acceptable amount to invest in these interventions. Furthermore, results from such explorative analyses can be used to evaluate budget impacts for the healthcare sector [4]. Regarding (3), decision-analytic models are useful when estimating the cost effectiveness of single or combined interventions both within and between intervention strategies such as surgery, chemotherapy, and screening.
The first objective of this study was to estimate the expected lifetime cost and survival of CRC treatment for an average 70-year-old CRC patient based on a general, validated decision-analytic model [5]. The second objective was to explore the applicability and flexibility of the model by performing several analyses of changing CRC treatment strategies, the consequences of increasing the number of patients receiving palliative chemotherapy (including antibodies), the consequences of decreasing the recurrence rate, and the effect of diagnosing CRC at an earlier stage (by screening or other measures). With these two objectives, we explored the general properties of the CRC decision-analytic model and how it contributed at all three levels as shown in Table 1.
Methods
We applied the perspective of the healthcare sector and included costs of diagnostic and staging investigations, surgery (major resection and palliative surgery without resection), treatment for complications, preoperative (neoadjuvant) and postoperative (adjuvant) treatment, follow-up, curative treatment of recurrence, palliative treatment of recurrence and primarily non-resectable disease, and visits to general practitioners. We measured the health outcomes in both life years (LYs) and quality-adjusted life years (QALYs).
The model
In brief, the costs and survival in this paper were estimated based on a semi-Markov model, details of which were published in [5]. The flow of CRC patients was simulated in the model from CRC diagnosis at the age of 70 years through periods of treatment and healthy periods until the patients were 100 years of age or had died from CRC or other causes (Fig. 1). Each arrow reflected the probability of an average CRC patient moving from one health state to another during one cycle or maintaining in the same health state (follow the loops). The patient entered the model at the time of primary diagnosis in one of the TNM stages (I, II, III or IV), and the first step included the costs of primary work-up and treatment during the first year after diagnosis. The following year, any patient who received curative treatment moved to the health state “disease free”, which means that the tumour had been resected and that there was no evidence of macro- or microscopic residual tumour (R0-resection)—locoregionally and no radiological evidence of distant metastases. Alternatively, the patient was not curable at the time of diagnosis (non-resectable disease) and moved to the palliative health state or experienced recurrence after an apparently curative resection or died of CRC or other causes. From ‘disease free’, the patients could die of other causes or move to one of the three recurrence states. The majority of patients entering one of the three recurrence states (local and/or distant recurrence) received palliative chemotherapy. Some patients underwent resection with curative intent, often combined with (neo)adjuvant (radio)chemotherapy, and some received only best supportive care. The probabilities of receiving the treatments depended on the type of recurrence.

Reproduced from [5] with kind permission from Sage publishers
Illustration of how the patient can move from one state to another in the model.
For the majority of the patients in stage IV, the intent of treatment was palliation. Patients not eligible for any specific anti-cancer therapy, due to old age or poor general health, received supportive care until entering ‘Dead by CRC’. Palliative treatment mainly consisted of chemotherapy and/or targeted therapy (antibodies), but a small proportion was also offered radiotherapy. The treatment algorithm for first, second, and third lines of palliative chemotherapy is illustrated in Fig. 2, which is a sub-model of the model in Fig. 1. The treatment depended on age and health status (fragile), and there were several treatment options in each treatment line. When the disease progressed during the initial palliative drug treatment (1st line), a new treatment was usually offered (second line), and when the patient experienced additional progression, a third line of treatment could be offered [6, 7]. FLIRI is a combination of irinotecan and 5-fluorouracil/folinic acid (5-FU/FA), the latter of which was based on a Nordic protocol (Nordic FLv). FLOX is a therapeutic combination of oxaliplatin and 5-FU/FA. The figures at each arrow in Fig. 2 indicate the conditional probability, and the figures in brackets express the joint (total) probability of receiving a certain type of treatment [5]. For each treatment in the decision tree, separate cost models were developed that included the costs of medication, CT scanning, complications, and treatment by nurses, pharmacists, and medical practitioners. The model was adjusted for non-compliance and discontinuation of chemotherapy, and this decision tree (Fig. 2) was the basis for estimating the average cost of palliative treatment.

Reproduced from [5] with kind permission from Sage publishers
The decision tree for palliative chemotherapy. Conditional probabilities without brackets. The numbers in brackets show the probabilities of patients receiving the treatment in the box given that the patients receive some kind of palliative treatment. 5-FU/FA Nordic FLv = 5-fluorouracil/folinic acid, EGFR-inh epidermal growth factor receptor inhibitors (cetuximab/panitumumab), FLIRI a combination of irinotecan and 5-FU/FA, FLOX a combination of oxaliplatin and 5-FU/FA, PS patient performance status.
In the model, the duration of one cycle was set to 1 year, and for each health state, there was a cost model estimating the cost of the health service provided per person per year. We estimated the total CRC cost and the survival of an average CRC patient diagnosed at the age of 70 years. Survival and QALYs were half-cycle corrected. For costs, standard half-cycle corrections were not modelled, but were modelled indirectly using empirical data to estimate CRC treatment cost considering compliance and mortality. Time dependency in the calculation of probabilities of recurrence and death was captured in the model by including tunnel states.
Data and data sources
We used Norwegian population-based data when possible. Transition probabilities were based on an observational study including 2049 patients diagnosed with CRC from 1993 to 2010 at Oslo University Hospital (referred to as OUS data) [8, 9]. The sample was population-based, and their ages correspond to CRC patients in general. The OUS data were also used to identify those treated with resection during primary treatment. Information from the Norwegian Patient Registry (referred to as NPR data) from 2003 to 2004, previously used in Aas et al. [10], was used to quantify hospital treatments, except primary surgical treatment, including hospital stays for complications and metastatic surgical treatment. The cost estimates from the NPR were average numbers and not adjusted for age. Radiotherapy and chemotherapy (both adjuvant and palliative) were based on treatment guidelines and expert opinions. Other data sources were national life tables, internationally published papers, and expert opinions (three co-authors—one surgeon, one oncologist, and one gastroenterologist). For complementary information about the assumptions and data used for the analyses not presented in the paper, see Online Resource 1.
We used the individual-level OUS data to estimate rates of recurrence, disease-free survival, and overall survival. We controlled for age and gender in the estimations, and for the model, we predicted the rates for a 70-year-old CRC patient [5].
The cost inputs for the treatments provided during the first year are presented in Online Resource 1, and the cost input of palliative chemotherapy is shown in Table 9 in Appendix 1 of Joranger et al. [5].
The probability of receiving an R0-resection after recurrence and all the conditional probabilities on the right side of squares A and Q in Fig. 2 were based on expert opinion [5]. To estimate QALYs, we assumed that the health-related quality of life (HRQoL) for patients with CRC and those without CRC was 0.74 and 0.80, respectively [11, 12].
We applied a 4% discount rate for costs, overall survival, and QALYs. In addition, we ran a separate analysis with zero discounting for overall survival [13, 14]. All cost were estimated in euros (€1 = NOK 7.79) and adjusted to 2016 euros using the consumer price index (2.62% for the period 2011–2016).
The Norwegian guidelines for economic evaluation of health interventions [13] recommend using NOK 500,000 per life year in full health (1 QALY) for analyses across sectors. Adjusted for inflation (2.34% yearly) [15], the Norwegian WTP threshold per QALY gained was then calculated to be €82,800 in 2016 euros.
This value was also used as a proxy for the WTP for a life year gained.
Estimation of costs and cost-effectiveness
The estimation of the total treatment cost (output) was mainly based on the CRC stage at the time of diagnosis, the recurrence rate for each stage, the type of recurrence, the probability of re-recurrence, the probability of receiving palliative chemotherapy, the probability of receiving certain kinds of palliative chemotherapy, the distribution between colon and rectal cancer in the population at different stages, the compliance when following up and completing chemotherapy, and the survival time.
For the analysis on changing treatment regarding chemotherapy and screening, we estimated the incremental cost, and for analysing the effect of reduced recurrence rate and the cost effectiveness of overall CRC treatment, we used the incremental cost-effectiveness ratio (ICER), which is defined by the differences in costs relative to differences in health outcomes.
The total cost of CRC treatment and the cost-effectiveness of overall CRC treatment was estimated by comparing the treatment for an average 70-year-old CRC patient (defined as the “base case”) to a population without CRC. For all the analyses of changes in treatment, the changes were compared with the base case.
Validation and uncertainty analysis
The model has been validated by [5] for face, internal, cross, and external validity. The external validation for relative survival was based on data from The Cancer Registry of Norway. The validation concluded that a satisfactory match was found with other models and real-life observations for both costs and survival time without any preceding calibration of the model. Because the model was partly based on data from 1993 to 2010, the validation was also done against observations and models based on data from the same time period.
We used one-way and multi-way sensitivity analyses to explore parameter-, methodological-, and model-structure uncertainty. To explore the total uncertainty concerning the use of expert opinion, we used probabilistic sensitivity analysis (PSA). In the PSA, we gave beta distributions to all the parameters based on expert opinions and assumed that the upper level of the 95% confidence interval was + 30% of the expected value and that the lower was − 30%. To explore the uncertainty in the estimation of survival for untreated patients in “Productivity in CRC treatment”, both deterministic analysis and PSAs were used.
Results
Base case cost and survival
Costs according to disease stage
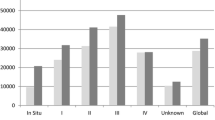
From a healthcare perspective, the total expected lifetime CRC costs and loss of life years (LYs) were reported for an average 70-year-old CRC patient according to the disease stage at the time of diagnosis (Table 2). Based on our model, a 70-year-old CRC patient had an expected lifetime CRC cost of €47,300. The expected costs increased with TNM stage as follows: stage I, €26,630; stage II, €38,130; stage III, €56,800; and stage IV, €69,890.
Type and phase of treatment
The treatments with the greatest impact on total lifetime costs (Table 2) were surgery of the primary tumour (€20,390) and palliative chemotherapy (€10,920). Costs related to diagnostic examinations, adjuvant treatment, and follow-up in general were modest for all stages. For stage IV, the main costs were “surgery—major resection” (primary tumour) (€19,230), “surgery—other” (€21,660), and “palliative chemotherapy” (€25,260). The palliative chemotherapy cost estimates were for the average patient that started with some kind of palliative chemotherapy treatment, and their treatment is shown in Fig. 2. “Surgery—major resection” was the major cost component for stages I and II. Variations between stages depended on differences in treatment, the mix of colon and rectum cases, and the proportion of patients experiencing cancer recurrence.
When we categorised treatment costs according to clinical pathway, starting with primary examinations and ending with palliative chemotherapy (Table 2), expected lifetime costs varied according to TNM stage at the time of diagnosis. Patients in stage IV had the highest expected costs both for primary treatment (€42,050) and palliative treatment (€25,260), while patients in stage III had the highest expected costs of treatment for recurrence (€6360).
The expected treatment cost of only the chemotherapy for the group of CRC patients receiving some kind of palliative chemotherapy was on average €40,850 per patient. This was estimated by multiplying the probability (in parentheses) of receiving the various treatment options shown in Fig. 2 with the costs of the respective chemotherapy regimens given in Table 1 in Online Resource 2. These estimates were then summarised to provide the expected total costs for these various treatments. Of this, epidermal growth factor receptor inhibitors (EGFR-inh) such as cetuximab/panitumumab and the related third line treatment with irinotecan in Fig. 2 jointly constituted 36.0% of the costs (equal to the sum of the third line scenarios in Table 2 in Online Resource 2), and bevacizumab and the related treatment FLIRI/FLOX jointly (both branch C, D and F, G in Fig. 2) constituted 34% of the costs (equal to the sum of 27.5%, 3%, 3%, 0.3% in Table 2 in Online Resource 2). Table 3 in Online Resource 2 shows the total treatment cost per patient when receiving all the chemotherapy treatments in one separate scenario/branch defined in Fig. 2. Costs were estimated for seven different branches, where the most expensive branches (C, D, and E in Fig. 2) cost €97,000 euro. Of the total cost of palliative CRC chemotherapy, this branch generated 40.7% of the cost when we adjusted for which treatment the patients actually received and the proportion of patients who did not undergo all treatments (equal to the sum of the first row in Table 2 in Online Resource 2).
Recurrence and palliative chemotherapy
Variations in treatment costs for a patient could also be estimated according to certain low- and high-cost treatment pathways. In the low-cost treatment pathway (stage I patients), we included the following cost components: (1) diagnostics, (2) resections without complications, and (3) 5-year follow-up. In the high-cost estimate (patients with recurrence), we included (1) diagnostics (2) treatment costs in the first year, (3) 1-year follow-up, (4) 1-year treatment for recurrence in the second year after being diagnosed with CRC, (5) 1-year follow-up after recurrence for those who achieved R0, and (6) palliative chemotherapy at the end of the second year, at the end of the third year, and at the end of the fourth year. The combination of palliative chemotherapy included in the high-cost treatment pathway was bevacizumab + FLIRI in the first line, FLOX in the second line, and EGFR-inh + irinotecan in the third line.
The expected costs for a low-cost treatment pathway (stage I without recurrence) were estimated to be €16,450, and the expected costs for a high-cost treatment pathway (with recurrence) were €125,830 and €142,540 for patients in stages I and IV, respectively (Table 2).
Survival, QALYs, and years lost
According to the model, the life expectancy for a CRC patient diagnosed at the age of 70 years was 9.3 years (7.0 years with discounting), implying a loss of 6.3 years (4.1 years discounted) compared to an average 70-year-old Norwegian (Table 2). The loss of discounted QALYs was 3.7 on average and 1.4, 2.6, 3.8, and 7.9 for stage I, II, III, and IV, respectively. Based on the model, we found that life expectancy was 14.0 years (1.6 years lost) for a patient in stage I and 1.5 years (14.1 years lost) for a patient in stage IV.
Uncertainty
According to the deterministic sensitivity analysis, for most input parameters, the model was insensitive to a 20% change. The expected total costs were most sensitive to changes in frequency of surgery and the use of bevacizumab in palliative treatment (see Online Resource 3).
We performed a PSA to simultaneously account for all uncertainty caused by parameters based on expert opinion and found that the 95% credible interval (CrI) for the total costs was ± 3% of the mean, and for the effect on life expectancy the 95% CrI was ± 0.5% of the mean (see Online Resource 3).
Changing treatment strategies
Scenarios of palliative chemotherapy
When health authorities estimate the costs of introducing new and costly drugs, such as EGFR-inh or bevacizumab, they may assume that 100% of the CRC patients will receive the treatment. Our analyses considered that these drugs were only relevant to subgroups of CRC patients [16, 17]. We assumed that 61% of all 70-year-old patients diagnosed at stage IV, or experiencing recurrence after R0 resection, would receive palliative chemotherapy [17]. The different treatment paths and related probabilities are shown in Fig. 2, and costs per treatment are shown in Table 1 in Online Resource 2. To account for higher compliance, we estimated the cost per patient (undiscounted) when fully treated according to the defined palliative chemotherapy scenarios compared to no palliative treatment (see Fig. 2 and Online Resource 2). The cost difference between the full treatment scenario “5-FU/FA (1st line) and EGFR-inh + irinotecan (2nd line)” (Q, R in Fig. 2) (€52,030) and the scenario “bevacizumab and FLIRI (1st line), FLOX (2nd line), and EGFR-inh + irinotecan (3rd line)” (C, D, E) (€97,000), which represents the strategy with bevacizumab, was €44,970 (see Table 3 in Online Resource 2). Furthermore, we found that using “bevacizumab and FLIRI” (C) rather than only “FLIRI” (J) in the first line would have an extra cost of €33,030 (see Table 3 in Online Resource 2).
Alternative chemotherapy schedules (protocols)—impact on costs
To show the importance of uncertainty in the input data and the possible impact of future decisions, we estimated the effect of changes in both prices and probabilities (see Table 1 in Online Resource 4). The use of bevacizumab varies between different countries. In the model, we assumed that 29% of patients receiving palliative chemotherapy were treated with this drug. We estimated the cost difference from the base case for the following new scenarios (treatment changes 1–4 in Table 3):
- 1.
All patients who receive palliative chemotherapy are treated with bevacizumab (all patients move through box B in Fig. 2).
- 2.
No patients receive bevacizumab (all patients going through box B in the base case move instead through I in Fig. 2).
- 3.
Patients receiving combination chemotherapy (FLIRI/FLOX) as the first line of treatment in the base case instead receive bevacizumab and FLIRI/FLOX (all patients who move through I in the base case move instead through B).
- 4.
The price of bevacizumab is reduced by 50%.
The treatment alternatives most sensitive to changes in treatment costs were the “EGFR-inh (cetuximab/panitumumab) + irinotecan treatment” and “bevacizumab + FLIRI treatment” (see Table 3). If we e.g. assume alternative 1 in Table 3 (All patients getting palliative chemotherapy receive bevacizumab), the expected total costs for a CRC patient would increase by 14%. This change in treatment strategy would thus increase the treatment costs in Norway by €27.8 million per year (assuming 4268 diagnosed CRC patients per year) and by €5.3 per capita per year. If bevacizumab was not offered (FLOX and FLIRI was used without bevacizumab, alternative 2), the expected total costs would decrease by 5% compared to the current strategy, and the Norwegian health sector’s expenditure would decrease by €10.9 million (€2.1 per capita). If those receiving “FLIRI/FLOX” as a first line of treatment were instead to receive “bevacizumab + FLIRI/FLOX” (alternative 3), then the costs would increase by 8% per patient and increase the health sector’s expenditure in Norway by €16.3 million (€3.1 per capita).
Increased use of chemotherapy in the elderly
CRC is common in elderly patients, and approximately 40% of CRC patients are 75 years of age or older. What then would be the effect on CRC costs of treating a greater number of elderly patients with palliative chemotherapy? One extreme scenario would be to assume that all patients would receive palliative chemotherapy. We estimated the change from the base case by analysing the following scenarios (treatment change 5–8 in Table 3):
- 5.
All patients who are not disease free after treatment receive palliative chemotherapy (more patients move into the sub-model illustrated by Fig. 2).
- 6.
Given we are in scenario 5 above, all patients in this scenario receive bevacizumab as a first line of treatment (all patients receiving palliative chemotherapy move through box B in Fig. 2).
- 7.
Ten percentage points compared to base case move from 5FU/FA-treatment (often elderly patients) to combination chemotherapy with bevacizumab (10% points move from box P to B).
- 8.
Ten percent more CRC patients receive palliative chemotherapy among those diagnosed with stage IV or recurrence.
One extreme scenario above is number 5—all patients who are not disease free after treatment would receive palliative chemotherapy regardless of age and general health. Based on the current pattern of chemotherapy prescription, the costs for an average CRC patient would increase by 9% (Table 3). If all patients received “bevacizumab as a 1st line of treatment” (scenario 6), the expected total costs would increase by 29%, and the health sector’s expenditure would increase by €58.2 million (€11.1 per capita).
Reduced recurrence rate
Recurrence of cancer implies more treatment and greater loss of life expectancy. We assumed a 5% point reduction in the 10-year recurrence rate from 32.5% (base case) to 27.5% for stages I–III. To achieve this, we used the same percentage reduction in the transition probabilities moving patients from the state of ‘disease free’ to the state of recurrence for all years and for all three stages. All other inputs were as in the base case. Reduced recurrence rate reduced the treatment costs because of fewer surgeries and other treatments for recurrence, less palliative treatment, and a reduced number of patients for follow-up after recurrence. However, reduced recurrence also caused more patients to complete the follow-up after the primary treatment. Furthermore, reduced recurrence caused increased survival.
According to the model, the 5% point reduction described above would reduce the costs by €2190 per patient (5.3%) and increase the overall survival by 0.68 years (0.43 years discounted). Out of the 4268 persons diagnosed with CRC in 2015, 80% were diagnosed with stage I, II, or III disease (OUS data). Hence, a reduction in the recurrence rate would imply 2310 LYs saved per year in Norway (0.68 years × 4268 CRC patients × 0.80) and reduce healthcare costs by €7.47 million per year (€1.44 per capita).
Given the Norwegian WTP threshold per QALY gained, society’s willingness to pay for interventions that could contribute to a 5% point reduction in recurrence was €28,540 per CRC patient when survival was discounted by 4% per year (€2190 + [0.43 year × 0.74 QALYs per LY × €82,800 per QALY) in stage I, II, or III (€43,850 with undiscounted survival). In total, this account for €97 million per year (€28,540 per patient × 4268 patients per year × 0.80 in stage I, II, or II) and €18.8 per capita.
Primary prevention
Prevention of CRC might be achieved by screening and removing precursor lesions, increased physical activity, modifications to diet and lifestyle (including smoking cessation and prevention of excessive body weight), and use of anti-inflammatory drugs. Preventive measures might reduce the number of cases in all CRC stages, and the outcome of preventive intervention for CRC can be estimated using the model. In our analysis, we assumed that prevention affects all stages with the same percentage reduction in the number of people who develop CRC. Thus, we used the cost estimates of the four CRC stages as an estimate of the cost reduction of saving one person from developing CRC and used the estimation of loss of life years for the same stage to estimate the number of years saved.
The reduction in costs caused by preventing one CRC case was estimated to be €47,300; see Tables 2 and 4. In addition, according to the model, each CRC case prevented gained 6.3 years (4.1 years discounted). Given the WTP threshold value, society’s willingness to pay for preventive interventions was estimated to be €353,660 per CRC case prevented (€47,300 + [3.7 QALY × €82,800 per year]) when survival was discounted by 4%.
Screening—gain from stage migration
To assess the effect of stage migration on healthcare costs, we used CRC screening as an example. Randomised controlled trials have been carried out for CRC screening in several countries, and our model estimates were based on results from the UK and Denmark [18, 19]. In both trials, faecal occult blood tests were used to detect cancer at an early, asymptomatic stage to improve survival and reduce the CRC treatment costs. Table 2 in Online Resource 4 shows that CRC patients diagnosed through a screening programme have a more favourable stage distribution than those in the control groups. This is a potential gain from screening, provided that the early-stage, screen-detected tumours do not represent overdiagnosis, e.g., tumours that would never have emerged as clinical tumours within the lifespan of the person. The stage migration effect was greater in the UK trial than in the Danish trial. Patients in the screening groups were 50–74 years old and 45–74 years old, respectively, in these trials.
Applying data from Denmark [19], the reductions in costs were €14.9 per screened individual and €7300 per CRC detected (both excluding the cost of screening). The corresponding results based on the UK trial [18] were €21.6 and €10,306, respectively. The changes in cost caused by screening were a result of stage migration from more advanced cancer when diagnosed due to symptoms (base case) to a less advanced and even pre-cancerous stage when detected pre-symptomatically at screening. In the model, stage migration reduced both the cost of primary treatment and the number of recurrences. When fewer patients were diagnosed with cancer at stages III and IV and did not experience recurrence, the number of patients receiving palliative treatment decreased. In cases where screening results in excessive overdiagnosis of early and non-cancerous lesions, the consequences for costs will be more complex.
Productivity in CRC treatment
To estimate the productivity of CRC treatment in general, we need to quantify the survival gained by CRC treatment. Therefore, we used the estimated life expectancy according to CRC stages (Table 2), compared this value to the life expectancy for a cohort of hypothetical patients without any CRC treatment, and estimated the gain to society per euro used for CRC treatment. Online Resource 5 presents the analysis of survival without CRC treatment as well as the model and the assumptions used.
We have not found any relevant survival data for a patient without treatment. Instead, we used a separate Markov model to estimate the survival for the group (see Online Resource 5). In this model, we followed the patients from the age of 70–100 years or until death, and we assumed the following transition probabilities from one stage to another: stages I–II 0.583 (CI used in the PSA: 0.3–0.9) per year, stages II–III 0.656 (0.3–0.9), and stages III–IV 0.747 (0.31–0.85). The assumptions were based on the literature, where the transition probabilities were estimated using calibrations [20,21,22]. For patients in stage IV, we assumed the total annual probability of CRC death and non-CRC death to be 0.582.
The gain in LYs from the overall CRC treatment was estimated to be 6.05 years. For all stages, and given the Norwegian WTP threshold per QALY, the gain was €5.2 per euro used for CRC treatment (€7.8 if survival was not discounted). For stages I, II, and III, the gain per euro used for CRC treatment was €12.7, €8.1, and €5.0, respectively.
These estimates depended partly on the estimated life expectancy for a cohort of hypothetical non-treated CRC patients, estimated separately with a Markov model. The parameter uncertainty for the transition parameters between CRC stages used in this separate model was considerable. Thus, we performed a PSA for this separate Markov model, and based on the upper level of expected survival time for untreated patients we estimated the gain for society to be €5.5 per euro used for CRC treatment and €3.6 when using the lower level (€5.7 if survival was not discounted).
Discussion
The results of the analyses
The estimated lifetime healthcare cost for an average 70-year-old CRC patient was €47,300 and varied with disease stage at diagnosis from €26,630 to €69,890. Compared with the empirical (“model-free”) Norwegian study by Aas [10], our overall cost estimate was 39% higher, but only 1.3% higher after adjusting for differences in the included costs and time horizon (see more in [5]). The increase in costs according to the disease stage was similar to increases reported by Ladabaum et al. [23] and Frazier et al. [21], while Brown et al. [24] found an increase in costs for stages I–III, but a decrease for stage IV. However, comparing our CRC, cost with those in non-Norwegian studies is difficult because of differences in unit costs and assumptions for the analyses [25]. Nevertheless, we compared our results with those of a recent Irish study by Tilton et al. that described the treatment regime and other important conditions in such detail that it allowed for adjustment based on relevant differences [26]. When adjusting for the exchange rate, the annual Irish inflation between 2008 and 2011, and important differences in unit prices and treatment regimens between the two studies, the cost difference between Tilton’s model and our model was − 3.0%, − 1.3%, 3.6%, and − 1.2% for stages I, II, III, and IV, respectively, all within the estimated credible intervals of the former study (see more in [5]).
The cost for CRC treatment estimated by the model appeared modest compared to the number of QALYs gained by the same treatment. For all stages and given the Norwegian WTP threshold per QALY gained, the gain to society was €5.2 per euro allocated for CRC treatment and €12.7, €8.1, and €5.0 for stages I, II, and III, respectively, per euro allocated for CRC treatment. These estimates depended heavily on the estimated survival time for non-treated patients (Online Resource 5, Fig. 2). However, the gain would still be €3.6 per euro spent on treatment for all stages, despite using the lower level of the estimated CrI. The public health service in Norway is often criticised for high costs, but our results indicate that the surplus to society seems to be considerable for CRC treatment.
A 20% change in the cost of the various palliative chemotherapies, including, for example, drug costs and time-use costs, had a minor effect on the total CRC costs (< 2%), while expanded use of palliative chemotherapy could increase the total costs up to 29% (€11.3 per capita). Two factors are especially important for a possible increase in cost—the use of bevacizumab or EGFR-inh and an increased use of palliative chemotherapy in elderly patients. The current trend to use EGFR-inh more frequently as a first line of treatment and the increased use of palliative chemotherapy in the elderly can, therefore, have a profound impact on cost [16, 27, 28]. Because many evaluations have time horizons of 10–30 years, PSA based on parameter probability distributions estimated from “yesterday’s data” can be misleading. Therefore, CRC evaluations with long time horizons need to not only focus on high-quality palliative chemotherapy data, but also make reasonable assumptions about changes in future palliative treatments and perform sensitivity analyses based on these assumptions and alternative scenarios.
We found that a 5% point reduction in the 10-year recurrence rate for stages I–III would reduce CRC costs by €2190 per patient and increase overall survival by 0.68 years per patient. Based on these findings and the declared acceptable WTP threshold value of €82,800 per QALY gained, the Norwegian health sector should be willing to pay €97 million in total per year to achieve this reduction in recurrence rate. Approximately, 3000 colorectal resections for malignancy are performed each year in Norway. Assuming that each colorectal surgeon should perform at least 15 resections each year to maintain their competence, a maximum of 200 surgeons is needed in this field [29]. A comprehensive training programme (initial colorectal surgery training and yearly follow-up training) could use modern educational tools (such as simulators, operations on animals, etc.) along with workshops and lectures by highly experienced and skilled colorectal surgeons, radiologists, and pathologists. Assuming that such a comprehensive training programme would cost €300,000 per surgeon and that the effect would be a reduction in recurrence rate by 5% points, the investment would be paid back after only 11 CRC operations per surgeon.
The estimates for a 5% point reduction in the 10-year recurrence rate are also relevant when estimating possible gains from post-cancer prevention such as lifestyle interventions (diet, physical activity, etc.). Some studies show significant effects of such interventions [30,31,32,33,34,35,36,37,38], but these effects are highly uncertain because of the scarcity of high-quality randomised controlled trials [37, 38]. When evaluating strategies for post-CRC cancer prevention, we also have to consider the possible effects on HRQoL, physical functioning, tolerance to interventions, morbidity, and non-CRC mortality [37, 38].
For the screening analysis, the estimates did not consider that some patients diagnosed with CRC in the screening group would have died of something else before their CRC had produced symptoms if they had not been screened. This implies overtreatment for the screening group, where some of the CRCs were unnecessarily discovered, which adds extra costs for the screening group that were not included in our estimates. To include this in the analysis, we would need data indicating the proportion of the population with undiagnosed CRC who die from non-CRC causes.
Strengths and weaknesses of the general model
The cycles in the model were set to 1 year. The precision level can be improved by shortening the cycle length, but this would make the model more complex and accentuate the trade-off between model complexity and accuracy. As a result of convex survival curves and half-cycle correction, we expected that this weakness would contribute to a slight overestimation of the mean survival.
The cohort used in the model was diagnosed at the age of 70 years. This age might have resulted in a higher survival rate than if we had used the average age in the OUS sample. In [5], the average age for stages I–IV at the year of diagnosis was 69.9, 72.3, 70.4, and 70.5 years, respectively, in the OUS sample. When comparing these patients with our 70-year-old patients (based on Weibull regressions), we found that the differences in overall 10-year survival were − 0.2%, 4.2%, 0.7%, and 0.03%, respectively, for the four stages.
Another weakness of the analysis was that some of the data used were relatively old. The data on, for example, recurrence and resections were based on observations in the period 1993–2010, survival data in palliative phase were mainly based on data from 1995 to 2002, background mortality data were from 2009, and certain parts of the frequency estimates for metastatic surgery and medical treatment for complications were from 2003 to 2004. The estimates for the use of chemotherapy in the palliative phase and all unit costs were from 2011 to 2012. The validation of the model showed good correspondence with other models and studies from the same time period as our model [5]. The CRC mortality is currently lower than those estimated by the model, and the 5-year relative survival of CRC in Norway increased by 7.7% points from the period 1998–2007 to 2013–2017 (Cancer in Norway 2017). We see the same trend for metastatic CRC in Norway, which does not seem to be in line with the trend in the Netherlands where Hamers et al. [39] concluded that the overall survival of real-life stage IV patients did not improve from 2008 to 2016.
The effects on total CRC cost of using relatively old data are uncertain, because lower recurrence implies lower CRC cost due to fewer surgeries and reduced palliative chemotherapy, while increased use of more expensive drugs, particularly in the palliative phase, implies higher CRC cost. Furthermore, if the threshold for receiving surgical treatment for metastatic cancer has changed (most likely increased), our cost estimates would be too low, particularly for stage IV. In “Scenarios of palliative chemotherapy”, we showed the significance of changes in palliative chemotherapy and found that increased use of bevacizumab and EGFR-inh was of great importance for the overall treatment cost. It was, therefore, mitigating that the model’s inputs for the palliative phase treatments were relatively up-to-date and based on expert opinions from 2011 to 2012. When developing the next version of the model, it will be important to update the input data.
Rectal and colon cancers are different with regard to survival and treatment. Therefore, optimally, the model should provide results for colon and rectum cancer separately. Even though we had access to a high-quality dataset to estimate recurrence rates, the dataset was too small to identify recurrence rates for rectal and colon cancer separately. Hence, the model was based on rectum and colon jointly. Furthermore, in addition to estimating the cost of CRC, one of the objectives of this study was to estimate the effect of changing treatment strategies. In palliation, this would not distinguish between rectal and colon cancers. Nevertheless, in the model, we adjusted for rectal and colon cancer by weighing the proportion of rectal versus colon cases in all health states. In addition, we accounted for the fact that more rectal cancer patients are eligible for radiotherapy and separated out colon and rectum cases concerning frequencies and unit cost of resections (see Table 1 in Online Resource 1) in each of the Duke stages. Although the model does not provide separate results for rectal and colon cancer, the model is capable of calculating these separately by making a model run for each cancer if the required data are available.
Our study showed that the model’s estimates of the total CRC cost are sensitive to changes in the chemotherapy treatment in the palliative phase. This means, for example, that in studies where we have to include future CRC costs (e.g., evaluation of screening), the uncertainty could be significant if the treatment strategies change a lot over time.
Future development of the general model should also include more detailed HRQoL measures and improvements to the palliative part of the model. In addition, the effect of CRC on HRQoL in the ‘disease-free’ health states should be considered.
Conclusions
The costs of CRC generally seem to be modest when comparing treatment cost and the number of years saved. The expected lifetime CRC costs increased with the stage of the disease at diagnosis and were higher among patient experiencing recurrence after a resection with a curative intent. Changes in the use of palliative chemotherapy had a major impact on the expected CRC costs. The current trend to use EGFR-inh more frequently as a first line of treatment and the increased use of palliative chemotherapy in the elderly can, therefore, have a profound impact on cost. Reducing the recurrence rate through improved surgical technique indicated a considerable cost-effectiveness potential.
The different applications of the model illustrate its flexibility and indicate how the general model might be used to evaluate a broad range of interventions, making the model useful for researchers, health policy makers, health authorities, innovators, and industry.
Availability of data and material
The Markov model and the data used are presented in a separate article (1). For modelling the Markov model, we used Excel 2016, and for the PSA we used @risk 7.5 for Excel from Palisade.
Abbreviations
- 5-FU/FA:
-
Nordic FLv = 5-fluorouracil/folinic acid
- CI:
-
Confidence interval
- COI:
-
Cost-of-illness
- CRC:
-
Colorectal cancer
- CrI:
-
Credible interval
- EGFR-inh:
-
Epidermal growth factor receptor inhibitors (cetuximab/panitumumab)
- FLIRI:
-
A combination of irinotecan and 5-fluorouracil/folinic acid
- FLOX:
-
A combination of oxaliplatin and 5-FU/FA
- FOBTs:
-
Faecal occult blood tests
- HRQoL:
-
Health-related quality of life
- LYs:
-
Life years
- NPR:
-
National Patient Registry
- OUS:
-
Oslo University Hospital
- PSA:
-
Probabilistic sensitivity analysis
- PS:
-
Patient performance status
- QALY:
-
Quality-adjusted life years
- WTP:
-
Willingness to pay
References
Jemal, A., Center, M.M., DeSantis, C., Ward, E.M.: Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Biomark. Prev. 19(8), 1893–1907 (2010)
Ferlay, J., Steliarova-Foucher, E., Lortet-Tieulent, J., Rosso, S., Coebergh, J.W.W., Comber, H., et al.: Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur. J. Cancer 49(6), 1374–1403 (2013)
Tarricone, R.: Cost-of-illness analysis. Health Policy 77(1), 51–63 (2006)
Sullivan, S.D., Mauskopf, J.A., Augustovski, F., Jaime Caro, J., Lee, K.M., Minchin, M., et al.: budget impact analysis—principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health 17(1), 5–14 (2014)
Joranger, P., Nesbakken, A., Hoff, G., Sorbye, H., Oshaug, A., Aas, E.: Modeling and validating the cost and clinical pathway of colorectal cancer. Med. Decis. Mak. 35(2), 255–265 (2014). https://doi.org/10.1177/0272989X14544749
Meltzer, D.: Accounting for future costs in medical cost-effectiveness analysis. J. Health Econ. 16(1), 33–64 (1997)
Drummond, M., Sculpher, M., Torrance, G., O’Brien, B., Stoddart, G.: Methods for the economic evaluation of health care programmes, 3rd edn. Oxford University Press, Oxford (2005)
Sjo, O.H., Lunde, O.C., Nygaard, K., Sandvik, L., Nesbakken, A.: Tumour location is a prognostic factor for survival in colonic cancer patients. Colorectal Dis. 10(1), 33–40 (2008)
Nesbakken, A., Nygaard, K., Westerheim, O., Mala, T., Lunde, O.C.: Local recurrence after mesorectal excision for rectal cancer. Eur. J. Surg. Oncol. 28(2), 126–134 (2002)
Aas E (2009) Cost-effectiveness of screening for colorectal cancer with once-only flexible sigmoidoscopy and faecal occult blood test. In: Oslo University, Health Economics Research Programme
Yabroff, K.R., Lawrence, W.F., Clauser, S., Davis, W.W., Brown, M.L.: Burden of illness in cancer survivors: findings from a population-based national sample. J. Natl. Cancer Inst. 96(17), 1322–1330 (2004). https://doi.org/10.1093/jnci/djh255
Saarni, S.I., Härkänen, T., Sintonen, H., Suvisaari, J., Koskinen, S., Aromaa, A., et al.: The impact of 29 chronic conditions on health-related quality of life: a general population survey in Finland using 15D and EQ-5D. Qual. Life Res. 15(8), 1403–1414 (2006)
Do, Health: Economic evaluation of health intervention: a guide. The Norwegian Directorate of Health, Oslo (2012)
Mo, Finance: Guid for cost-benefit analysis. The Treasury Department, Oslo (2005)
Finance Mo: Principles and requirements for the preparation of socio-economic analyzes. Ministry of Finance, Oslo (2014)
Razenberg, L.G.E.M., Creemers, G.-J., Beerepoot, L.V., Vos, A.H., van de Wouw, A.J., Maas, H.A.A.M., et al.: Age-related systemic treatment and survival of patients with metachronous metastases from colorectal cancer. Acta Oncol. 55(12), 1443–1449 (2016)
Sorbye, H., Pfeiffer, P., Cavalli-Björkman, N., Qvortrup, C., Holsen, M.H., Wentzel-Larsen, T., et al.: Clinical trial enrollment, patient characteristics, and survival differences in prospectively registered metastatic colorectal cancer patients. Cancer 115(20), 4679–4687 (2009)
Scholefield, J.H., Moss, S.M., Mangham, C.M., Whynes, D.K., Hardcastle, J.D.: Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut 61(7), 1036–1040 (2012)
RCPH (2007) Screening for colorectal cancer in Vejle and Copenhagen County: Research Centre for Prevention and Health (RCPH)
Tappenden, P., Chilcott, J., Eggington, S., Sakai, H., Karnon, J., Patnick, J.: Option appraisal of population-based colorectal cancer screening programmes in England. Gut 56(5), 677–684 (2007)
Frazier, A.L., Colditz, G.A., Fuchs, C.S., Kuntz, K.M.: Cost-effectiveness of screening for colorectal cancer in the general population. JAMA 284(15), 1954–1961 (2000)
Tappenden, P., Eggington, S., Nixon, R., Chilcott, J., Sakai, H., Karnon, J.: Colorectal cancer screening options appraisal: cost-effectiveness, cost-utility and resource impact of alternative screening options for colorectal cancer. University of Sheffild, Sheffild (2004)
Ladabaum, U., Phillips, K.A.: Colorectal cancer screening: differential costs for younger versus older Americans. Am. J. Prev. Med. 30(5), 378–384 (2006)
Brown, M.L., Riley, G.F., Potosky, A.L., Etzioni, R.D.: Obtaining long-term disease specific costs of care: application to medicare enrollees diagnosed with colorectal cancer. Med. Care 37(12), 1249–1259 (1999)
Yabroff, K.R., Borowski, L., Lipscomb, J.: Economic studies in colorectal cancer: challenges in measuring and comparing costs. JNCI Monogr. 2013(46), 62–78 (2013)
Tilson, L., Sharp, L., Usher, C., Walsh, C., Whyte, S., O’Ceilleachair, A., et al.: Cost of care for colorectal cancer in Ireland: a health care payer perspective. Eur. J. Health Econ. 13(4), 511–524 (2012)
Van Cutsem, E., Cervantes, A., Adam, R., Sobrero, A., Van Krieken, J.H., et al.: ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 27(8), 1386–1422 (2016). https://doi.org/10.1093/annonc/mdw235
Tejpar, S., Stintzing, S., Ciardiello, F., et al.: Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the crystal and fire-3 trials. JAMA Oncol. 3(2), 194–201 (2017)
Norderhaug, I.T.H.: Pasientvolum og kvalitet ved koloncancerkirurgi. Nasjonalt kunnskapssenter for helsetjenesten, Oslo (2009)
Meyerhardt, J.A., Giovannucci, E.L., Holmes, M.D., Chan, A.T., Chan, J.A., Colditz, G.A., et al.: Physical activity and survival after colorectal cancer diagnosis. J. Clin. Oncol. 24(22), 3527–3534 (2006)
Meyerhardt, J.A., Heseltine, D., Niedzwiecki, D., Hollis, D., Saltz, L.B., Mayer, R.J., et al.: Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J. Clin. Oncol. 24(22), 3535–3541 (2006)
Meyerhardt, J.A., Giovannucci, E.L., Ogino, S., Kirkner, G.J., Chan, A.T., Willett, W., et al.: Physical activity and male colorectal cancer survival. Arch. Intern. Med. 169(22), 2102–2108 (2009)
Lynch, B.M., Cerin, E., Owen, N., Aitken, J.F.: Associations of leisure-time physical activity with quality of life in a large, population-based sample of colorectal cancer survivors. Cancer Causes Control 18(7), 735–742 (2007)
Haydon, A.M., MacInnis, R.J., English, D.R., Giles, G.G.: Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut 55(1), 62–67 (2006)
Meyerhardt, J.A., Niedzwiecki, D., Hollis, D., Saltz, L.B., Hu, F.B., Mayer, R.J., et al.: Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA 298(7), 754–764 (2007)
Huxley, R.R., Ansary-Moghaddam, A., Clifton, P., Czernichow, S., Parr, C.L., Woodward, M.: The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int. J. Cancer 125(1), 171–180 (2009)
Rock, C.L., Doyle, C., Demark-Wahnefried, W., Meyerhardt, J., Courneya, K.S., Schwartz, A.L., et al.: Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 62(4), 242–274 (2012)
Ravasco, P., Monteiro-Grillo, I., Camilo, M.: Individualized nutrition intervention is of major benefit to colorectal cancer patients: long-term follow-up of a randomized controlled trial of nutritional therapy. Am. J. Clin. Nutr. 96(6), 1346–1353 (2012)
Hamers, P., Bos, A.C.R.K., May, A.M., Punt, C.J.A., Koopman, M., Vink, G.R.: Recent changes in overall survival of real-life stage IV colorectal cancer patients. J. Clin. Oncol. 37(15_suppl), 3522 (2019)
Acknowledgements
We acknowledge the Department of Health, Nutrition and Management (HEL), The Faculty of Health Sciences, and Oslo and Akershus University College of Applied Sciences for funding Paal Joranger’s doctorate.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors contributed to the work, are aware of, and agree to the submission. Any other person or body with an interest in the manuscript, such as our funders and employers, are also aware and agreed on the submission. Paal Joranger, Eline Aas, and Arne Oshaug declare no support from any organisation for the submitted work, no financial relationships in the previous 3 years with any organisations that might have an interest in the submitted work, and no other relationships or activities that could appear to have influenced the submitted work. In 2017, Geir Hoff received payment from Amgen Norway for giving a lecture at a medical conference. Halfdan Sorbye received Grants and personal fees from Merck, Roche, and Amgen and personal fees from Sanofi during the study. Arild Nesbakken received funding from Helse Sør-Øst for the clinical studies on colorectal cancer (OUS-Aker series). He is a member of a research group in OUS, which has patents on one diagnostic and two prognostic genetic tests for colorectal cancer. He received payment from Amgen Norway for giving a lecture at a medical conference 2018. Financial support for this study was provided entirely by the authors’ employers, which are listed above. The funding agreements ensured the authors’ independence in designing the study, interpreting the data, and writing and publishing the article. The authors declare that they have no competing interests.
Ethics approval and consent to participate
The observational study from 1993 to 2010, including 2049 patients diagnosed with CRC at Oslo University Hospital, was approved by the Regional Ethics Committee (Norway) for Medical Research (REK approval 1.2005.1629). The study with data collected from the National Patient Register (NPR) was approved by the Regional Ethics Committee (Norway). The reference number is S-02113 (2013/83).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Joranger, P., Nesbakken, A., Sorbye, H. et al. Survival and costs of colorectal cancer treatment and effects of changing treatment strategies: a model approach. Eur J Health Econ 21, 321–334 (2020). https://doi.org/10.1007/s10198-019-01130-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-019-01130-6