Abstract
Predator–prey interactions can cross ecosystem boundaries and the outcome of these interactions is seen in prey defensive behavior. We aimed to test how the presence of a semi-aquatic predator alters the behavior and foraging microhabitat of Dendropsophus minutus tadpoles when they are either in groups or alone. We hypothesized that in the presence of a predator, Thaumasia fishing spider, tadpoles will be (1) less active; (2) forage far from the predator and; (3) forage evenly when in groups. We measured activity and foraging microhabitat as the proportion of time spent moving, and the total percentage of food removed from the upper and lower inner surfaces of the aquarium, respectively. The presence of the spider reduced tadpole activity by 24% compared to treatments without predators. Contrary to our expectations, solitary tadpoles were 34% more active than tadpoles in groups, and larger larvae were less active than smaller ones. The presence of the fishing spider decreased tadpoles activity, but the presence of conspecifics did not dilute the predator effect. Larger larvae are under more substantial selective pressure than smaller ones. Finally, our experiment empirically demonstrates that predator effects are transferable, generating a cascading system, and affecting the recipient ecosystem in various manners.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation is a significant selective force of characteristics that increase the chances of survival of the individual. Prey animals can alter their morphology, their life-history strategies, and their behavior as a response against predators (Lima and Dill 1990; Skelly and Werner 1990; Dijk et al. 2016). Behavioral changes induced by predators are generally rapid, reversible (Relyea 2003) and effective in reducing mortality risk (Sansom et al. 2009). In amphipods, for example, behavioral responses such as a change in microhabitat and decreased activity can delay the time to the first predator attack (Wisenden et al. 1999). In experimental conditions, Pardosa spiders have their chance of survival almost tripled when they assume a vertical position on the substrate (Persons et al. 2002). Although the most evident impact of behavioral defense is the decreased risk of predation experienced by an individual, behavioral changes induced by predators may interfere with the result of competitive interactions and have consequences at the trophic network level (Werner and Anholt 1996; Breviglieri et al. 2017). Therefore, understanding how prey responds to predators is the first step to infer all possible implications of predator–prey interaction for the populations and the community of which they are part.
Tadpoles are part of the non-reproductive larval stage of the annual life cycle. The absence of necessary behaviors for sexual activity summarizes their set of behaviors to activities that increase survival, growth, and development for metamorphosis (Altig and Mcdiarmid 1999). A variety of both vertebrate and invertebrate animals prey upon tadpoles which, in turn, exhibit multiple behavioral defenses (Relyea 2001; Van Buskirk 2001). Such strategies can be induced by chemical, visual and mechanical cues from predators (Van Buskirk and Arioli 2002; Takahara et al. 2012; Gazzola et al. 2017). When exposed to predator trails, tadpoles may respond with clustering, distancing, and microhabitat shift (Hews and Blaustein 1985; Laurila et al. 1997; Gazzola et al. 2018). In addition, tadpoles often respond with decreased activity, which results in an effective reduction of mortality (Lawler 1989). Although aggregations increase tadpoles’ conspicuity, they can decrease the risk of predation per individual through the dilution effect (Watt et al. 1996; Spieler 2005). Even in non-aggregated species, the presence (i.e., dilution) and behavior (i.e., escape) of conspecifics in the environment could bring greater security by acting as an additional channel for threat detection. As an additional benefit, the presence of conspecifics could increase the efficiency of tadpole feeding by decreasing the need for other defensive behaviors that can affect foraging (i.e., immobility), and by promoting the suspension of food present in the background by collective activity (Katz et al. 1981).
Interactions between aquatic predators, generally insects and other vertebrates, and tadpoles are widely known in the literature, and several previous studies have reported the diversity of tadpoles’ response to them (e.g., Relyea 2001; Nomura et al. 2011). Semi-aquatic predators, on the other hand, are known for their consumptive effects on adult anuran populations (Formanowicz et al. 1981), but little is known about the interaction between tadpoles and these predators, besides some anecdotal predation reports (Menin et al. 2005). Semi-aquatic predators, such as fishing spiders, could play an essential role in the demographic dynamics of different species of anurans because they consume both adults and larvae. In an experiment, Jara (2008) observed that fishing spiders in the genus Thaumasia Perty (Araneae, Pisauridae) consumed one tadpole per hour. Besides its high consumption capacity and direct impact on the tadpole population, non-consumptive effects, such as life-history changes, have also been induced by these fishing spiders. When exposed to the indirect clues of Thaumasia sp. (Araneae, Pisauridae) fed with metamorphs, tadpoles emerged later and larger (Vonesh and Warkentin 2006). Thus, due to the considerable density of spiders in the natural environment, they can impact the survival of tadpoles and act as a selective force in the evolution of defense mechanisms, which would be displayed in the presence of these predators. However, in ephemeral environments, where most of the anuran larvae develop, there is also the risk of desiccation due to rapid water evaporation. This context generates a system of conflicting demand between behaviors that maximize foraging and behaviors that decrease the chance of predation. Thus, reducing activity and switching from superficial to deeper environments—which could be effective against fishing spiders (i.e., the predator is not able to dive deep), but have an impact on tadpole foraging efficiency—can be attenuated if other factors, such as the presence of conspecifics, act as an additional protective barrier.
In this work, we aimed to test how the presence of Thaumasia spiders alter the activity and foraging of Dendropsophus minutus (Anura, Hylidae) tadpoles in different social contexts to understand how fishing spiders affect the behavior of tadpoles. We hypothesize that the presence of the predator (1) reduces tadpole activity, decreasing the chance of encounter and/or detection, (2) generates a change in the use of microhabitat by tadpoles, which can be observed as a more prolonged time foraging at the bottom of the puddle, maintaining greater distance from the predator, but (3) when in groups, these effects are diluted and, in this way, tadpoles in groups will move more and use the microhabitat more uniformly than solitary conspecifics.
Methods
Study system
The experiment took place between 19-Feb-2018 and 26-Feb-2018, during the rainy season, in Emas National Park, located in the southwest of the state of Goiás, central-western Brazil (18°15′ 50.2″ S 52°53′31.7″ W). Both prey and predator are relatively common and coexist in temporary pools found in the park.
We collected the tadpoles used in the experiments in an ephemeral puddle (18°15′42.2″ S 52°53′ 17.5″ W). Dendropsophus minutus larvae have a nektonic habit (personal observation), which increases the chance of encounters between the larvae and the fishing spiders in the natural environment. We placed tadpoles together in a 10-L tank, which we kept at the Emas National Park lodge and fed them ad libitum with algae-based fish food (sera® Micron) once a day in natural daylight regime. The tadpoles were then selected based on their stage of development (i.e., we excluded tadpoles with well-developed legs, above Gosner’s stage 38) and size (between 2.5 cm and 3.5 cm) and separated in a tank for the next day’s experiment. The puddle from which we collected the tadpoles was approximately 45 cm deep at its most profound site, and vegetation predominated on its margins. We deprived the tadpoles of food for 24 h before the experiments began to induce foraging during the experimental trials and to avoid tadpoles from eating their feces or the feces of other tadpoles in group treatments. Since tadpoles scrape the substrate and filter water to obtain food, we did not use the water from the puddle in the experiment or for tadpole maintenance to reduce the amount of suspended organic matter. The water used for tadpoles maintenance and the experiment came from the Formoso River, located within the limits of the Emas National Park, of which pH was similar to that of the pool from which we collected the tadpoles used herein. However, we filtered the water using a zooplankton sieve (mesh = 23 µm) before using it in the aquariums to promote oxygenation and removal of organic matter. After the experiment, we euthanized all tadpoles and brought them to the Laboratório de Ecologia e Funcionamento de Comunidades at the Universidade Federal de Goiás, where we determined tadpoles’ development stages and total length. The average size of tadpoles used in the experiment was 27.26 ± 2.05 mm (min = 22, max = 32 mm), and the development stage (sensu Gosner 1960) varied between 27 and 37.
We collected Thaumasia sp. individuals during the night on the surface of the same puddle where we collected tadpoles and in the Capivara Lake (18°16′16.9″ S 52° 50′34.2″ W). There are reports of predation of fish and tadpoles by female spiders in this genus, and because they are semi-aquatic predators, they become a suitable predator model for this study (e.g., Machado and Lipinski 2014). The length of the cephalothorax of spiders used in the experiment varied between 4 and 6 mm (mean value: 4.74 ± 0.45 mm). Spiders were fed ad libitum with tadpoles to avoid predation during the experimental trials.
Experimental design
We conducted a draw to assign the following treatments to each aquarium: 1 = with both a solitary spider and a tadpole (TS); 2 = with a spider and three tadpoles (TG); 3 = no spider and a solitary tadpole (CS); and 4 = no spiders and three tadpoles (CG). First, we randomly placed fasting tadpoles into glass aquariums (15 × 10 × 13 cm) containing 1300 ml of water each for 1 h and 30 min. After this acclimatization period, we placed glass slides (7.5 × 2.5 cm) containing fish food against the walls of the aquarium. We set up the slides vertically on the central portion of a wall, in the proportion of one blade per tadpole (Sousa et al. 2014) (Fig. 1). Subsequently, we added predators and covered the top of the aquarium with a transparent plastic sheet. We covered the outer side of the aquariums’ walls with brown paper to prevent external sources of disturbance from influencing the behavior of tadpoles. The total duration of treatments was 20 h and 30 min, and we used both spiders and tadpoles only once. We conducted the experiment at room temperature (average water temperature = 22 ± 0.82 °C and average air temperature = 24.54 ± 0.61 °C) for three consecutive days. In each day, we run four replicates for each treatment (TS, TG, CS, and CG) and all aquariums were washed, the water changed, and slides replaced between trials. We discarded any observation where the spider or any tadpoles were dead at the end of the experiment, or if the food has come off the slides. The final number of replicates for each treatment was 11 for treatment TS, 9 for TG, 10 for CS, and 11 for CG.
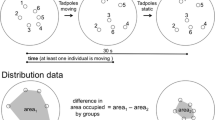
The experimental design used to measure defensive responses displayed by tadpoles: a three tadpoles with a predator (TG), b a single tadpole with a predator (TS), c three tadpoles without predator (CG) and d a single tadpole without predator (CS). We added a single individual of Thaumasia sp. spider in all predator-present treatments
To test the effect of the predator’s presence on tadpole activity, we started filming the interior of the aquariums in the morning, 18 h after the start of the experiments. Tadpoles of D. minutus generally forage within this period (personal observation). We measure the amount of time (in seconds) each tadpole spent moving, which included tail movement and vertical and horizontal displacements. Each filming was 25-min long, but we discarded the first 10 and the last 5 min to reduce the influence of stress of the tadpoles due to our presence during the setting of the equipment for filming. To test the effect of the predator’s presence on the foraging behavior of tadpoles, we photographed the slides and used the ImageJ (Rasband 2012) particle analysis tool for quantifying the percentage of food removed (Fig. 2).
Statistical analysis
We used a permutational analysis of covariance (permutational ANCOVA; 1000 permutations) to predict whether the presence of conspecifics or predators affected the percentage of food removed in both upper and lower halves of the glass slides as well as the overall percentage of food consumed by tadpoles. In addition, we used tadpole body length as a covariate. We then built models for each response variable separately. Thus, we tested whether, in the presence of the predator, the tadpoles (1) spend less time in motion, (2) eat less in the upper half of the glass slides and, (3) when in groups, individuals moved more and scraped food more evenly from the glass slides. For tadpoles in groups, we calculate the average percentage of food consumed in the three slides, the average time spent moving and the average size of the three tadpoles. We conducted all statistical analyses using the lmPerm v.2.1.0 package in R (R Development Core Team 2008). All results show the mean ± standard deviation.
Results
We found no interaction between the predator presence and presence of conspecifics to explain the activity or food consumption performed by the tadpoles of D. minutus (Table 1). The presence of the spider reduced tadpole activity by 24% compared to treatments without predators (with Spider: 292 ± 201 s; without Spider: 384 ± 179 s; p = 0.018, Table 1, Fig. 3). Contrary to our expectations, solitary tadpoles were 34% more active than tadpoles in groups (loners: 406 ± 218 s; group: 269 ± 136 s; p = 0.007, Table 1) and larger larvae were less active than smaller ones (p = 0.0018, Table 1, Fig. 4).
The relation between tadpole size and tadpole activity (black line), tested in an ANCOVA model (size is the covariate). Data for all tadpoles used in the experiment. Open circle—solitary tadpoles, without spiders. Filled circle—solitary tadpoles, with a spider. Open square—tadpole in a group, with spiders. Filled square—tadpole in a group, without spider
Tadpoles removed less food from the upper half of the slides when the predator was present (p < 0.000, Table 1, Fig. 5). Food removal from the slides’ upper half was almost twice higher in the absence of the predator (predator absent: 60.17 ± 16.59%; predator present: 32.78% ± 24%). Total food removal and removal from the lower half of the glass slides were similar between treatments, regardless of the presence of either the spider or conspecifics (Table 1). However, the size of the tadpole had a positive effect on the total amount of food removed from the lower half of the glass slides, with larger tadpoles removing more food than smaller conspecifics (Table 1).
Effects of the predator and conspecifics on food removal from the upper half of the glass slides by the tadpoles. The horizontal line represents the median. The lower and upper parts of the box are the first and third quartiles. The vertical line indicates the highest and lowest adjacent values. The black circles indicate potential outliers
Discussion
We found evidence that Dendropsophus minutus tadpoles modulate their foraging behavior, by reducing their activity and altering their selection of microenvironments, as a function of predators outside the aquatic environment. The overall behavior of the larva was dependent on size. However, the presence of conspecifics did not dilute the predator effect. Decreased activity is a typical behavioral response in tadpoles when exposed to predators (e.g., Lawler 1989; Hokit and Blaustein 1995; Gazzola et al. 2015), which is particularly effective against ambush predators such as Thaumasia sp. (personal observation). The decrease in foraging activity can decrease encounter rates between predator and prey and, consequently, reduce the risk of predation. Vonesh and Warkentin (2006) did not observe behavioral changes in tadpoles in response to the presence of spiders but found changes in larvae’s size at metamorphosis. The absence of behavioral changes, in this case, could be related to the fact that they fed spiders with metamorphs while we used tadpoles to serve as prey to the fishing spiders. Vonesh and Warkentin (2006) suggest that tadpoles are capable of differentiating stage-specific predators if the information used by tadpoles for this differentiation is the presence of conspecifics traits in the predators excretes, which may explain the contrasting results we obtained. The predator diet is one of the clues tadpoles use to assess predation risk, and they can exhibit more extreme responses to those predators that feed on conspecifics (Laurila et al. 1997).
The active time also varied with the tadpole size. Larger tadpoles, in general, were less active. Size offers protection against predators for whom handling large prey is costly (Formanowicz 1986). However, for predators that have poison, size does not seem to offer additional protection to prey (Jara 2008). In a scenario where larger larvae are more vulnerable, that is, where there are predators that are not limited by prey size, decreased activity associated only with size may be a consequence of the selection pressure in this population. Thus, larger, and very active tadpoles would be more quickly predated, with only the less active individuals remaining in the pool (e.g., Watkins 1996). We predict that larger tadpoles are more active when they are in groups exposed to predators with limited-prey-size strategy. On the other hand, the opposite would happen to tadpole groups exposed to predators that are not limited by prey size, as we found herein.
Tadpoles in groups were also less active than solitary tadpoles. Nicieza (1999) noted that tadpoles with siblings were more active than solitary ones; however, tadpoles in the presence of non-siblings tadpoles or when solitary had similar activity levels. In our study, the effect of the presence of unrelated individuals seems to negatively correlate with tadpole activity, with tadpoles exhibiting defensive behaviors in the presence of conspecifics. The defensive behavior displayed by individuals in groups may be a consequence of the stress caused by the short inter-individual distance, accentuated by the lack of kinship. However, this hypothesis needs further research to be confirmed. In addition, if unrelated tadpoles can detect a threat, this can generate a system of reinforcement to immobility, since tadpole activity is a measure of predation risk. In this scenario, the first tadpole that moves can draw the predator’s attention, thus, reducing the risk to the other individuals.
Moreover, we observed that tadpoles switched between microhabitats when the fishing spider was present, foraging mostly in the lower half of the glass slide. Such change in the use of microhabitat as an antipredator strategy has been reported in tadpoles before (e.g., Formanowicz and Bobka 1989; Lawler 1989) and it can mitigate the costs of cryptic behavior by allowing individuals to forage in safer sites, protected against predators. Bridges (2002) noted that tadpoles, in the presence of predators, decreased activity while feeding. The combination of both strategies—decreased activity and switch in the use of microhabitat—could decrease, for example, the costs of starvation risk and limitation to low-quality resources, separately. However, experimental conditions do not reflect all the variables that tadpoles experience in the natural environment (i.e., different types of predators, competitors, availability of resources). Decreased activity and change in the use of microhabitat can be part of an escalation system in defense strategies, depending on the risk of predation. They can be used not only as a compliment but also as an alternative in a system for reducing the costs of defense and optimizing foraging activity.
Both the total amount of food removed and the average amount of food removed from the lower half of glass slides by larger tadpoles were greater, a pattern already expected since they have higher consumption capacity. However, prey size did not affect the amount of food removed in the upper half of the slides. This result indicates that larger tadpoles are under stronger selection pressure, regarding not only their activity but also the foraging site. This suggests that predation at the air–water interface may have a substantial impact on the regulation of larvae behavior and possibly the community dynamics in temporary ponds. Luhring (2013) tested the effects of top–down predator pressure on tadpoles in the nutrient cycle and found that the gross primary productivity in tanks where predators were present was similar to those tanks with no tadpoles. Even though non-consumptive top–down effects are not yet fully understood, we suggest the presence of the fishing spider may be capable of defining the outcomes of competitive interactions. Thaumasia spiders prefer larger tadpoles (Jara 2008) and this preference may create more opportunities for smaller tadpoles to access food, who were at a disadvantage due to both interference and exploitation competitions. It is worth noting that in our study the total amount of food consumed by tadpoles was similar between treatments (with and without predator) even when tadpoles switched between microhabitats and decreased activity. However, under natural conditions, these strategies can affect weight gaining and time for metamorphosis, since the density of competitors and predators is high, and resources are scarce.
In conclusion, tadpoles exhibited defensive behavior against a semi-aquatic predator. However, the presence of conspecifics did not result in diluted predator effects and, consequently, mitigation of behavioral responses. Contrary to our expectations, the presence of conspecifics resulted in the display of typical defensive behavior, which was exacerbated in larger tadpoles. Moreover, the differential consumption of tadpoles could result in cascading effects in aquatic ecosystem transported by the presence of the semi-aquatic spider predator.
References
Altig R, McDiarmid RW (1999) Tadpoles: the biology of anuran larvae. University of Chicago Press
Breviglieri CPB, Oliveira PS, Romero GQ (2017) Fear mediates trophic cascades: nonconsumptive effects of predators drive aquatic ecosystem function. Am Nat 189:490–500
Bridges CM (2002) Tadpoles balance foraging and predator avoidance: effects of predation, pond drying, and hunger. J Herpetol 36:627–635
De Sousa VTT, Nomura F, Venesky MD, Rossa-Feres DC, Pezzuti TL, Andrade GV, Wassersug RJ (2014) Flexible feeding kinematics of a tropical carnivorous anuran tadpole. J Zool 293:204–210
Dijk B, Laurila A, Orizaola G, Johansson F (2016) Is one defence enough? Disentangling the relative importance of morphological and behavioural predator-induced defences. Behav Ecol Sociobiol 70:237–246
Formanowicz DR Jr (1986) Anuran tadpole/aquatic insect predator-prey interactions: tadpole size and predator capture success. Herpetologica 42:367–373
Formanowicz DR Jr, Bobka MS (1989) Predation risk and microhabitat preference: an experimental study of the behavioral responses of prey and predator. Am Midl Nat 121:379–386
Formanowicz DR, Stewart M, Townsend K, Harvey F, Brussard P (1981) Predation by giant crab spiders on the Puerto Rican frog Eleutherodactylus coqui. Herpetologica 37:125–129
Gazzola A, Brandalise F, Rubolini D, Rossi P, Galeotti P (2015) Fear is the mother of invention: anuran embryos exposed to predator cues alter life-history traits, post-hatching behaviour and neuronal activity patterns. J Exp Biol 218:3919–3930
Gazzola A, Balestrieri A, Ghitti M, Paganelli D, Galeotti P (2017) Behavioural and life history responses to predation risk by common frog tadpoles exposed to two predators during ontogeny. Acta Ethol 20:235–241
Gazzola A, Balestrieri A, Martín J, Pellitteri-Rosa D (2018) Is It Worth the risk? Food deprivation effects on tadpole anti-predatory responses. Evol Biol 45:67–74
Gosner KL (1960) A simplified table for staging anuran embryos on larvae with notes on identification. Herpetologica 16:183–190
Hews DK, Blaustein AR (1985) An investigation of the alarm response in Bufo boreas and Rana cascadae tadpoles. Behav Neural Biol 43:47–57
Hokit DG, Blaustein AR (2010) Predator avoidance and alarm-response behaviour in kin-discriminating tadpoles (Rana cascadae). Ethology 101:280–290
Jara FG (2008) Differential vulnerability of Physalaemus pustulosus tadpole size classes to predation by the water spider Thaumasia sp. (Physauridae). Amphib Reptil 29:432–437
Katz LC, Potel MJ, Wassersug RJ (1981) Structure and mechanisms of schooling in tadpoles of the clawed frog, Xenopus laevis. Anim Behav 29:20–33
Laurila A, Kujasalo J, Ranta E (1997) Different antipredator behaviour in two anuran tadpoles: effects of predator diet. Behav Ecol Sociobiol 40:329–336
Lawler SP (1989) Behavioural responses to predators and predation risk in four species of larval anurans. Anim Behav 38:1039–1047
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Luhring TM (2013) Complex life-histories and biogeochemical cycles: interactions between amphibian life-history strategies and elemental cycling. PhD dissertation. University of Missouri-Columbia, USA
Menin M, Rodrigues DDJ, de Azevedo CS (2005) Predation on amphibians by spiders (Arachnida, Araneae) in the Neotropical region. Phyllomedusa J Herpetol 4:39–47
Nicieza AG (1999) Context-dependent aggregation in Common Frog Rana temporaria tadpoles: influence of developmental stage, predation risk and social environment. Funct Ecol 13:852–858
Nomura F, Prado VHM, Silva FR, Borges RE, Dias NYN, Rossa-Feres DC (2011) Are you experienced? Predator type and predator experience trade-offs in relation to tadpole mortality rates. J Zool 284:144–150
Persons MH, Walker SE, Rypstra AL (2002) Fitness costs and benefits of antipredator behavior mediated by chemotactile cues in the wolf spider Pardosa milvina (Araneae: Lycosidae). Behav Ecol 13:386–392
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rasband WS (2012) ImageJ. U.S. Bethesda: National Institutes of Health
Relyea RA (2001) The relationship between predation risk and antipredator responses in larval anurans. Ecology 82:541–554
Relyea RA (2003) Predators come and predators go: the reversibility of predator-induced traits. Ecology 84:1840–1848
Sansom A, Lind J, Cresswell W (2009) Individual behavior and survival: the roles of predator avoidance, foraging success, and vigilance. Behav Ecol 20:1168–1174
Skelly DK, Werner EE (1990) Behavioral and life-historical responses of larval American toads to an odonate predator. Ecology 71:2313–2322
Spieler M (2005) Can aggregation behavior of Phrynomantis microps tadpoles reduce predation risk? Herpetol J 15:153–157
Takahara T, Kohmatsu Y, Maruyama A, Doi H, Yamanaka H, Yamaoka R (2012) Inducible defense behavior of an anuran tadpole: cue-detection range and cue types used against predator. Behav Ecol 23:863–868
Van Buskirk J (2001) Specific induced responses to different predator species in anuran larvae. J Evol Biol 14:482–489
Van Buskirk J, Arioli M (2002) Dosage response of an induced defense: how sensitive are tadpoles to predation risk? Ecology 83:1580–1585
Vonesh JR, Warkentin KM (2006) Opposite shifts in size at metamorphosis in response to larval and metamorph predators. Ecology 87:556–562
Watkins TB (1996) Predator-mediated selection on burst swimming performance in tadpoles of the pacific tree frog, Pseudacris regilla. Physiol Zool 69:154–167
Watt PJ, Nottingham SF, Young S (1997) Toad tadpole aggregation behaviour: evidence for a predator avoidance function. Anim Behav 54:865–872
Werner EE, Anholt BR (1996) Predator-induced behavioral indirect effects: consequences to competitive interactions in anuran larvae. Ecology 77:157–169
Wisenden BD, Cline A, Sparkes TC (1999) Survival benefit to antipredator behavior in the amphipod gammarus minus (Crustacea: Amphipoda) in response to injury-released chemical cues from conspecifics and heterospecifics. Ethology 105:407–414
Acknowledgements
The present work was carried out with the support of the Coordination of Improvement of Higher Level Personnel—Brazil (CAPES)—funding Code 001 and the Graduate Program in Ecology and Evolution of the Federal University of Goiás. FN thanks funding by Conselho Nacional de Desenvolvimento Científico e Tecnológico -CNPq (301232/2018-0, 441214/2016-9 and 420051/2016-3). We acknowledge Dr. L Signorelli and Dr. V Moraes, for suggestions and field assistance. We also thank the professors Dr. R Daud and Dr. F Teresa for their insightful comments and suggestions. All applicable institutional and national guidelines for the care and use of animals were followed.
Author information
Authors and Affiliations
Contributions
JLM and FN conceived and designed the experiments. JLM performed the experiments. JLM and FN analyzed the data. JLM and FN wrote the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
de Lima Mamede, J., Nomura, F. Dendropsophus minutus (Hylidae) tadpole evaluation of predation risk by fishing spiders (Thaumasia sp.: Pisauridae) is modulated by size and social environment. J Ethol 39, 217–223 (2021). https://doi.org/10.1007/s10164-021-00696-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-021-00696-0