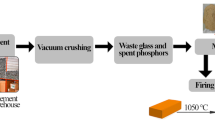
Abstract
In this study, utilization of waste fluorescent lamp as mineral filler in asphalt mixture was investigated. The phosphorus powder of fluorescent lamps was removed by sieving and washing of the samples. Toxicity of waste lamps was investigated. Glass sample was used in the ratio of 25%, 50%, 75% and 100% to mineral filler. Optimum bitumen ratio was determined using super-pave mixing method which requires volumetric properties of the mixtures. Finally, indirect tensile tests were carried out on the dry and wet samples. Results of toxicity test indicated that glass of waste lamp was not classified as hazardous waste after washing. Indirect tensile test show that dry resistance values of the sample containing waste glass were similar to control sample. However, after the samples were conditioned, indirect tensile test show that wet values were significantly decreased by increasing glass content. As a result, when the glass of waste fluorescent lamp was used as mineral filler, the asphalt mixtures became more sensitive to moisture. Since this sensitivity will not be important, it is recommended to use waste fluorescent lamp glasses as a mineral filler for the “binder layer” that is not directly exposed to contact with water.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollution and waste problem are among the first issues that we need to look for solutions due to the increasing population and consumption habits. The latest policy in this regard is zero waste. New and strict sanctions for the control and reduction of wastes are adopted by the countries of the world due to zero waste policies [1]. Therefore, reducing waste at its source, reuse and recycling applications are gaining importance every day. Furthermore, achieving a circular economy by promoting the 3Rs (Reduce, Reuse and Recycle) has an important place among government policies today. Glass waste can be released in large volumes among municipal solid wastes [2] and promising element of solid waste recycling, and investigations on the possibility of using waste glass in different ways are increasing in the literature. Globally, amount of the waste glass is estimated to be nearly ten million tons per year [3].
One of the wastes with a high amount of glass is waste fluorescent lamps (WFLs). Glass corresponds to 90–95% of the total weight of end-of-life fluorescent lamps [4,5,6]. WFLs belong to category of e-waste which are generated due to the short life resulted from increased company competition [7] and the annual production worldwide of e-wastes are approximately 1.5 billion units [6, 8]. Considering the zero waste policies, it should be stored separately from other recyclable solid wastes and should be received from the source for certain periods by licensed hazardous waste collection companies due to its mercury content. Mercury is described as one of the most toxic elements [9, 10], especially the form of methylmercury form can cause poisoning in humans [11]. Significant methylmercury poisoning events occurred in Minamata in 1956, affecting thousands [12, 13], Niigata Province in 1969, and Ontario in 1965 [14]. A convention had been held after the Minamata disaster as named The Minamata Convention on Mercury, recognized that mercury was a global chemical because of its ability to bioaccumulate in the environment and its persistence in ecosystems. Under the convention, the members should take precautions regarding the temporary storage of mercury in an environmentally friendly manner, taking into account any directives. Members shall cooperate with each other and with relevant intergovernmental and other organizations, as appropriate, to build capacity for the environmentally sound temporary storage of mercury and mercury compounds [12].
The total average value of mercury mass in WFLs varies with lamp type and year of manufacture [15]. The mercury content of WFLs has been reported as 6–12 mg/lamp [4] and between 0.72 and 115 mg/lamp [16] for T8 and T12 type lamps. The average mercury content is also stated as about 30 mg/lamp [17]. Other types of WFLs are compact fluorescent lamps (CFLs). According to data reported by Rey–Raap and Gallardo [18], the mercury concentration in CFLs is in the range of 2.37 ± 0.50 mg/g (Average of CFL from various manufacturers, production year and locations). As these authors concluded [3, 19], at the end of the lamp's life, 13.66% of the mercury is dispersed in the glass during operating hours, while the phosphor powder (PP) contains 85.76% mercury (the remaining 0.58% is in the vapor phase). As a result, this glass waste is classified as hazardous according to both European Union [6], USEPA [20] and Turkey regulations. In the code 20 01 21-Fluorescent tubes and other wastes containing mercury must also be collected according to the Turkish regulation 28300/2012 and should be processed in various streams in recycling facilities but are disposed of by burying [21]. According to our estimates based on regional and national fluorescent lamp sales and associated with official values of disposed spent fluorescent lamps, the disposal rate is increased in Turkey by the years. However, the controlled disposing of WFL is only 17% of total consumption in 2012. The remains (83%) usually end up in dumpsters or trash containers and ultimately, in landfills [21]. Before hazardous wastes containing mercury can be landfilled according to disposal restriction standards (LDR). The LDR standard for lamps is 25 μg/L, as determined by The Toxicity Characteristic Leaching Procedure test (TCLP) [21, 22]. Therefore, the aim of the study is to develop an alternative disposal and recycle method of glasses by landfilling. The increase in the number of applicable recycling research will contribute to the development of solutions for uncontrolled wastes.
The primary use of recycled glass is construction industry which can consume enormous amount of these materials [23]. Most of them indicated that alkali–silica reaction is considered as a major obstacle that restrains the use of recycled glass in concrete [2]. Recent studies deal with how to overcome this obstacle [23, 24]. Another important utilization of recycled glass is asphalt pavements. There are numerous studies in the literature that utilize recycled glasses as an aggregate for cement concrete production. Wartman et al. [25] stated that crushed glass can be used as aggregates, because it is abundantly available, environmentally friendly, and relatively cheap to natural aggregates. Nicholls and Lay [26] reported that up to 30% addition of crushed glass into asphalt mixture does not alter workability and compaction performance. Other studies also showed that utilization of recycled glass at lower ratios has no negative impact on the performance of asphalt mixtures [27,28,29,30]. A study by Arabani et al. [31] reveals that utilization of crushed glass having the maximum size of 4.75 mm with 3–5% anti-stripping aging in the asphalt mixtures had higher modulus of elasticity, lower sensitivity to temperature changes, and a longer fatigue life than the conventional mixtures. Many researchers found that glass powder (GP) as filler does not decline the performance of asphalt mixtures if optimum glass ratio in the mixture is obtained [32]. Optimal glass content seems to be in between 7 and 15% [29, 31, 33]. Simone et al. [34] also observed that incorporation of GP as filler increased the stiffness of asphalt mastics. As a result, this option may result in formation of asphalt mixes having better bearing capacity and rutting resistance [34].
Waste glass does not break down easily on its own, so it can remain in the field for many years [23]. Studies have shown that there is a potential amount of glass that can be recovered from WFLs [19, 35]. Waste lamps are considered as highly hazardous and risky for recycling. Recycling of used lamps may generate glass, metal and pulverized phosphorous thus the amount of waste that would end up in the landfills [36]. The most critical obstacle in the recycling of the lamp is removal of the mercury deposited in PP attached to the surface of the glass [37,38,39]. There is only one study in the literature which have utilized glass of spent fluorescent lamps as filler in asphalt mixture. The study presented that incorporating used fluorescent lamps (2–5%) can provide an alternative resource for low to moderate traffic road surfaces after subjected to tests including the Marshall, indirect tensile stiffness modulus, wheel track, indirect tensile fatigue and dynamic creep test [40].
In this study, utilization of glass of used fluorescent lamps in the asphalt mixtures was investigated using a different approach. The superpave method was used for asphalt design in the study. A parallel issue is the recent depletion of scarce raw materials used in construction of road, forcing highway authorities to seek sustainable and new resources [40]. Due to reuse and recycle of wastes policies, reusing glass waste as part of electronic waste in asphalt pavements instead of more traditional mineral fillers can be an economical and environmental solution.
Materials and methods
Preparation of samples
Waste lamps were collected from official buildings located in Isparta and provided by a company operating in Turkey. The company has a license from the Ministry of Environment and Urbanization to collect WFLs. Waste tubular fluorescent (type T8 and T12) and compact fluorescent lamps (CFLs) were selected to be used owing to their high rate of current consumption (Turkey in 2018; 70% CFL, 30% tubular lamps) [41] and their relatively higher mercury content. Tubular lamps are classified as hazardous waste and their use is decreasing day by day [42]. For this reason, CFLs samples and mixture samples (type T8 and T12) were used in toxicity tests. Mix sample was prepared by mixing 30% pulverized tubular lamps and 70% pulverized CFLs samples to simulate a more realistic situation. The amount of mercury vapor released during the cracking processes was measured with the Jerome 431-XE mercury vapor detector. This device detects elemental mercury vapor and measures in the range of 0.003–0.999 mg. Samples are shown in Table 1 for TCLP tests. The methods and procedures applied to the samples are given in Table 2.
The washing process was applied efficiently separate PP from fluorescent lamp glasses. The washing process was carried out by mixing 20 g of each sample in 400 ml of distilled water, at room temperature, for 18 ± 2 h, at 265 rpm in a magnetic stirrer, and the filtrate containing PP was separated with washing from the glass sample. Glass samples without PP were dried at room temperature for 24 h [42]. While mercury metal has the property of evaporation at any temperature, Raposo et al. [43] determines that the mercury that can be found in the glass samples of WFLs begins to be released the environment above 25 °C. This is because mercury is bound in the structure of the fluorescent lamp glass. For this reason, it was predicted that there was no mercury loss during the drying process at room temperature (20 °C) for 24 h.
TCLP testing
The TCLP tests were performed in 500 mL polypropylene flasks with mechanical stirrers. 400 ml of acetate buffer solution with a pH of approximately 4.66 ± 0.01 was added to 20 g glass samples. The sample was stirred at room temperature for 20 h at 30 rpm and then filtered through a 0.60 μm glass microfiber filter with the help of a vacuum pump. The mercury content was determined using atomic absorption spectrophotometer (attached with flow injection system) (PerkinElmer-FIMS 400) [4, 44,45,46].
Aggregates, filler and bitumen
Both aggregates and bitumen used in the study were obtained from Isparta Municipality Asphalt Construction Site. Aggregates used in the study consisted of clean, strong and durable grains and did not contain clay soils, plant materials, etc. Gradation of sample aggregates was determined using a sieve according to specifications.
Apparent specific gravity (ASG), volume specific gravity (VSC), water absorption rate (WAR) and volume specific gravity on a saturated basis of the aggregates were determined according to ASTM C 128-88 [47]. Apparent specific gravity of the filler was determined according to ASTM C 854-10 [48]. Penetration tests on asphalt cement samples were carried out according to TS 118 [49] using penetrometer. TS 119 [50] was applied to determine the ductility of the asphalt mixtures. Softening point test according to TS 120 [51] was also carried out to determine the consistency of the bitumen. Viscometer was used to determine the high-temperature fluidity characteristics of the bitumen. The pycnometer method was used for the determination of the specific gravity of asphalt mixture (TS EN 15326) [52]. As the bitumen material, 0.074 mm pore diameter (200 mesh) was sieved and the material that passed under the sieve was used.
Design of bituminous mixtures with the superpave method
In Superpave method, design of asphalt mixture considers the air voids, voids in the mineral aggregate (VMA), and voids filled with asphalt (VFA). The asphalt binder content in the mixture is determined using the percent air voids. VMA is called as the sum of the volumes of the air voids and the unabsorbed binder in the compacted specimen. VFA is the ratio of VMA containing asphalt binder. It is widely accepted that these volumetric properties are effective in predicting the asphalt performance [53].
The sample, which was not classified as hazardous waste because of TCLP, was used as fillers in asphalt pavements. Prepare test samples, a mixture of 1200 g is prepared in aggregates determined according to the percentage of material on the gradation sieve to be used for the design and dried in an oven at 110 °C until it reaches a constant weight. Two samples were prepared from the combinations of fluorescent flat glass used as aggregate and filler material. Glass of WFLs was used as 25%, 50%, 75% and 100% of the mineral filler, respectively. Bitumen ratios of 4%, 4.5%, 5%, 5%, 6% and 6.5% were used for each series of four. As a result, 16 samples were prepared for glass of WFLs instead of using mineral fillers. Before preparing the samples, the mold is placed in an oven for 1.5–2 h and the temperature is 150–160 °C.
Asphalt cement is also kept in an oven to become fluid. Then, liquid asphalt is poured on the gradation according to certain proportions and mixed with the aggregate in the presence of heat, and this process is continued until there are no white spots in the aggregates. Then, the thoroughly mixed gradation is poured into the mold and compressed with a machine under predetermined conditions.
After the compaction to calculate the air void contents, VMA contents, VFA contents and specific gravity values, the following equations were used:
Va = (1 – Gmb / Gmm) *100
Gmb = wD/WSSD − Wsub
Gmm = Wagg + Wb / Veff + Vb
Veff = Vagg − VBA
where Va (%); air void content, Gmb (g/cm3); bulk specific gravity, Gmm (g/cm3); maximum theoretical specific gravity, wD (g); dry weight, WSSD (g); saturated surface dry weight, Wsub (g) weight submerged in water, Wagg (g); weight of aggregate, Wb (g); weight of binder, Veff (cm3); effective volume of aggregate, Vb (cm3); volume of binder, Vagg (cm3); volume of aggregate and VBA (cm3); volume of absorbed asphalt [53,54,55]. When the air voids are calculated the corresponding VMA and VFA values are calculated using the following equations:
VMA = 100 − [(Gmb ∗ Ps)/Gsb]
VFA = VMA – Pa
where VMA (%); voids in mineral aggregate, Ps (%); aggregate content by weight of mix, Gsb (g/cm3); bulk specific gravity of the aggregate, VFA (%); voids filled with asphalt and Pa (%); percent of air voids [56, 57].
Moisture sensitivity of asphalt mixtures
Moisture sensitivity of asphalt mixes is defined as the resistance to damage in contact with water, as moisture densifies in the asphalt mixture, it can damage the bond between the asphalt binder and the aggregate. Moisture susceptibility in asphalt mixture is assessed using AASHTO T283 [58] tests. Compressed samples are first kept in an oven at 40 °C for 72 h. After the samples are taken out of the oven, they are divided into 2 groups equally and kept at room temperature until the temperature drops to 25 °C. Indirect tensile strength is determined for unconditioned samples at 25 °C. The second group of samples is kept in a 25 °C water bath for 24 h. After that samples were placed in a water-filled container with 2.5 cm of water on top and vacuum was applied until the saturation rate is 55–80%. Vacuumed samples were wrapped with cling film and kept in a deep freeze at −18 °C for 16 h. Then, the samples are kept in a water bath at 60 °C for 24 h followed by water bath in 25 °C for 2 h. Finally, they were subjected to indirect tensile test (IDT). The sensitivity of samples to water is determined by Indirect Tensile Strength Ratios (TSR). Unconditioned (IDTdry) and conditioned (IDTwet) for samples are calculated with the following equation.
TSR = IDTwet / IDTdry
TSR = Indirect tensile strength ratio
IDTwet = Average indirect tensile strength of the conditioned group (kPa)
IDTdry = Average indirect tensile strength of the unconditioned group (kPa)
Asphalt mixtures with an indirect tensile strength ratio of less than 0.8 are less resistant to moisture, but mixtures greater than 0.8 presents better resistance to moisture damage [59].
The indirect tensile test
To determine the tensile properties of asphalt concrete, an indirect tensile test is performed. The indirect tensile test (IDT) involves the fracture of the cylindrical test specimen by compressing under load, which is applied vertically to the cylindrical test sample. This test simulates the fracture properties of road surfaces. IDT value is calculated using the following equation [58]:
IDT = 2 Pmax./\(\pi\) T D
where:
Pmax = Maximum applied load (kN),
T = Thickness of the sample,
D = Diameter of sample.
Results and discussion
Mercury levels in the working environment
The amount of mercury released from a broken fluorescent compact lamp was reported to be above 1 mg [45] and 1/3rd of the total amount of mercury in the lamp would disperse into the environment within 8 h [60]. The breaking, dismantling and sieving of WFLs carried out under vacuum. Mercury released into the environment was measured 0.011–0.015 mg Hg/m3 for the 6 h working time. The mercury emissions of the indoor working environment did not exceed OSHA (Occupational Safety & Health Administration) standard (exposure threshold of 0.1 mg Hg/m3) [61].
Results of TCLP analysis
The test results of the samples prepared for the TCLP tests were compared with the storage limits in the field. The mean values of these data are USEPA (200 μg/L land storage limit-LDR: Land Disposal Restriction) [45]. The comparison with the limit specified in the Regulation on the Control of Hazardous Wastes (< 20 μg/L) and the Regulation on the Regular Storage of Wastes (200 μg/L) is shown in Table 3.
In the TCLP tests performed before and after the PP is separated, it has been determined that 75% of the toxicity can be reduced when the PP is separated by the sieving method in the CFL sample, and in addition, the toxicity can be reduced by 69% in the mixture sample by the sieving method. Using the PP washing procedure, the toxicity of the glass samples was reduced below the USEPA limit value (0.2 mg/L) and the Hazardous Waste Control Regulation limit value (0.02 mg/L). Since the TCLP value (0.017 mg/L) of the washed mixture sample is not in the hazardous waste class, it was decided to use the washed fluorescent lamp sample to be tested as fillers on the road pavement.
Physical properties of mineral aggregate and apparent specific gravity of mineral filler
The test results performed to determine the specific gravity of the fine and coarse aggregate used in this study are given in Table 4. The results were found to be within the limits specified in the specification. The specific gravity test results of the mineral fillers used are given in Table 5.
Properties of bitumen and aggregate gradation used in samples
The specific gravity of bitumen was determined as 0.97 gr/cm3. Other properties of bitumen used in the study are given in Table 6. The aggregate gradation used in the experiment was selected according to the limits determined in the General Directorate of Highways wear layer Type-1 conditioning. The gradation values used are given in Fig. 1.
Determination of optimum bitumen ratio
Percentage of bitumen was determined as 4.5% for the control samples. When glass of WFL (25%, 50%, 75%, and 100%) was used as filler, optimum bitumen percentages were determined as 5.29%, 4.78%, 5.13% and 4.94%, respectively, using the graphs of VMA and VFA graphs.
One of the important properties of bituminous hot mixes is the air void ratio. In Superpave method, the asphalt content is determined at 4% air voids. The most important reason for defining this limit range is to prevent a possible deterioration in hot bituminous mixtures. Another reason is to ensure the water impermeability of the lower layers of the hot bituminous mixtures, sufficient stability and to reduce the oxidation of bitumen. If oxidation occurs, it reduces the penetration value of the binder and causes the coating to become brittle and brittle after a while [62].
As illustrated in Fig. 2a–d, when the bitumen ratio increases, the air void ratio decreases. With increasing density, physical properties such as durability, impermeability and stability also increase. In high density mixtures, aging which occurs because of bitumen oxidation and ultraviolet rays is slower and, therefore, an increase in durability and a decrease in deterioration due to peeling. Figure 3a–d illustrates that as the ratio of bitumen increases, the practical specific gravity also increases. The relationship between the weight ratio of bitumen and the practical specific gravity is almost directly proportional.
Another important feature of mixtures is the void ratio between mineral aggregate (VMA) which is the air voids between aggregate particles, including bitumen filled voids in compacted pavement mixes. The VMA represents the usable volume required for the bitumen and air space in the mixture. Therefore, as VMA increases, the thickness of the bitumen film on the aggregates increases and thus it can be said that the durability of the mixture will increase [63]. In the Highways Technical Specification of 2013, the lowest VMA value for aggregates used in the wear layer is determined as 14%. The relationship between the VMA and the ratio of bitumen is given in Fig. 4a–d When fluorescent lamp glass was used as filler, VMA values were found to be higher than 14% for all samples tested. In general, VMA values are decreasing with increasing bitumen ratio.
The VFA is an important measure of relative durability. Low VFA means there is not enough asphalt to provide durability. The VFA also affects the plasticity and coating friction coefficient in hot bituminous mixtures [63]. Most specifications require VFA of 70–80% during the design phase. In the Highways Technical Specification (2013), VFA (%) value should be between 65% and 75%. The relationship between the VFA and the ratio of bitumen is given in Fig. 5a–d. As shown in figures increase in the bitumen ratio cause an increase in the VFA value. When glass of WFL (25%, 50%, 75%, and 100%) was used as filler, optimum bitumen ratios that meet VFA values were determined as 5.3%, 4.78%, 5.12% and 4.94%, respectively.
Indirect tensile strength test results
Results of Indirect Tensile Test (IDT) conducted according to AASHTO T-283 of both wet and dry samples were presented in Fig. 6 As shown in the figure the IDT values of control samples are higher than other samples. As can be seen in Fig. 6, the IDTwet values decrease proportionally to the amount of waste glass used in the study. Performance of samples containing waste glass is inferior to the control samples. As stated by Paul et al. [32] if the optimum ratio of glass is used in the asphalt mixture, the performance would not decline. Many researchers indicated that optimal glass content is between 7% and 15% [29, 33, 64]. Some investigations also found that small amount of glass addition to the asphalt mixture may improve some properties of the mixtures [34, 40]. GP was attributed to low asphalt absorption by the glass and high silica content in its composition [65,66,67].
Indirect Tensile Strength (IDTwet, IDTdry) which show the sensitivity of the samples to moisture shown in Fig. 7. TSR of the samples containing waste glass as filler did not confirm the limit value of 0.8 which is specified in the standards. TSR is evidence of the strength loss resulting from damage caused by “stripping” under laboratory controlled accelerated water conditioning. The results of study indicate that asphalt samples manufactured using waste glass will suffer from long-term susceptibility to stripping. To overcome the effects of water damage, hydrated lime which is an anti-stripping additive is suggested in all asphalt mixtures. In addition, this issue can be looked at with another positive approach. The bituminous course of a road pavement is generally constructed in two sub-layers. The upper part is called as bituminous surface course, and the lower part called binder course. The bituminous course of a road pavement is generally constructed in two sub-layers. The upper part is called as bituminous surface course, and the lower part called binder course. While the bituminous surface course is exposed to natural factors, such as rainwater and traffic, the binder course is not directly exposed to these effects [68]. For this reason, since the waste material used in the study will not come into direct contact with water, it can be used effectively in the binder course and is suitable for a sustainable use.
As a continuation of the study, new studies which aiming to determine the optimum asphalt mixtures can be research by mixing other wastes such as waste of tire rubber, coking sulfur paste [69, 70] or other wastes which have been reported successful results with WFLs in asphalt production in the literature. On the other hand, there are different literature studies on the recycling of waste fluorescent lamps to control them. According to Asari et al. [71] stated that technologies and systems should be developed to ensure the use of mercury to be recovered by establishing a closed-circuit recycling system in Japan for fluorescent lamp waste management. In addition to promoting public participation and collection systems in the system, it is of great importance to establish regulatory measures, new technologies for alternatives to the use of mercury, and new recycling business models. To manage solid waste management more effectively and to recycle some of the material for its economic value, there is a tremendous demand for recycled waste nowadays, which was not there before [72]. Therefore, researchers are trying to create many alternative elements from various types of solid waste.
Conclusions
WFLs can cause serious harm to both the environment and human health due to the mercury metal they contain. Removal of phosphor dust by washing process, the mercury metal can be reduced and the WFL glasses can be recovered without being evaluated as hazardous waste. The use of waste glass in different layers on the road pavement prevents environmental pollution and can make serious contributions to the economy. In the TCLP tests performed before and after the PP is separated, it has been found that when the PP is separated by the sieving method in the glass samples, the toxicity was reduced by 75%. Since the TCLP value (0.017 mg/L) of the washed samples is lower than limit value (0.02 mg/L), the glass of WFLs tested as filler for the asphalt mixtures. In the continuation of the study, the mercury in the form of PP separated by washing from fluorescent lamps can be removed by various leching studies and PP can be used as a non-hazardous waste in highway applications for lighting purposes.
The appropriate binder ratios were determined using superpave mixing method. The optimum binder ratios were found as 4.5%, 5.30%, 4.78%, 5.12% and 4.94% for the control sample, samples containing 25%, 50%, 75% and 100% waste glass, respectively. The binder ratio of the samples containing waste glass was higher than control sample which may increase the cost of the mixture.
The results of the study indicates that IDTdry strength of the samples containing waste glass close to the control sample, while IDTwet strength values were decreasing with increasing glass ratio. The test results show that the use of waste glass makes the sample more sensitive to moisture, that is, its strength will be significantly reduced when exposed to water and moisture. When the TSR ratios were examined, it was seen that the values obtained were below the limit value requested in the specification. As a result, when the glass of WFL was used as mineral filler, the asphalt mixtures became more sensitive to moisture. Since this sensitivity will not be important, it is recommended to use WFL glasses as a mineral filler for the “binder layer” that is not directly exposed to contact with water. Alternatively, to overcome the effects of water damage, hydrated lime which is an anti-stripping additive can be used.
References
Coskun S (2021) Effect of the Covid-19 pandemic period on zero waste awareness: a scale development survey in Turkey. Glob Nest J. 23:581–589. https://doi.org/10.30955/gnj.004152
Mallum I, MohdSam ARM, Lim NHAS, Omolayo N (2021) Sustainable utilization of waste glass in concrete: a review. SILICON. https://doi.org/10.1007/s12633-021-01152-x
Wu S, Yang W, Xue Y (2004) Preparation and properties of glass-asphalt concrete. Key laboratory for silicate materials science and engineering of ministry of education, Wuhan University of Technology, Wuhan
Coskun S, Civelekoglu G (2015) Recovery of mercury from spent fluorescent lamps via oxidative leaching and cementation. Water Air Soil Pollut 226(6):1–13. https://doi.org/10.1007/s11270-015-2391-9
Lee CH, Popuri SR, Peng YH, Fang SS, Lin K, Fan KS, Chang TC (2015) Overview on industrial recycling technologies and management strategies of end-of-life fluorescent lamps in Taiwan and other developed countries. J Mater Cycles Waste Manag 17:312–323
Novais RM, Ascensao G, Sebra MP, Labrincha JA (2016) Waste glass from end-of-life fluorescent lamps as raw material in geopolymers. Waste Manag 52:245–255. https://doi.org/10.1016/j.wasman.2016.04.003
Wang C, Wang H, Cao Y (2019) Waste printed circuit boards as novel potential engineered catalyst for catalytic degradation of orange II. J Clean Prod 221:234–241
Wagner TP (2011) Compact fluorescent lights and the impact of convenience and knowledge on recycling rates. Waste Manag 31:1300–1306
Smocovich MC (2000) Emergencias químicas provocadas por mercurio y sus compuestos: su prevención y control. Tesis por la Universidad Nacional de General San Martín
Wong CSC, Duzgoren-Aydin N, Aydin A, Wong MH (2006) Sources and trends of environmental mercury emissions in Asia. Sci Total Enrivon 368:649–662
Diez S (2009) Human health effects of methylmercury exposure. Rev Environ Contam and Toxicol 198:111–132. https://doi.org/10.1007/978-0-387-09647-6_3
United Nations Environment Programme (2019). Minamata Convention on mercury text and annexes. https://www.mercuryconvention.org/sites/default/files/2021-06/Minamata-Conventionbooklet-Sep2019-EN.pdf
Yorifuji T, Tsuda T, Inoue S, Takao S, Harada M, Kawachi I (2013) Critical appraisal of the 1977 diagnostic criteria for Minamata Disease. Arch Environ Occup H 68(1):22–29
Harada M, Hanada M, Tajiri M, Inoue Y, Hotta N, Fujino T (2011) Mercury poisoning in first nations groups in Ontario, Canada: 35 years of Minamata disease in Canada. J Minamata Stud 3:3–30
NEMA (2000) Environmental impact analysis: spent mercury-containing lamps, 4th edn. NEMA, Rosslyn
Truesdale RS, Beaulieu SM, Pierson TK (1993) Management of used fluorescent lamps: preliminary risk assessment. Final report. Research Triangle Institute, USA.
Jang M, Hong SM, Park JK (2005) Characterization and recovery of mercury from spent fluorescent lamps. Waste Manag 25:5–14
Rey-Raap N, Gallardo A (2012) Determination of mercury distribution inside spent compact fluorescent lamps by atomic absorption spectrometry. Waste Manag 32:944–948. https://doi.org/10.1016/j.wasman.2011.12.001
Pitarch AM, Reig L, Gallardo A, Soriano L, Borrachero MV, Rochina S (2021) Reutilisation of hazardous spent fluorescent lamps glass waste as supplementary cementitious material. Constr Build Mater 292:123424. https://doi.org/10.1016/j.conbuildmat.2021.123424
USEPA (2007) Fluorescent lamp disposal and recycling in EPA Region 2 A guide for businesses in NJ, NY, PR and VI. https://www3.epa.gov/region02/waste/spent-lamp.pdf
Coskun S, Ozgur C, Guncan A, Coskunsu S, Civelekoglu G (2014) Management of mercury risk originated from spent fluorescent lamps in Turkey. Conference: 4th international conference on industrial and hazardous waste management, Chaina, Crete, Greece
Durão WA, Castro CA, Windmöller CC (2008) Mercury reduction studies to facilitate the thermal decontam-ination of phosphor powder residues from spent fluorescent lamps. Waste Manag 28:2311–2319
Tamanna N, Sutan NM, Lee DTC, Yakub İB (2013) Utilization of waste glass in concrete. 6th international engineering conference, energy and environment (ENCON 2013), Kuching, Sarawak, Malaysia
Sharifi NP, Jafferji H, Reynolds SE, Blanchard MG, Sakulich AR (2016) Application of lightweight aggregate and rice husk ash to incorporate phase change materials into cementitious materials. J Sustain Cem -Based Mater 5(6):349–369. https://doi.org/10.1080/21650373.2016.1207576
Wartman J, Grubb DG, Nasim ASM (2004) Select engineering characteristics of crushed glass. J Mater Civ Eng 16(6):526. https://doi.org/10.1061/(ASCE)0899-1561(2004)16:6(526)
Nichols JC, Lay, J (2002) Crushed glass in macadam for binder course and road base layers. Proceedings of 4th European symposium on performance of bituminous and hydraulic materials in pavements, BITMAT 4, University of Nottingham, U.K., 11–12 April 2002, A.A.Balkema Publishers, Netherlands pp. 197–212
Su N, Chen JS (2002) Engineering properties of asphalt concrete made with recycled glass. Resour Conserv Recycl 35(4):259–274. https://doi.org/10.1016/S0921-3449(02)00007-1
Wu SP, Yang WF, Xue YJ (2003) Design and preparation of steel slag SMA. J Wuhan Univ Technol-Mat Sci Edit 18(3):86–88
Jony HH, Al-Rubaie MF, Jahad IY (2011) The effect of using glass powder filler on hot asphalt concrete mixtures properties. Eng Tech Journal 29(1):44–57
Saltan M, Uz V, Betul O (2015) Use of glass waste as mineral filler in hot mix asphalt. Sci Eng Compos 22(3):271–277. https://doi.org/10.1515/secm-2013-0135
Arabani M, Mirabdolazimi SM, Ferdowsi B (2012) Modeling the fatigue behaviors of glasphalt mixtures. Sci Iran 19(3):341–345. https://doi.org/10.1016/j.scient.2012.02.021
Paul D, Suresh M, Pal M (2021) Utilization of fly ash and glass powder as fillers in steel slag asphalt mixtures. Case Stud Constr Mater 15:e00672. https://doi.org/10.1016/j.cscm.2021.e00672
Ogundipe OM, Nnochiri ES (2020) Evaluation of the effects of waste glass in asphalt concrete using the Marshall test. Eng Rev 40(2):24–33. https://doi.org/10.30765/er.40.2.04
Simone A, Mazzotta F, Eskandarsefat S, Sangiorgi C, Vignali V, Lantieri C, Dondi G (2017) Experimental application of waste glass powder filler in recycled dense-graded asphalt mixtures. Road Mater Pavement Des 20:592–607. https://doi.org/10.1080/14680629.2017.1407818
Wijesekara RGS, Navarro R, Matsumura M (2011) Removal and recovery of mercury from used fluorescent lamp glass by pyrolysis. J Natl Sci Found 39(3):235–324. https://doi.org/10.4038/jnsfsr.v39i3.3627
Coskun S, Civelekoglu G (2014) Characterisation of waste fluorescent lamps to investigate their potential recovery in Turkey. Int J Glob Warm 6(2/3):140–148. https://doi.org/10.1504/IJGW.2014.061004
Raap NR, Gallardo A (2013) Removal of mercury bonded in residual glass from spent fluorescent lamps. J Environ Manage 115:175–178. https://doi.org/10.1016/j.jenvman.2012.11.012
Rhee SW, Choi HH, Park HS (2013) Performance evaluation of material separation from spent fluorescent lamps using the thermal end-cutting method. J Mater Cycles Waste Manag 15:503–509. https://doi.org/10.1007/s10163-013-0172-3
Choi Y, Rhee SW (2017) Evaluation of energy consumption in the mercury treatment of phosphor powder from spent fluorescent lamps using a thermal process. Sustainability 9(11):2013
Gedik A, Selcuk S, Lav AH (2021) Investigation of recycled fluorescent lamps waste as mineral filler in highway construction: a case of asphaltic pavement layers. Resour Conserv Recycl 168:106290
Republic of Turkey Ministry of Environment, Urbanization and Climate (2019) Hazardous waste licensed companies. https://eizin.cevre.gov.tr/Rapor/BelgeArama.aspx. Accessed 26 Feb 2019
Coskun S (2013) Mercury recovery from waste fluorescent lamps and process optimization. Dissertation, Sulayman Demirel University
Raposo C, Windmöller CC, Durao WA (2003) Mercury speciation in fluorescent lamps by thermal release analysis. Waste Manag 23(10):870–886. https://doi.org/10.1016/S0956-053X(03)00089-8
USEPA (1992) Toxicity characteristic leaching procedure (TCLP), Method 1311. USEPA Web Publishing. https://www.epa.gov/sites/default/files/2015-12/documents/1311.pdf. Accessed 15 Feb 2020
Li Y, Jin L (2011) Environmental release of mercury from broken compact fluorescent lamps. Environ Eng Sci 28(10):5. https://doi.org/10.1089/ees.2011.0027
Ozgur C, Coskun S, Guncan A, Civelekoglu G (2014) Investigation of the recovery potential of mercury from spent tubular fluorescent lamps by electrowinning process. Fresenius Environ Bull 23(12A):3199–3201
ASTM C 128-88 (1992) Test method for specific gravity and adsorption of fine aggregate. Annual Book of ASTM Standards, USA
ASTM D854-10 (2010) Standard methods for specific gravity of soils by water pycnometer. ASTM International, West Conshohocken
TS 118 EN 1426 (2002) Bitümler ve bitümlü bağlayıcılar – İğne batma derinliği tayini. Türk Standartları Enstitüsü, Ankara
TS 119 (1964) Bitümlü maddelerin duktilite deneyi için metot. Türk Standartları Enstitüsü, Ankara
TS 120 EN 1427 (2002) Bitümler ve bitümlü bağlayıcılar- Yumuşama noktası tayini–Halka ve bilya metodu. Türk Standartları Enstitüsü, Ankara
TS EN 15326 (2010) Bitüm ve bitümlü bağlayıcılar-Yoğunluk ve özgül kütle tayini-Kapiler kapaklı piknometre yöntemi, Türk Standartları Enstitüsü, Ankara
Karahancer S, Eriskin E, Capali B, Saltan M, Terzi S (2018) Superpave volumetric mix design of hot mix asphalt: case study of Isparta. J Eng Res Des 6(1):108–117. https://doi.org/10.21923/jesd.306098
Vavrik WR, Carpenter SH (1998) Calculating voids at a specified number of gyrations in the superpave gyratory compactor. In: Transportation Research Record 1630, TRB. National Research Council, Washington
Tam WO (2005) The handbook of highway engineering. Texas
Bonaquist R, Christensen DW (2005) Practical procedure for developing dynamic modulus master curves for pavement structural design. Trans Res Rec 1929:208–217. https://doi.org/10.1177/0361198105192900
DeVol JR, Krause JK, Laughlin SP, Willoughby K, McLean I (2007) Superpave gyratory compactor internal angle of gyration study. No. WA-RD 677.1. Washington State Department of Transportation, Olympia
AASHTO T283 (2011) Standard method of test for resistance of compacted asphalt mixtures to moisture-ınduced damage. American Association of State and Highway Transportation Officials. Washington, DC
Ahmadzade P, Altaş T, Geçkil T, (2008) Asfalt betonun siyah karbonun filler olarak kullanımı. IMO Teknik Dergi 297:4493–4507
Aucott M, McLinden M, Winka M (2003) Release of mercury from broken fluorescent bulbs. J Air Waste Manag Assoc 53:143–151. https://doi.org/10.1080/10473289.2003.10466132
Occupational Safety and Health Administration (2000). Title 29. Code of Federal Regulations, Part 1910.1000, OSHA Table Z-2. U.S. Government Printing Office, Washington, DC
Umar F, Ağar E (1994) Road pavement. İstanbul Technical University, Civil Engineering Faculty Publications, 1st Edn. İstanbul, Turkey
Gurer C, Selman GS (2016) Investigation of properties of asphalt concrete containing boron waste as mineral filler. Mater Sci-Medzg 22(1):118–125. https://doi.org/10.5755/j01.ms.22.1.12596
Arabani M, Hamedi GH (2011) Using the surface free energy method to evaluate the effects of polymeric aggregate treatment on moisture damage in hot mix asphalt. J Mater Civ Eng 23(6):802–818
Choudhary J, Kumar B, Gupta A (2020) Utilization of solid waste materials as alternative fillers in asphalt mixes: a review. Constr Build Mater 234:117271. https://doi.org/10.1016/j.conbuildmat.2019.117271
Choudhary J, Kumar B, Gupta A (2021) Utilization of waste glass powder and glass composite fillers in asphalt pavements. Adv Civ Eng. https://doi.org/10.1155/2021/3235223
Sanij HK, Meybodi PA, Hormozaky MA, Hosseini SH, Olazar M (2019) Evaluation of performance and moisture sensitivity of glass-containing warm mix asphalt modified with zycothermTM as an anti-stripping additive. Constr Build Mater 197:185–194. https://doi.org/10.1016/j.conbuildmat.2018.11.190
Carteret RD, Comport L, Metcalf J, Rebbechi J (2009) Guide to pavement technology part 8: Pavement Construction. https://austroads.com.au/publications/pavement/agpt11/media/AGPT08-09_Guide_to_Pavement_ Technology_Part_8_Pavement_Construction.pdf ISBN 978-1-921551-58-1.
Yu H, Deng G, Wang D, Zhang Z, Oeser M (2020) Warm asphalt rubber: a sustainable way for waste tire rubber recycling. J Cent South Univ 27(11):3477–3498
Zhao Y, Li G, Li T, Wang H, Zhang S, Zhang Y (2021) Performance and mechanism of solid waste coking sulfur paste modified asphalt mixture before and after curing. J Cent of South Univ 28(7):2179–2192
Asari M, Fukui K, Sakai S (2008) Life-cycle flow of mercury and recycling scenario of fluorescent lamps in Japan. Sci Total Environ 393(1):1
Sahin CK, Solak EB, Sava B, Onay BA (2022) Case study of Egirdir zero waste park for living and learning. Eur J Appl Sci 10(4):591–603
Acknowledgements
We would like to thank Associate Professor Şebnem KARAHANÇER and Selcan Özen for his contributions to the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anwari, R.A., Coskun, S. & Saltan, M. Research on recycle of waste fluorescent lamp glasses and use as mineral filler in asphalt mixture. J Mater Cycles Waste Manag 25, 258–271 (2023). https://doi.org/10.1007/s10163-022-01525-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-022-01525-3