Abstract
In this work, the recovery of mercury from spent fluorescent lamps by oxidative leaching followed by cementation process was studied. Two different reactive solutions (NaOCl/NaCl and KI/I2) during oxidative leaching were investigated whereas at the cementation process, metallic powders of iron (Fe), copper (Cu), and zinc (Zn) were used as reducing agents to capture mercury in solid phase. Mercury could be transferred to the solution with an efficiency of 96 % from the spent lamp samples through the NaOCl/NaCl reagent. The optimal leaching conditions were determined as 2-h contact time, 120 rpm agitation speed, pH 7.5, and 50 °C of temperature. The reducing agent, Zn, provided 99 % of the cementation. The optimal process conditions were observed to be as 5-min contact time, pH 1, and 5 g L−1 of reducing agent concentration. This combined approach appears to be technically effective for the recovery of mercury from spent fluorescent lamps.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The significance of the mercury recovery processes becomes prominent taking the threat of mercury pollution on human health into consideration. The main goals in the recovery of the fluorescent lamps are both the removal of the hazardous wastes properly and the economic reuse of resources such as glass, metal, and pulverized phosphorous. Metal recovering would reduce the amount of waste that would end up in the landfills, thus reducing the final elimination costs and the potential environmental risks (Coskun and Civelekoglu 2014).
The high efficiencies for mercury recovery from spent fluorescent lamps were reported using processes such as thermal desorption (pyrometallurgical) (Fujiwara and Fujinami 2004; US EPA 2007; Durão et al. 2008; Chang et al. 2009), chemical leaching (hydrometallurgical) (Klasson et al. 1998; Jang et al. 2005; Tunsu et al. 2014), and stabilization/solidification (Piao and Bishop 2006; US EPA 2007; Wang et al. 2012). The main goal of these processes is the removal of mercury from the lamps, converting it into less toxic chemical compounds or recovering it as metal in its pure state. Thermal desorption is the most widely used technology to treat mercury-containing wastes and many full-scale or pilot-scale applications have been summarized in the literature. Nevertheless, the process was reported to generate various hazardous products in spent fluorescent lamp recovery operations, such as mercury-contaminated phosphorous powder, mercury-contaminated filters, crushed glass, and aluminum end caps (Jang et al. 2005; Rhee et al. 2014). Furthermore, thermal desorption is a highly energy-consuming process due to high temperature (ranging between 600 and 800 °C) requirements during operation (Durão et al. 2008).
An acidic/oxidizing environment is required for capturing mercury in a solution in the form of Hg(II) ion. Usually, strong acid solutions (i.e., HCl, H2SO4, HNO3) at very low pH were preferred to extract mercury as Hg(II) from spent fluorescent lamps (Raap and Gallardo 2013; Tunsu et al. 2014). However, although the efficiencies of these mercury extractions were relatively high (>85 %), reusing of the reagents was limited, and thus the environmental impact and health risks imposed by the acidic reagents would be considerable. Moreover, the strong acids used in the process were reported to result in the extraction of other metals than mercury into the leaching solution (Kalb et al. 1999). Another undesirable aspect of this process is the metal oxide or the metal hydroxide precipitation according to pH adjustment requirement. The buffer solution used to maintain the environmental pH at 5.5 during acidic leaching (HCl/HNO3) was reported to cause the formation of yellow gel-like precipitates with aluminum or iron hydroxide content (Bussi et al. 2010). Therefore, oxidative leaching using sodium hypochlorite/sodium chloride (NaOCl/NaCl) and potassium iodide/iodine (KI/I2) was conducted as efficient methods to extract mercury as an Hg(II) complex (mercury tetrachloride, HgCl4 2−, and HgI4 2−) as given by Eq. 1 (Twidwell and Thompson 2001) and Eq. 2 (Klasson et al. 1998).
These oxidizing agents were generally used in mercury recovery from the process sludge in chlorine-alkaline facilities (Twidwell and Thompson 2001) and from industrial wastes (Klasson et al. 1998) with high mercury content. The recovery efficiencies were in the range of 85 to 99 %.
Mercury, which is extracted into solution via chemical leaching, is still toxic due to the presence of Hg(II) compounds. Mercury needs to be reduced to its elemental state (Hg0) following chemical leaching. Processes such as cementation, electroplating/electrorecovery, ultrafiltration, ion exchange, solvent extraction, and biodegradation/bioreduction were reported to be used for such a purpose, and mercury would be neutralized and then recovered through the hybrid use of these processes (Chaturabul et al. 2015). The most frequently used process for the recovery of mercury from industrial wastes is cementation. The main goal of this process is the selective precipitation of the mercury species in the leaching solution into solid phase using another reducing chemical. The powdered metals lower the solution potential during cementation causing the ionic mercury in the charged leaching solution to be reduced into its elemental state. Redox reactions, which allow metal substitution, were reported to facilitate the removal and recovery of precious metals from wastewaters and solutions (Anacleto and Carvalho 1996; Djokic 1996; Nosier and Sallam 2000; Ku et al. 2002). Neutral iron (Fe), zinc (Zn), and copper (Cu) metals in powdered form were reported as successful reducing agents for the cementation of mercury (Anacleto and Carvalho 1996). The following reactions were reported to take place between mercury and these reducing agents at 25 °C during the process (Ku et al. 2002; Noubactep 2010).
In the present study, the hydrometallurgical extraction of mercury with oxidative leaching and followed by its cementation using three different reducing agents was investigated. The pertinent parameters facilitating high-efficiency mercury extraction and recovery from spent fluorescent lamps were identified.
2 Material and Methods
2.1 Collection, Breaking, and Pulverization of Fluorescent Lamps
Spent fluorescent lamps of type T8 and T12 were selected to be used in the study owing to their high rate of consumption around the world and their relatively higher mercury content. The letter “T” indicates that the shape of the bulb is tubular, and the proceeding number is the diameter of the lamp in eighths of an inch (i.e., T12 lamps are 12/8" or 38 mm in diameter). Spent lamps were individually broken in a laminar hood and the glass was separated from the phosphorous powder for each lamp sample. The glass samples stripped from phosphorous powder were fractioned into three different size categories using standard Retsch sieves (larger than 100–500–1000 μm), and the free phosphorous powder samples were fractioned into two size groups, those with particle size larger or smaller than 45 μm for testing the effect of particle size on the efficiency of chemical leaching experiments. The samples in which the glass could not be separated from the phosphorous powder were pulverized in a mill (Retsch/RM 200) for both types of lamps individually, and two different chemical leaching tests were conducted on those samples. Since spent fluorescent lamps would be collectively processed under normal conditions, a 50-50 % pulverized mixture of the T8 and T12 lamp samples was prepared to simulate a more realistic situation and the oxidative leaching tests were also conducted on this sample.
2.2 Determination of the Initial Mercury Concentration
From each test case, 20-g samples, glass, phosphorous powder, pulverized glass + phosphorous, and the 50-50 % mixture (pulverized T8 and pulverized T12 glass + phosphorous powder), were mixed with 25-mL water and 25-mL aqua regia (HCl/HNO3, v/v—3/1) in polypropylene flasks using a magnetic stirrer at 200 rpm (Heidolph MR Hei-Tec 3001) at room temperature for 18 ± 2 h. Each sample was filtered through funnels using black band filters (1238 Filter Lab) following the mixing stage. The determination of the mercury content was conducted as reported in method 7471B (mercury in solid or semisolid waste-manual cold vapor technique) in US EPA’s Test methods for evaluating solid waste-physical/chemical method (SW-846) (US EPA 1998). The mercury content of the sample solutions was determined using atomic absorption spectrophotometer (AAS) (PerkinElmer-FIMS 400, attached with flow injection automated system). The concentration of the mercury vapor emission was controlled by a portable detector (Jerome 431-XE) to maintain reliable working conditions.
2.3 Oxidative Leaching Tests Using NaOCl/NaCl and KI/I2
NaOCl (6–14 % active chlorine, Merck 105614), NaCl (extra pure, Merck 106400), KI (99.9 % purity, Merck 105044), and I2 (99.9 % purity, Merck 104763) were used as the oxidative chemical leaching reagents to extract the mercury. The leaching tests were conducted based on a 23 factorial design (Table 1). The central point tests were used to evaluate the experimental error of leaching process and therefore the standard error (SE) for the effects. The individual effects of the three design parameters as well as their combinatorial effects were evaluated for the varying size fractions of glass, free phosphorous powder, pulverized glass + phosphorous powder, and the pulverized mixture samples. The experimental matrix design was utilized in the chemical leaching tests according to Yates Algorithm (Montgomery 1991).
Since a wide range of pH and reaction times were reported in the literature (Twidwell and Thompson 2001; Hummer et al. 2006; Bussi et al. 2010), the impact of these parameters on the effectiveness of oxidative leaching was investigated by conducting preliminary tests for determining the concentrations of the reagents. According to these preliminary results, the leaching tests for each reactive solution were conducted at an agitation rate of 120 rpm, pH = 7.5, and a process time of 2 or 5 h for the NaOCl/NaCl and KI/I2 solutions, respectively. The leaching tests were performed in 250-mL polypropylene flasks placed in temperature-controlled water baths (GFL 1086) with mechanical stirrers. During the leaching tests, the pH of the solutions was monitored using a digital pH meter (WTW multi, 340i). The solutions were filtered (20 μm, pure cellulose filter papers) and analyzed for their mercury content to quantify leaching efficiency. Each sample was diluted by a factor of 1:10 using nitric acid solution (pH = 2) to avoid the precipitation of metals and then stored at 4 °C for further analysis using AAS.
2.4 Cementation Experiments
The results of the leaching experiments indicated a high yield of mercury leaching (as high as 96 %) from pulverized lamp sample mixtures (50-50 % pulverized T8 glass + phosphorous powder and pulverized T12 glass + phosphorous powder mixture) under the following conditions: the leaching temperature at 50 °C, a solid/volume ratio of 1/2, and a leaching solution composition of 0.5 M NaOCl/0.2 M NaCl. All leaching tests were carried out using this combination, which yielded the most optimal leaching efficiency for mercury, prior to conducting the cementation experiments.
Neutral Zn (Merck 1087, 95 % purity, particle size <45 μm), Cu (Merck 1027, 99.7 % purity, particle size <63 μm), and Fe (Merck 1038, 99.5 % purity, particle size 10 μm) metals in powder form were used in the cementation experiments that were conducted to reduce the ionic mercury extracted into the leaching solution (Hg2+) to a less harmful elemental state (Hg0) and thus recover the metal. The effects of the amount of the reducing agent, pH, and the cementation time on process yield were evaluated on the basis of 23 full factorial design (Table 2). The central point tests were used to evaluate the experimental error of cementation process and therefore the SE for the effects.
The experimental matrix was designed according to Yates Algorithm (Montgomery 1991). Constant operating conditions of 25 °C of temperature and 150 rpm rate of water bath agitation were used during cementation as reported in the literature (Ku et al. 2002). The experiments were carried out in 250-mL polypropylene flasks placed in temperature-controlled water baths (GFL 1086) with mechanical agitation. The solutions were filtered (20 μm, pure cellulose filter papers) and the concentration of mercury in the filtered solutions was determined using AAS in order to quantify the efficiency of cementation.
The solid phase remaining on the filter papers were washed with acetone and then dried in the lyophilizer (Virtis Benchtop SLC) at a temperature below −50 °C and under 13.3-Pa pressure for 24 h. Due to the relative ease of vaporization for mercury, heat drying of the samples in the oven was not preferred. The dried solid residues and the pure powder samples of the reducing agents were analyzed by X-ray diffraction (XRD) (Philips, X’Pert PRO MPD) and scanning electron microscope coupled with an energy-dispersive X-ray spectrophotometer (SEM-EDX) (FEI Quanta250 FEG) to investigate the mineralogical and elemental compositions.
3 Results and Discussion
3.1 Mercury Vapor Emission
The breaking, the dismantling, and the sieving of the T8 and T12 fluorescent lamps were carried out under vacuum in the laboratory. The mercury vapor released into the environment was measured in the range of 0.013–0.016 mg Hg/m3 for the 6-h working time. The mercury emissions of the indoor working environment did not exceed the exposure threshold limit value of 0.025 mg Hg/m3 as reported by Occupational Safety and Health Administration (OSHA).
3.2 Leaching Experiments
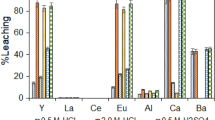
The effect of particle size on the efficiency of chemical leaching was determined in the oxidative leaching tests. Glass samples having three different size fractions which were subject to mercury leaching using solutions of NaOCl/NaCl and KI/I2 were shown in Figs. 1 and 2, respectively.
Figures 1 and 2 indicate that the highest leaching efficiencies were attained at factors “B” and “BC” and “0” (central point) in the leaching experiments in which the spent T12 and T8 fluorescent lamp glass and phosphorous powder + glass samples were leached using either reactive solution. Investigation for the leaching efficiencies for each factor separately indicated that the leaching efficiency mostly increased with decreasing particle diameter. The leaching efficiency increased with increasing reactive solution concentration for either one of the reactive solutions (factor B). This observation was also reported previously in the literature (Bussi et al. 2010). Increasing the solid matter content or the temperature alone was not sufficient to increase the leaching efficiency (factors “C” and “AC”).
The free phosphorous powder (PP) samples of two different size fractions were leached using the same solutions, and the leaching efficiencies were shown in Fig. 3. Decreasing the particle size in phosphorous powder samples was also shown to increase efficiency as it was observed for the glass samples. The efficiency was determined to be relatively higher for the phosphorous powder samples of size smaller than 45 μm with regard to all factors than those samples of larger size. The central point tests provided the optimal leaching conditions for both lamp types. The highest leaching efficiency (>95 %) was achieved at factor 0 central point tests (Fig. 3a, b). The lowering of the efficiency with regard to the factors “A” and AC in the phosphorous powder samples indicated that increasing the solid/liquid ratio alone or together with temperature adversely affected the mercury extraction. Increasing temperature alone (factor “C”) resulted in low leaching efficiencies, too (Fig. 3a, b).
A 50-50 % pulverized mixture of the T8 and T12 lamp samples was prepared and leached using 2 different reactive solutions (KI/I2 and NaOCl/NaCl), and the obtained mercury leaching efficiencies were displayed in Fig. 4a. The percent effect of the main factors (solid/liquid ratio, reactive concentration, and temperature) on the leaching of the mixture sample was evaluated using the ANOVA method. The negative and the positive effects of each factor were shown in Fig. 4b. F test statistics was used to evaluate factor coefficients for significance at 95 % (p = 0.05) confidence level. The calculated SE values using three central point experiments of KI/I2 and NaOCl/NaCl leaching were 1.42 and 3.05, respectively.
The factors “AB” and “ABC” were determined to be statistically insignificant (p > 0.05) in mercury leaching using either solution, and in addition factors A and BC were insignificant using NaOCl/NaCl, and these factors were excluded from the figure (Fig. 4b). Factor B, which positively affected the mercury leaching efficiency in either solution, indicated a high reactive concentration (1 M/0.1 M). Increasing the solid/liquid ratio did not have a significant effect on leaching efficiency whereas increasing the temperature along with the solid/liquid ratio (factors C and AC) had an adverse effect when the NaOCl/NaCl solution was used (factors A, AB, and ABC) (Fig. 4b). The results displayed in Fig. 4a indicate that the leaching efficiency was lower with respect to factors C and AC (77 and 66 %) than with respect to factors B and AB (87 and 93 %). Increasing the temperature (90 °C) and the solid matter ratio (1/1; 100 g) also adversely affect the leaching efficiency in the KI/I2 leaching solution (factors C and AC) and increasing the reactive concentration still improved leaching efficiency, although very marginally (3.77 %) despite increasing of the temperature (factor BC) (Fig. 4b). The maximum leaching efficiencies were determined at factor 0 (central point test) for using either reactive solution (96 % in NaOCl/NaCl and 95 % in KI/I2). A solid matter ratio of 1/1 at 100 g was shown to have an adverse effect on efficiency in KI/I2 solution as displayed in Fig. 4b (factor A, −3.33 %) whereas the adverse effect was removed at a solid matter ratio of 1/2 with 50 g and the leaching efficiency was determined to be above 90 % (factor 0) (Fig. 4a). Conducting the central point experiment at 50 °C rather than at 90 °C had a positive effect on the experimental efficiency, and a solution concentration of 0.5 M/0.2 M was determined to be satisfactory for the domain of mercury leaching efficiency. A high concentration of hypochlorite would also be undesirable for the next process, which would be cementation. High hypochlorite concentration would cause the precipitation of Fe(OH)3 if Fe powder were to be used as the reducing agent during cementation. This condition would have caused the elemental mercury, which was recovered during cementation, to dissolve back again, thus adversely affecting the cementation efficiency (Twidwell and Thompson 2001; Bussi et al. 2010).
The optimal leaching conditions for the 50 % pulverized mixture of glass + phosphorous powder from spent T8-T12 lamps were achieved by the central point tests that were conducted at a temperature of 50 °C, a solid/liquid ratio of 1/2 and at reactive solution concentrations of 0.5 M NaOCl/0.2 M NaCl (96 % mercury leaching efficiency) and 0.5 M KI/0.2 M I2 (95 % mercury leaching efficiency). Although the mercury yield efficiencies of KI/I2 or NaOCl/NaCl were very similar, NaOCl/NaCl was preferred as “leaching reactive” based on low cost and recovery/reuse ability of this reagent.
3.3 Cementation Experiments
The mercury complexes that were leached into the solution under optimal conditions were then transferred into the solid phase in its elemental state during cementation, and the cementation efficiencies were displayed in Fig. 5a. ANOVA method was used to evaluate the effect of the main factors on the process of cementation for each metal powder separately, and the results were summarized in Fig. 5b. F test statistics was used to evaluate factor coefficients for significance at 95 % (p = 0.05) confidence level. The calculated SE values using three central point experiments of Fe, Cu, and Zn cementation were 9.78, 6.71, and 3.65, respectively.
Factors A, AB, AC, and ABC were statistically insignificant (p > 0.05) during cementation using Zn powder and factor B was determined to be insignificant when Cu powder was used. Factor C, described as short reaction time (5 min), strongly acidic environment (pH = 1), and high reducing agent concentration (0.5 g/L), was determined to positively affect cementation efficiency when either one of the three agents was used in the process (Fig. 5b). Figure 5a shows that the efficiency was very low under the conditions described by factor A, low pH, and longer process time, regardless of the reducing agent that was used in the process. The negative effect of factor A (−12.97 %) was specifically apparent for the runs in which Fe was used (Fig. 5b). This situation was thought to stem from the redissolution of mercury that was cemented on Fe powder back into the solution in prolonged process times. Shorter reaction times were therefore nominated to be among the designated optimal cementation conditions.
Low efficiencies were attained in the cementation tests using Cu as the reducing agent under conditions given by factors “1,” A, B, or AB as displayed in Fig. 5a. The redox potential requirements might not have been met in these experiments since minimal amounts of reducing agent (0.1 g/L) were used. In highly acidic environments, hydrogen ions also tend to be reduced in redox reactions and the presence of hydrogen ions might have caused a competitive environment for the mercury ions necessitating the presence of more of the reducing agent in the test environment to maintain the required redox potential. Achieving a very high mercury cementation (recovery) efficiency of 99 % through the use of Fe or Zn at their highest concentration (5 g/L) supports this notion (Fig. 5a; factor C). The efficiency was calculated to be lower (ca. 80 %) using Cu powder. Higher pH (pH = 5) was shown to affect the cementation efficiency adversely when high reducing agent concentrations were utilized (factor BC) as displayed in Fig. 5b. The mercury recovery efficiency using Zn was determined as 99 and 98.64 % under acidic conditions indicated by the factors C and AC, respectively, whereas the recovery efficiency was lowered by 40 and 60 % under weakly acidic conditions (pH = 5) indicated by the factors BC and ABC, respectively (Fig. 5a). High pH and short reaction time (factor B) was shown to affect cementation efficiency negatively, by −16.07 and −18.74 %, when either Fe or Zn was used as the reducing agent, respectively (Fig. 5b). These results are in accordance with those reported in the literature. Formation and precipitation of metal hydroxide complexes were reported to prevent metal cementation at higher pH (Ku et al. 2002; Noubactep 2010). Factor B (reaction time of 5 min, pH = 5, reducing agent concentration of 0.1 g/L) did not affect the cementation process when Cu was used as the reducing agent although factor BC (reaction time of 5 min, pH = 5, reducing agent concentration of 0.5 g/L) negatively affected the cementation process (−7.87 %) supporting the notion that metal hydroxides form at higher pH values and that the process would be adversely affected by this chemical phenomenon.
The redox potentials [E 0 (V)] of the metals that were selected for hydrometallurgical cementation were reported as 0.854, 0.763, −0.337, and −0.440 V for Hg2+, Zn2+, Cu2+, and Fe2+, respectively (Ku et al. 2002; Noubactep 2010). A metal having a high redox potential reacts with a reducing agent possessing a low redox potential in an electrochemical reaction. The difference in the redox potentials between the metal and each reducing agent needs to be calculated in order to determine the effectiveness of cementation. ΔE was calculated as +1.617 V, +1.294 V ve, and +1.191 V for Zn, Fe, and Cu, respectively. These results indicated that the most effective redox potential is that for the reduction of mercury using Zn followed by Fe. Zn metal would be selected as the preferred reducing agent since the reproducibility of the high-efficiency performance could be ensured as shown by this analysis.
After cementation, the Hg-Zn alloy in solid phase were washed with acetone and dried in the lyophilizer. XRD and SEM-EDX analyses were then carried out to identify and characterize elemental mercury and the mercury in the solid state. The results of the XRD and SEM-EDX analyses for the Zn-Hg sample were shown in Figs. 6 and 7, respectively.
Diffraction patterns of Zn and Hg compounds (including elemental mercury) were cross-referenced with the Inorganic Crystal Structure Database (ICSD). As shown in Fig. 6, mercury was present in its solid phase as kleinite (Hg6Cl3N3H2O). Elemental mercury was also detected in the SEM-EDX analysis of the cemented solid samples. Figure 7 shows an SEM-EDX image of the elemental analysis of pure Zn powder and Zn-Hg compound. The particle size range was shown to vary between 2 and 12 μm. The present analysis indicated that approximately 86.88 % of total weight was identified as Zn, 12.06 % of the sample was identified as O by weight, and a modest 1.06 % was identified as Hg (Fig. 7c). SEM image of pure Zn was identified to form a porous powder structure comprised of nanoparticles. This porous structure of the powder was thought to be caused by the cementation reactions. Investigation of the results obtained by the SEM-EDX analysis indicated that the amount of Hg, which was reduced during cementation, was determined as 1.06, 0.41, and 0.72 % when the reducing agents Zn, Cu, and Fe were used, respectively. Zn metal was determined as the most suitable reducing agent as indicated by the presented results.
4 Conclusions
The efficiency of two different reactive solutions during oxidative leaching was comparatively investigated. NaOCl and NaCl mixture solution was more effective in the removal of mercury. Increasing the reactive concentration under optimal conditions did not affect leaching efficiency although contrary findings were reported in the literature. Decreasing solid particle size was shown to increase leaching efficiency in the tests conducted on both the glass samples and the phosphorous powder samples. Zinc was determined as the most suitable reducing agent during cementation. The higher pH and very short reaction periods adversely affect cementation efficiency due to the formation of metal hydroxide complexes and the occurrence of precipitation. Oxidative leaching processes may replace the acidic solutions for eco-friendly recovery of mercury that would also be less hazardous on human health. Although this combined leaching and cementation approach appears to be technically effective for the recovery of mercury from spent fluorescent lamps, the economical evaluations should be carried out prior to each specific full-scale application.
References
Anacleto, A. L., & Carvalho, J. R. (1996). Mercury cementation from chloride solutions using iron, zinc and aluminium. Minerals Engineering, 9, 385–397.
Bussi, J., Cabrera, M. N., Chiazzaro, J., Canel, C., Veiga, S., & Florencio, C. (2010). The recovery and recycling of mercury from fluorescent lamps using photocatalytic techniques. Journal of Chemical Technology and Biotechnology, 85, 478–484.
Chang, T. C., You, S. J., Yu, B. S., Chen, C. M., & Chiu, Y. C. (2009). Treating high-mercury-containing lamps using full-scale thermal desorption technology. Journal of Hazardous Materials, 162, 967–972.
Chaturabul, S., Srirachat, W., Wannachod, T., Ramakul, P., Pancharoen, U., & Kheawhom, S. (2015). Separation of mercury(II) from petroleum produced water via hollow fiber supported liquid membrane and mass transfer modeling. Chemical Engineering Journal, 265, 34–36.
Coskun, S., & Civelekoglu, G. (2014). Characterisation of waste fluorescent lamps to investigate their potential recovery in Turkey. International Journal of Global Warming, 6(2/3), 140–148.
Djokic, S. S. (1996). Cementation of copper by aluminum in alkaline solution. Journal of Electrochemical Society, 143, 1300–1305.
Durão, W. A., de Castro, C. A., & Windmöller, C. C. (2008). Mercury reduction studies to facilitate the thermal decontamination of phosphor powder residues from spent fluorescent lamps. Waste Management, 28, 2311–2319.
Fujiwara, K., & Fujinami, K. (2004). Mercury recovery method. U.S. Patent 2004/6800112 B2.
Hummer, H., Gonzala, L. S. S., Fernandes, A. L. V., & Yallouz, A. V. (2006). Treatment of mercury bearing fluorescent lamps by using an electrochemical process. Thesis, Centre Tecn. Miner. Leoben.
Jang, M., Hong, S. M., & Park, J. K. (2005). Characterization and recovery of mercury from spent fluorescent lamps. Waste Management, 25, 5–14.
Kalb, P. D., Adams, J. W., Milian, L. W., Penny, G., Brower, J., & Lockwood, A. (1999). Mercury bakeoff: technology comparison for the treatment of mixed waste mercury contaminated soils at BNL. Tucson: Waste Management Congress.
Klasson, K. T., Koran, L. J., Gates, D. D., & Cameron, P. A. (1998). Removal of mercury from solids using potassium iodide/iodine leaching process. The Scientific Report. Tennessee: OAK Ridge National Laboratory.
Ku, Y., Wu, M. H., & Shen, Y. S. (2002). Mercury removal from aqueous solutions by zinc cementation. Waste Management, 22, 721–726.
Montgomery, D. C. (1991). Design and analysis of experiments (3rd ed.). New York: Wiley.
Nosier, S. A., & Sallam, S. A. (2000). Removal of lead ions from wastewaters by cementation on a gas-sparged zinc cylinder. Separation and Purification Technology, 18, 93–101.
Noubactep, C. (2010). Elemental metals for environmental remediation: learning from cementation process. Journal of Hazardous Materials, 181, 1170–1174.
Piao, H., & Bishop, P. L. (2006). Stabilization of mercury-containing wastes using sulfides. Environmental Pollution, 139, 498–506.
Raap, N. R., & Gallardo, A. (2013). Removal of mercury bonded in residual glass from spent fluorescent lamps. Journal of Environmental Management, 115, 175–178.
Rhee, S. W., Choi, H. H., & Park, H. S. (2014). Characteristics of mercury emission from linear type of spent fluorescent lamp. Waste Management, 34(6), 1066–1071.
Tunsu, C., Ekberg, C., & Retegan, T. (2014). Characterization and leaching of real fluorescent lamp waste for the recovery of rare earth metals and mercury. Hydrometallurgy, 144–145, 91–98.
Twidwell, L. G., & Thompson, R. J. (2001). Recovering and recycling Hg from chlor-alkali plant wastewater sludge. Journal of The Minerals Metals & Materials Society, 53, 15–17.
US EPA. (1998). Comments related to industry source reduction efforts. In Response to comments document, final rule on hazardous waste lamps. Washington, DC: United States Environmental Protection Agency, DCN FLEP-00156.
US EPA. (2007). Treatment technologies for mercury in soil, waste, and water, final report. Washington, DC: United States Environmental Protection Agency.
Wang, J., Feng, X., Anderson, C. W. N., Xing, Y., & Shang, L. (2012). Remediation of mercury contaminated sites—a review. Journal of Hazardous Materials, 221, 1–18.
Acknowledgments
This work was funded by a research grant from the Scientific and Technical Research Council of Turkey (TUBITAK) (project no. 110Y264) and Research Projects Funding Unit of the Suleyman Demirel University (project no. BAP 2376-D-10).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coskun, S., Civelekoglu, G. Recovery of Mercury from Spent Fluorescent Lamps via Oxidative Leaching and Cementation. Water Air Soil Pollut 226, 196 (2015). https://doi.org/10.1007/s11270-015-2391-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2391-9