Abstract
We experimentally tested whether the presence of a free benthic predator (Odonata naiads) alters the displacement time, the position occupied in the water column, and the proportion of food consumed by benthic and nektonic tadpoles. The presence of predators did not influence the displacement time or the proportion of food consumed by any of the two species. In the presence of predators, benthic tadpoles avoided the benthic microhabitat, increasing their time in the middle of the water column. This behavior was unexpected since the previous studies indicate that the morphology of benthic tadpoles restricts them to the bottom of water bodies. We, thus, hypothesize that such a drastic behavior change was a consequence of the real risk of predation to which the tadpoles were exposed. Our results are in accordance with the threat-sensitivity hypothesis, in which prey behave flexibly when exposed to different degrees of predation threats. Nektonic tadpoles, however, slightly increased their permanence in the water column in the presence of the same benthic predators. Therefore, we provide support for the hypothesis that predators induce greater behavioral changes in prey that exhibit patterns of microhabitat use similar to theirs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tadpoles are surrounded by predators from both outside (e.g., spiders and birds; Jara, 2008; Martins et al., 2021) and inside waterbodies (e.g., Odonata naiads and fish; Mettler et al., 2021; Iglesias-Carrasco et al., 2022). The distribution of tadpoles and their predators in the aquatic environment is affected by several factors, including luminosity, temperature, oxygen levels, vegetation cover, depth, type of substrate, hydroperiod, as well as quality and availability of nutrients (Beiswenger, 1977; Noland & Ultsch, 1981; Collins & McIntyre, 2015; de Melo et al., 2018; Kloh et al., 2023). Thus, microhabitats have attributes that can favor the occurrence of both prey and predators. Predator–prey interactions lead to adaptations of both predators and prey, as the former improve their capture ability, and the latter develop anti-predatory strategies (e.g., Van Buskirk, 2001; Teplitsky et al., 2005). Some prey responses are behavioral, for example, tadpoles typically use refuge (e.g., Babbitt & Tanner, 1998; Hartman & Lawler, 2014) and reduce foraging in the presence of predators (e.g., Rae & Murray, 2019; Mamede & Nomura, 2021). Others are morphological, such as changes in width and length of the tail and the body (e.g., Relyea et al., 2021; Sergio et al., 2021).
Organisms that live in the bottom of water bodies, such as benthic tadpoles (Altig & Johnston, 1989; McDiarmid & Altig, 1999), respond to the presence of predators usually with immobility or reduced swimming activity (e.g., Azevedo-Ramos et al., 1992; Schalk, 2016). At midwater, these organisms swim more actively, like nektonic tadpoles (Altig & Johnston, 1989; McDiarmid & Altig, 1999), and use aquatic vegetation as a refuge against predators, which increases their chances to survive (e.g., Kopp et al., 2006). Thus, predator–prey interactions can lead to important behavioral adjustments for both preys and predators. However, species less likely to interact, such as those that inhabit different microhabitats, may have a weak influence on each other’s behavior.
In this study, we tested the hypothesis that predators have a greater impact on the behavior of prey species that inhabit the same microhabitats as their predators, compared to prey species that occupy different microhabitats. More specifically, we tested how a free benthic predator (Odonata naiads) altered the displacement time, the position occupied in the water column, and the proportion of food consumed by benthic and nektonic tadpoles. Because a previous study has found that benthic tadpoles tend to remain at the bottom of water bodies regardless of the presence of benthic predators (de Souza et al., 2022), we predicted that these tadpoles would reduce their activity rates as a strategy to avoid predation and, hence, reduce their food consumption. Nektonic tadpoles, on the other hand, can avoid these predators by foraging at different water depths (Altig & Johnston, 1989; McDiarmid & Altig, 1999), so we predicted they would be less affected by the presence of the benthic predator.
Materials and methods
Species, collection, and acclimation
We selected tadpoles of two species, one benthic, Physalaemus nattereri (Steindachner, 1863), and one nektonic, Scinax fuscovarius (Lutz, 1925), both common and abundant at the study region (e.g., Vasconcelos et al., 2011). Benthic tadpoles of P. nattereri present a globular body, low fins, small and dorsal eyes (Rossa-Feres & Nomura, 2006), and occur at the bottom, near, or among the vegetation of shallow water bodies (Schulze et al., 2015). Nektonic tadpoles of S. fuscovarius present a compressed body, high fins, a flagellum at the tip of the tail, and big, lateral eyes (Rossa-Feres & Nomura, 2006). They occur, generally, at midwater of deep water bodies (60 cm of depth), usually next to or among vegetation (Schulze et al., 2015). Despite differences in morphology and habit, these species have a similar diet, composed mainly of algae. However, P. nattereri tadpoles also consume Ciliophora protozoa and Acari (Rossa-Feres et al., 2004).
We also selected Odonata naiads, from species of Micrathyria Kirby, 1889, as they are efficient predators of tadpoles (e.g., Gascon, 1989; Arribas et al., 2018). They usually remain inactive, wait until the prey approaches, and execute a quick blow (Pritchard, 1965). This behavior can be considered as a “sit and wait” foraging strategy (Wellborn et al., 1996).
Tadpoles and Odonata naiads were collected with wire mesh (32 cm diameter; 3 mm mesh size) on February 25, 2019, and March 11, 2019, in two temporary ponds located in a pasture (pond 1: 20° 50′ 48.7′′ S, 49° 28′ 27.4′′ W and pond 2: 20° 50′ 50.3′′ S, 49° 28′ 29.5′′ W) in the city of Mirassol, southeast of Brazil. Tadpoles and Odonata naiads were separately transported in plastic bags containing water from the ponds where they were collected and accommodated in isothermal expanded polystyrene boxes to avoid overheating.
Collections were performed with authorization from the Institute of the Environment and Renewable Natural Resources (IBAMA) and the Chico Mendes Institute for Biodiversity Conservation (ICMBio)—System of Authorization and Information on Biodiversity (SISBio) license number: 18163-1 to D.C.R.-F. Maintenance of tadpoles in the laboratory and the experimental design were approved by the Ethics Committee on the Use of Animals—CEUA (no 187/2018), in accordance with the National Animal Experimentation Control Council (CONCEA).
Animals were acclimatized in the laboratory for 2–5 days before the beginning of the experiment. During this period, tadpoles and Odonata naiads were kept in polyethylene aquaria filled with constantly aerated dechlorinated water. The experiment was conducted in a controlled photoperiod (12 h of light and 12 h of darkness) and air temperature (28 °C–30 °C), which kept the water temperature around 26 °C, simulating the natural condition of the ponds in which the animals were collected. Tadpoles were fed ad libitum with a commercial food based on algae and krill (Sera Micron®), and Odonata naiads were fed with tadpoles that would not be used in the experiments.
Prey sample sizes were as follows: P. nattereri (n = 40) and S. fuscovarius (n = 40), and for the predator Micrathyria sp. (n = 100). We selected tadpoles and Odonata naiads with similar sizes (P. nattereri: 19.36 ± 2.86 mm; S. fuscovarius: 24.73 ± 2.30 mm; and Micrathyria sp.: 17.63 ± 2.30 mm; data presented as means ± SD). Odonata naiads had adequate size to predate tadpoles, as evidenced in preliminary tests (YCMS, personal observation). We also selected tadpoles in developmental stages 26–33 (Gosner, 1960), in which the major changes in tadpole morphology are related to body growth, not to developmental changes (McDiarmid & Altig, 1999).
Experimental design
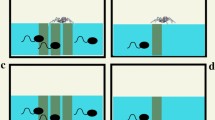
We performed the experiments on February 28, 2019, and March 3, 2019, with S. fuscovarius tadpoles, and on March 14, 2019, and March 17, 2019, with P. nattereri. We used glass aquaria (30 cm × 20 cm × 30 cm, l × w × d) filled with 12 L of dechlorinated water. We covered three faces of each aquarium with a blue plastic sticker to reduce possible stress that other colors could cause and to avoid visual contact among individuals (Maia & Volpato, 2013). The uncovered face of the aquaria (30 cm × 30 cm) was marked with two horizontal dashed lines to delimit water depths: bottom (15–10 cm of depth) and midwater (10–0 cm of depth); the depth was measured starting from top to bottom. These dashed lines facilitated the determination of tadpoles’ position along the water column. To test the influence of predator on the foraging behavior of tadpoles, we prepared a mixture of water and the same commercial food used during tadpoles’ acclimatization (Sera Micron®) at a concentration of 100 mg mL−1 (e.g., Venesky et al., 2013). We brushed 18 mL of this solution on the upper part of the glass slides (29 cm × 19 cm), making a uniform layer of food over an area of 20 cm × 5 cm of the slide (Fig. 1). We placed the slides on laboratory benches and waited for 24 h to use them. This period was long enough for the mixture to dry naturally and become firmly adhered to the slide surface.
The treatment corresponded to an aquarium with five free predators, one tadpole, either of P. nattereri or S. fuscovarius, two slides with food (one at each extreme of the aquaria), and four artificial plants. The plants were included to simulate aquatic vegetation that nektonic tadpoles of S. fuscovarius use in natural microhabitats (e.g., Schulze et al., 2015). The control had the same items, except for the predators (Fig. 2). We utilized free predators to create more realistic ecological dynamics for the experiment (e.g., Underwood, 2009). Predators have specific behaviors when hunting prey, and, by being free, they can exhibit these behaviors, making predator–prey interaction more realistic (e.g., Lima, 2002). Each trial was replicated 20 times per species. Thus, in total, there were 20 control and 20 treatment trials for each species.
The experiment started with the addition of a focal tadpole in each aquarium. We waited for 5 min to start observing the tadpoles’ behavior and to minimize potential stressful behaviors that could result from handling the individuals. Observations were performed between 10:00 and 11:00 h, the period of the day when tadpoles were determined to be more active (YCMS, personal observation). The time to observe tadpole behavior was established with the aim of preventing tadpoles acclimating to the predators’ presence or degradation of chemical cues in water (e.g., Peacor, 2006; Ferrari et al., 2007). Both situations could reduce the accuracy of predation risk assessment by the tadpoles and influence their behavioral response. Each aquarium was individually observed for 180 s, always by the same person (YCMS) positioned 80 cm away. At this distance, neither predators nor tadpoles exhibited stress signals, such as agitation, which indicated that the observer’s presence did not influence their behavior. The observer was sitting in a chair positioned in front of the aquarium. The height and distance of the observer to the aquarium were the same to ensure that the observation was performed in a standardized way. The experiment ran for a total of 3 h after the observation period to quantify food consumption. Then, the slides with food were removed and left to dry naturally for 24 h for further analyses of food consumption. Due to logistical reasons, only 10 treatment units and 10 control units were observed per day for each species. Thus, the experiment had a total duration of 4 days, two for tadpoles of S. fuscovarius and two for tadpoles of P. nattereri. Tadpoles and Odonata naiads were used only once for each trial, and the observation order of control and treatment groups was randomized.
After the experiment, tadpoles were euthanized by immersion in 10% lidocaine anesthetic solution and preserved in solution of 1:1 alcohol 70% and formaldehyde 15%. Odonata naiads were conserved in alcohol 70%. We deposited the preserved specimens at the Amphibians Scientific Collection (DZSJRP Amphibia-Tadpoles) from the Department of Zoology and Botany of UNESP, campus of São José do Rio Preto, Brazil (DZSJRP 0003.01, 0361.01, 0003.INV, and 0361.INV).
Data analysis
To evaluate predator influence on the displacement time of the tadpole (Supplementary Material, Table 1), we quantified the time in seconds, considering only whole numbers, that the tadpole swam during the observation period (180 s). We used a chronometer to pause the time when tadpoles stopped displacing. As such, we considered the displacement time of each tadpole as the sum of all moments that tadpoles displaced during the observation period.
To evaluate predator influence in the position occupied in the water column by the tadpole (Supplementary Material, Table 1), we noted the depth occupied by the tadpole in the water column during the observation period (180 s). However, the tadpoles did not change their initial depth along the water column during the observation period. Therefore, we considered this a binary variable: bottom or column.
To evaluate predator influence on the proportion of food consumed by the tadpoles (Supplementary Material, Table 1), we quantified the percentage of food removed from the glass slides offered to tadpoles. For this, we removed the slides after 3 h of the experiment and left them on laboratory benches to dry naturally. Then, the slides were digitized (HP LaserJet M1132) and analyzed using the Particle Analysis tool from ImageJ® software (Schneider et al., 2012). This tool counts pixels in a selected area and provides the proportion of particles in the image, or, in our case, the proportion of food removed from the slides by each tadpole (e.g., de Souza et al., 2022).
Statistical analysis
To test whether predators differentially influenced the behavior of benthic and nektonic tadpoles, we used generalized linear models with an adequate probability distribution for each response variable (Zuur et al., 2009). In all analyses, we included the day that the tadpoles were submitted to the experiment as an explanatory variable. Because displacement time was measured as the number of seconds (i.e., a discrete variable), we modeled it using the negative binomial distribution. The position occupied in the water column and the proportion of food consumed were modeled using the binomial and beta distributions, respectively. We assessed the fit of our models by visual inspection of randomized quantile residuals (Dunn & Smyth, 1996). We, then, tested for the effect of the day when the trials were conducted and presence of predators through likelihood ratio tests (Zuur et al., 2009). All models were fitted using the glmmTMB function from the “glmmTMB” package (Brooks et al., 2017) in the R software (R Core Team, 2022).
Results
Predators remained mostly at the bottom of the water column (Supplementary Material, Fig. 1). Four P. nattereri tadpoles were consumed by predators during the experiment (Supplementary Material, Video 1). Scinax fuscovarius tadpoles used the vegetation to support themselves, attaching the oral disk to the tip of the leaves (Supplementary Material, Fig. 2). They also consumed food along the water column (Supplementary Material, Fig. 3a and 3b).
For both species, the presence of predators did not influence the displacement time of tadpoles, but the day when trials were conducted did (P. nattereri, day 1: 21.18 ± 5.50 s and day 2: 0.00 ± 0.00 s; Table 1 and Fig. 3a; S. fuscovarius, day 1: 22.90 ± 40.07 s and day 2: 0.00 ± 0.00 s; Table 1 and Fig. 3b). Also, predators led to some changes in tadpoles’ spatial distribution. When predators were present, 18% of P. nattereri tadpoles occupied the water column whereas none moved away from the bottom when predators were absent (Table 1 and Fig. 3c). For S. fuscovarius, 86% of the tadpoles occupied the water column when predators were present, whereas in the absence of predators, only 60% did (Table 1 and Fig. 3d).
Mean displacement time of a P. nattereri and b S. fuscovarius tadpoles in response to the presence or absence of predators between experimental treatments. Mean proportion of tadpoles in the water column for c P. nattereri and d S. fuscovarius in response to the presence or absence of predators between experimental treatments. Error bars are standard errors. Mean values and standard errors were estimated from generalized linear models. Significant treatment highlighted with an asterisk
Unexpectedly, none of the benthic tadpoles consumed food during the whole experiment. The proportion of food consumed by S. fuscovarius was similar in the presence and absence of predators (Table 1 and Fig. 4).
Discussion
We corroborated our hypothesis that prey with similar habits to their predators would present greater behavioral changes than those with different habits. We observed that the presence of benthic predators significantly increased the use of the water column by benthic P. nattereri tadpoles, which is unusual for this species, whereas nektonic tadpoles of S. fuscovarius only marginally increased their use of the water column. However, the presence of predators did not affect the activity levels of tadpoles of either species, nor affect food consumption of S. fuscovarius tadpoles. P. nattereri tadpoles did not consume food during the experiment.
The presence of predators led tadpoles of P. nattereri to increase their permanence in the water column. This was unexpected considering their restrictive morphology (e.g., globular body and low fins; Altig & Johnston, 1989; McDiarmid & Altig, 1999) and physiology (e.g., negative buoyancy; Gee & Waldick, 1995; Tu et al., 1999). Furthermore, field observations show that benthic tadpoles tend to remain at the environment’s bottom (e.g., do Prado et al., 2009; Schulze et al., 2015). As tadpoles of P. nattereri remained at the bottom in the absence of predators, their change in water column use can be understood as a predator avoidance strategy. Greatly differing from this result, when exposed to the same but caged predators (Micrathyria sp.), tadpoles of P. nattereri did not exhibit displacement and remained immobile (de Souza et al., 2022). In this study, the high number of predators and the real risk of predation may have represented stimuli for anti-predatory behavior, forcing tadpoles to move to other depths of the water column. The different behaviors of P. nattereri when exposed to caged and free predators are in accordance with the threat-sensitivity hypothesis, in which prey behave flexibly toward different degrees of predator threats (e.g., Helfman, 1989; Bishop & Brown, 1992).
Several studies have shown that behavioral changes, such as the decrease in activity level, are a common anti-predatory response of benthic tadpoles (e.g., Ramamonjisoa et al., 2019; Scribano et al., 2020). Contrary to this expectation, in this study, the displacement time of P. nattereri tadpoles did not differ in the presence or absence of benthic predators. However, it is possible that the change in water column use by P. nattereri tadpoles helped them to avoid predators with no detriment to their activity levels, as suggested by the threat-sensitivity hypothesis.
Benthic tadpoles did not consume food either in treatment or in control groups. Swimming up and down to rasp food from substrate surfaces may represent an energetic challenge for tadpoles, as suggested by Annibale et al. (2019). Thus, the position that we chose to offer food for tadpoles may have constrained P. nattereri’s feeding behavior. Furthermore, we observed (DCRF and FSA) that P. nattereri tadpoles consume more food at nighttime, as such, it is possible that the period, when the trials were conducted, also affected tadpoles feeding behavior.
Corroborating our hypothesis, the presence of benthic predators did not affect the displacement time or the food consumption rates of nektonic tadpoles. The slight increase in the permanence of nektonic tadpoles in the water column when predators were present may reflect a weak influence of the benthic predator on behavioral aspects of the nektonic tadpoles. This is likely due to the low encounter rate between these organisms in microhabitats, since they have distinct habits (benthic vs nektonic) in natural conditions. This hypothesis is also in accordance with the threat-sensitivity hypothesis, in which prey behave flexibly toward different degrees of predator threats (e.g., Helfman, 1989; Bishop & Brown, 1992). As such, our study demonstrates empirically that predation pressure is strong on prey with habits similar to those of their predators, but weak on those with different habits.
The literature about the predator–prey interaction is extensive, especially on morphological and behavioral changes induced by predators on tadpoles (e.g., McCollum & Van Buskirk, 1996; Relyea, 2001; Jara & Perotti, 2010; Gazzola et al., 2023). Even so, our knowledge of how such relationships influence species distributions within the same habitat is limited to inferential studies, and behavioral aspects of Neotropical tadpoles are still little explored (e.g., Rossa-Feres et al., 2015; Annibale et al., 2023). Thus, our study contributes to a better comprehension of how the coexistence between predators and prey influences species’ spatial distribution. For example, tadpoles that are restricted to microhabitats where their morphology does not favor better locomotor performance, such as benthic tadpoles of P. nattereri that migrate to the water column to avoid predators, may be less capable of exploring available food sources or may not have access to the best food sources. Therefore, it is possible that these individuals reach metamorphosis with smaller sizes or experience slower development. Moreover, the behavioral repertoire of P. nattereri tadpoles highlights the limited knowledge about how these tadpoles assess the risk of predation, indicating a need for further experimental studies.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files.
References
Altig, R. & G. F. Johnston, 1989. Guilds of anuran larvae: relationships among developmental modes, morphologies, and habitats. Herpetological Monographs 3: 81–109. https://doi.org/10.2307/1466987.
Annibale, F. S., V. T. T. de Sousa, C. E. de Sousa, M. D. Venesky, D. D. C. Rossa-Feres, F. Nomura & R. J. Wassersug, 2019. Influence of substrate orientation on tadpoles’ feeding efficiency. Biology Open 8: bio037598. https://doi.org/10.1242/bio.037598.
Annibale, F. S., R. J. Wassersug, D. D. C. Rossa-Feres, F. Nomura, C. A. Brasileiro, A. F. Sabbag, Y. Zeng & J. R. Phillips, 2023. The case for studying tadpole autecology, with comments on strategies to study other small, fast-moving animals in nature. Austral Ecology 48: 855–876. https://doi.org/10.1111/aec.13367.
Arribas, R., J. C. Touchon & I. Gomez-Mestre, 2018. Predation and competition differentially affect the interactions and trophic niches of a Neotropical amphibian guild. Frontiers in Ecology and Evolution 6: 28. https://doi.org/10.3389/fevo.2018.00028.
Azevedo-Ramos, C., M. Van Sluys, J. M. Hero & W. E. Magnusson, 1992. Influence of tadpole movement on predation by odonate naiads. Journal of Herpetology 26: 335–338. https://doi.org/10.2307/1564891.
Babbitt, K. J. & G. W. Tanner, 1998. Effects of cover and predator size on survival and development of Ranautricularia tadpoles. Oecologia 114: 258–262. https://doi.org/10.1007/s004420050444.
Beiswenger, R. E., 1977. Diel patterns of aggregative behavior in tadpoles of Bufo americanus, in relation to light and temperature. Ecology 58: 98–108. https://doi.org/10.2307/1935111.
Bishop, T. D. & J. A. Brown, 1992. Threat-sensitive foraging by larval threespine sticklebacks (Gasterosteus aculeatus). Behavioral Ecology and Sociobiology 31: 133–138.
Brooks, M. E., K. Kristensen, K. J. van Benthem, A. Magnusson, C. W. Berg, A. Nielsen, H. J. Skaug, M. Mächler & B. M. Bolker, 2017. glmmTMB balances speed and flexibility among packages for zero-inflated Generalized Linear Mixed Modeling. The R Journal 9: 378–400.
Collins, S. D. & N. E. McIntyre, 2015. Modeling the distribution of odonates: a review. Freshwater Science 34: 1144–1158. https://doi.org/10.1086/682688.
de Melo, L. S. O., M. V. Garey & D. de Cerqueira Rossa-Feres, 2018. Looking for a place: how are tadpoles distributed within tropical ponds and streams? Herpetology Notes 11: 379–386.
De Souza, Y. C. M., F. S. Annibale, L. G. Carvalheiro, T. S. Vasconcelos & D. D. C. Rossa-Feres, 2022. Differential behavioral responses of benthic and nektonic tadpoles to predation at varying water depths. Canadian Journal of Zoology 100: 526–538. https://doi.org/10.1139/cjz-2021-0236.
Do Prado, V. H., M. G. Fonseca, F. V. De Almeida, O. N. Junior & D. D. C. Rossa-Feres, 2009. Niche occupancy and the relative role of micro-habitat and diet in resource partitioning among pond dwelling tadpoles. South American Journal of Herpetology 4: 275–285. https://doi.org/10.2994/057.004.0311.
Dunn, P. K. & G. K. Smyth, 1996. Randomized quantile residuals. Journal of Computational and Graphical Statistics 5: 236–244. https://doi.org/10.2307/1390802.
Ferrari, M. C., F. Messier & D. P. Chivers, 2007. Degradation of chemical alarm cues under natural conditions: risk assessment by larval woodfrogs. Chemoecology 17: 263–266. https://doi.org/10.1007/s00049-007-0381-0.
Gascon, C., 1989. Predator-prey size interaction in tropical ponds. Revista Brasileira De Zoologia 6: 701–706. https://doi.org/10.1590/S0101-81751989000400016.
Gazzola, A., B. Guadin, A. Balestrieri & D. Pellitteri-Rosa, 2023. Effects of predation risk on the sensory asymmetries and defensive strategies of Bufotes balearicus tadpoles. Animal Cognition 26: 491–501. https://doi.org/10.1007/s10071-022-01687-5.
Gee, J. H. & R. C. Waldick, 1995. Ontogenetic buoyancy changes and hydrostatic control in larval anurans. Copeia 1995: 861–870. https://doi.org/10.2307/1447034.
Gosner, K. L., 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16: 183–190.
Hartman, R. & S. Lawler, 2014. Evidence for contemporary evolution of behavioural responses to introduced fish. Animal Behaviour 97: 213–220. https://doi.org/10.1016/j.anbehav.2014.09.021.
Helfman, G. S., 1989. Threat-sensitive predator avoidance in damselfish-trumpetfish interactions. Behavioral Ecology and Sociobiology 24: 47–58. https://doi.org/10.1007/BF00300117.
Iglesias-Carrasco, M., C. Cabido & T. J. Ord, 2022. Natural toxins leached from Eucalyptus globulus plantations affect the development and life-history of anuran tadpoles. Freshwater Biology 67: 378–388. https://doi.org/10.1111/fwb.13847.
Jara, F. G., 2008. Differential vulnerability of Physalaemus pustulosus tadpole size classes to predation by the water spider Thaumasia sp. (Physauridae). Amphibia-Reptilia 29: 432–437.
Jara, F. G. & M. G. Perotti, 2010. Risk of predation and behavioural response in three anuran species: influence of tadpole size and predator type. Hydrobiologia 644: 313–324. https://doi.org/10.1007/s10750-010-0196-9.
Kloh, J. S., C. C. Figueredo, D. B. Provete & P. C. Eterovick, 2023. Taste for pollen comes in different shapes: consumption by tadpoles from three divergent ecomorphotypes. Journal of Zoology 320: 42–52. https://doi.org/10.1111/jzo.13051.
Kopp, K., M. Wachlevski & P. C. Eterovick, 2006. Environmental complexity reduces tadpole predation by water bugs. Canadian Journal of Zoology 84: 136–140. https://doi.org/10.1139/z05-186.
Lima, S. L., 2002. Putting predators back into behavioral predator–prey interactions. Trends in Ecology & Evolution 17: 70–75. https://doi.org/10.1016/S0169-5347(01)02393-X.
Lutz, A. 1925. Batraciens du Brésil. Comptes Rendus et Mémoires Hebdomadaires des Séances de la Société de Biologie et des ses Filiales. Paris 93(2): 211–214.
Maia, C. M. & G. L. Volpato, 2013. Environmental light color affects the stress response of Nile tilapia. Zoology 116: 64–66. https://doi.org/10.1016/j.zool.2012.08.001.
Mamede, J. & F. Nomura, 2021. Dendropsophus minutus (Hylidae) tadpole evaluation of predation risk by fishing spiders (Thaumasia sp.: Pisauridae) is modulated by size and social environment. Journal of Ethology 39: 217–223. https://doi.org/10.1007/s10164-021-00696-0.
Martins, Í. M., A. D. S. Vasconcellos, T. Mota & P. C. Eterovick, 2021. Detectability is in the eye of the beholder – the role of UV reflectance on tadpole detection and predation by a passerine bird. Behavioral Ecology and Sociobiology 75: 1–14. https://doi.org/10.1007/s00265-021-02983-9.
McCollum, S. A. & J. Van Buskirk, 1996. Costs and benefits of a predator-induced polyphenism in the gray treefrog Hyla chrysoscelis. Evolution 50: 583–593. https://doi.org/10.1111/j.1558-5646.1996.tb03870.x.
McDiarmid, R. W. & R. Altig, 1999. Research: materials and techniques. In McDiarmid, R. W. & R. Altig (eds), Tadpoles: The Biology of Anuran Larvae The University of Chicago Press, Chicago: 7–23.
Mettler, C. A., M. Aguirre-Morales, J. Harmeson, W. L. Robinson & B. E. Carlson, 2021. Effects of the herbicide metolachlor and fish presence on pond mesocosm communities. The American Midland Naturalist 186: 199–214. https://doi.org/10.1674/0003-0031-186.2.199.
Noland, R. & G. R. Ultsch, 1981. The roles of temperature and dissolved oxygen in microhabitat selection by the tadpoles of a frog (Rana pipiens) and a toad (Bufo terrestris). Copeia 1981: 645–652. https://doi.org/10.2307/1444570.
Peacor, S. D., 2006. Behavioural response of bullfrog tadpoles to chemical cues of predation risk are affected by cue age and water source. Hydrobiologia 573: 39–44. https://doi.org/10.1007/s10750-006-0256-3.
Pritchard, G., 1965. Prey capture by dragonfly larvae (Odonata; Anisoptera). Canadian Journal of Zoology 43: 271–289. https://doi.org/10.1139/z65-026.
R Core Team, 2022. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. Accessed from https://www.r-project.org/
Rae, J. & D. Murray, 2019. Pathogen vs. predator: ranavirus exposure dampens tadpole responses to perceived predation risk. Oecologia 191: 325–334. https://doi.org/10.1007/s00442-019-04501-1.
Ramamonjisoa, N., C. Oiire, X. J. Zheng & S. Kimura, 2019. Predation decreases cohort foraging activity and growth, yet increases individual size variation in prey. Evolutionary Ecology 33: 233–242. https://doi.org/10.1007/s10682-019-09977-0.
Relyea, R. A., 2001. Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82: 523–540. https://doi.org/10.2307/2679877.
Relyea, R. A., P. R. Stephens & J. I. Hammond, 2021. Phylogenetic patterns of trait and trait plasticity evolution: Insights from tadpoles. Evolution 75: 2568–2588. https://doi.org/10.1111/evo.14338.
Rossa-Feres, D. D. C. & F. Nomura, 2006. Characterization and taxonomic key for tadpoles (Amphibia: Anura) from the northwestern region of São Paulo State, Brazil. Biota Neotropica 6: 1–26. https://doi.org/10.1590/S1676-06032006000100014.
Rossa-Feres, D. D. C., J. Jim & M. G. Fonseca, 2004. Diets of tadpoles from a temporary pond in southeastern Brazil (Amphibia, Anura). Revista Brasileira De Zoologia 21: 745–754. https://doi.org/10.1590/S0101-81752004000400003.
Rossa-Feres, D. D. C., M. D. Venesky, F. Nomura, P. C. Eterovick, M. F. V. Candioti, M. Menin, F. A. Juncá, L. C. Schiesari, C. F. B. Haddad, M. V. Garey, L. A. dos Anjos & R. Wassersug, 2015. Taking tadpole biology into the 21st century: a consensus paper from the First Tadpoles International Workshop. Herpetologia Brasileira 4: 48.
Schalk, C. M., 2016. Predator-induced phenotypic plasticity in an arid-adapted tropical tadpole. Austral Ecology 41: 409–416. https://doi.org/10.1111/aec.12327.
Schneider, C. A., W. S. Rasband & K. W. Eliceiri, 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. https://doi.org/10.1038/nmeth.2089.
Schulze, A., M. Jansen & G. Köhler, 2015. Tadpole diversity of Bolivia’s lowland anuran communities: molecular identification, morphological characterization, and ecological assignment. Zootaxa 4016: 1–111. https://doi.org/10.11646/zootaxa.4016.1.1.
Scribano, G., A. Balestrieri, A. Gazzola & D. Pellitteri-Rosa, 2020. Strong behavioural defensive responses of endemic Rana latastei tadpoles induced by a native predator’s odour. Ethology 126: 922–930. https://doi.org/10.1111/eth.13072.
Sergio, C., R. Luca & F. Olivier, 2021. Plasticity and flexibility in the anti-predator responses of treefrog tadpoles. Behavioral Ecology and Sociobiology 75: 1–14. https://doi.org/10.1007/s00265-021-03078-1.
Steindachner, F. 1863. Über einige neue Batrachier aus den Sammlungen des Wiener Museums. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Classe 48: 186–192.
Teplitsky, C., S. Plénet, J. P. Léna, N. Mermet, E. Malet & P. Joly, 2005. Escape behaviour and ultimate causes of specific induced defences in an anuran tadpole. Journal of Evolutionary Biology 18: 180–190. https://doi.org/10.1111/j.1420-9101.2004.00790.x.
Tu, M. C., C. W. Chu & K. Y. Lue, 1999. Specific gravity and mechanisms for its control in tadpoles of three anuran species from different water strata. Zoological Studies 38: 76–81.
Underwood, A. J., 2009. Components of design in ecological field experiments. Annales Zoologici Fennici 46: 93–111.
Van Buskirk, J., 2001. Specific induced responses to different predator species in anuran larvae. Journal of Evolutionary Biology 14: 482–489. https://doi.org/10.1046/j.1420-9101.2001.00282.x.
Vasconcelos, T. D. S., T. G. Dos Santos, D. D. C. Rossa-Feres & C. F. Haddad, 2011. Spatial and temporal distribution of tadpole assemblages (Amphibia, Anura) in a seasonal dry tropical forest of southeastern Brazil. Hydrobiologia 673: 93–104. https://doi.org/10.1007/s10750-011-0762-9.
Venesky, M. D., D. C. Rossa-Feres, F. Nomura, G. V. De Andrade, T. L. Pezzuti, V. T. T. De Sousa, C. V. Anderson & R. J. Wassersug, 2013. Comparative feeding kinematics of tropical hylid tadpoles. Journal of Experimental Biology 216: 1928–1937. https://doi.org/10.1242/jeb.082040.
Wellborn, G. A., D. K. Skelly & E. E. Werner, 1996. Mechanisms creating community structure across a freshwater habitat gradient. Annual Review of Ecology and Systematics 27: 337–363. https://doi.org/10.1146/annurev.ecolsys.27.1.337.
Zuur, A. F., E. N. Ieno, N. J. Walker, A. A. Saveliev & G. M. Smith, 2009. Mixed effects models and extensions in ecology with R, Springer, New York.
Acknowledgements
We thank Luiz H. Florindo for supporting this study with equipment and infrastructure, Paulo De Marco Jr for identifying Odonata naiads, and Carlos E. de Sousa for helping us during collections.
Funding
This study was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (88887.509980/2020-00 to Y.C.M.S.) and by Conselho Nacional de Desenvolvimento Científico e Tecnológico (Proc. 304760/2021-8 to D.C.R-F.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Handling editor: Gary Bucciarelli
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 674 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Souza, Y.C.M., Annibale, F.S., Pelinson, R.M. et al. Behavioral responses of benthic and nektonic tadpoles to the presence of a benthic predator. Hydrobiologia (2024). https://doi.org/10.1007/s10750-024-05652-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-024-05652-w