Abstract
Rare-earth elements are used in neodymium magnets, and these elements are critical to Japanese industry. In this study, we focused on the electrochemical behavior of neodymium magnets for the recovery of rare-earth elements using molten salt electrolysis. The influence of the rare-earth elemental composition of the neodymium magnets on their anodic polarization behavior and oxidation mechanism was studied. The use of potentiostatic electrolysis enabled selective leaching of rare-earth elements from neodymium magnets in the potential range from −1.8 to −0.8 V. The oxidation potential limits the oxidation stage, enabling rare-earth elements to be leached from mixed neodymium magnets simultaneously.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The separation of rare-earth elements using chemical separation methods is difficult. Typically, multistep cycles of solvent extraction are used to isolate each of the elements because of their similar chemical characteristics. Neodymium magnets contain amounts of neodymium, praseodymium, and dysprosium. Dysprosium, which is more expensive than neodymium and praseodymium, is added to neodymium magnets to improve their heat resistance. Neodymium magnets comprise a main phase and a boundary phase; the main component of the main phase is Nd2Fe14B alloy, while that of the boundary phase is rare-earth elements. Neodymium magnets are used in numerous consumer electronics and electric- and hybrid-powered vehicles. Large amounts of neodymium are wasted each year when products that contain these magnets are discarded. Processes for the recovery of rare-earth elements from neodymium magnets have been developed; these methods include hydrometallurgical treatments [1,2,3], hydrothermal processing [4], molten metal extraction [5,6,7,8], molten salt extraction [9], chloride volatility processing [10], and glass slag processing [11]. Rare-earth oxides are recovered from these processes, and these oxides are subsequently reduced to rare-earth metals using molten salt electrolysis. We have reported a recovery process for rare-earth elements from neodymium magnets using molten salt electrolysis, where these elements were recovered as alloys [12]. Our process is able to recover rare-earth alloy from neodymium magnets using single electrolysis in a single reactor.
In this study, we focus on the differences in the electrochemical behaviors of rare-earth elements in neodymium magnets during molten salt electrolysis. We conducted anodic polarization experiments to determine the optimal electrochemical leaching conditions and observed the leaching behaviors of various rare-earth elements.
Materials and methods
Experimental equipment
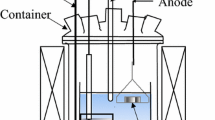
The experimental apparatus is shown in Fig. 1. The electrolysis potential was controlled using a potentiostat (HZ-5000; Hokuto Denko). The reactor was made of Pyrex glass and was purged with Ar gas. A eutectic salt mixture of 59 mol% LiCl and 41 mol% KCl (melting point 626 K), which melted at 723 K, was used in the electrolysis bath. The cathode electrode was a glassy carbon rod. The anode electrode was a neodymium magnet, a neodymium rod, a dysprosium rod, an iron wire, or an aluminum wire. The composition of the neodymium magnets used in the experiments is shown in Table 1. The nickel coating of neodymium magnet was removed before electrolysis. The anode electrode was connected directly using a tantalum wire for obtaining anodic polarization curves. In potentiostatic electrolysis experiments, the neodymium magnet was inserted into a bucket constructed from tantalum plate to avoid diffusion of the magnet residue from the electrolyte. The magnet was cut to dimensions of 5 × 5 × 2 mm3, and the weight of each magnet was 1 g. The tantalum bucket contained two neodymium magnets with a total weight of 2 g. The reference electrode was an Ag/AgCl (0.1 mol%) electrode in a eutectic composition of LiCl–KCl; this electrode was placed in a mullite tube. The electrolysis bath was maintained at 473 K for 24 h under vacuum to eliminate water.
Analytical method
The compositions of the neodymium magnet, electrolysis bath, and anodic residues were analyzed by inductively coupled plasma atomic emission spectrometry (ICP-AES; Optima-3300DV, PerkinElmer). Samples for ICP analysis were dissolved in aqua regia. The surface morphology of the samples was observed using scanning electron microscopy (SEM, JSM-6330F, JEOL). The electron microscope was equipped with an energy-dispersive X-ray spectrometer. The electrodeposited products were identified using X-ray diffraction (XRD) carried out on a diffractometer (XRD-6000, Shimadzu, Japan) equipped with a thin-film analytical unit.
Results and discussion
Anodic polarization
The scan rate of anodic polarization was 5 mV s−1. The anodic polarization curves of the elements in the neodymium magnet are shown in Fig. 2. The oxidation currents of neodymium and dysprosium were generated at approximately −2.2 V and their oxidation potentials were the lowest among the elements in the neodymium magnet. Neodymium magnets were oxidized at approximately −1.8 V. The iron oxidation potential was noted at −0.7 V. Iron is the main component of neodymium magnets, and leaching of iron must be avoided in the recovery process. The aluminum oxidation potential was −1.3 V. Although aluminum is corroded by chloride, aluminum leaching is unavoidable under these conditions. The use of potentiostatic electrolysis enabled selective leaching of rare-earth elements from the neodymium magnet in the potential range from −1.8 to −0.8 V.
Leaching behavior of rare-earth elements from neodymium magnets
Rare-earth elements were first leached from the boundary phase, and the leaching rate of rare-earth elements from the main phase was slower than that from the boundary phase in previous work [12]. The leaching of rare-earth elements from magnet A was categorized into three stages as shown in Fig. 3. The leaching behavior of rare-earth elements in each stage was observed using potentiostatic electrolysis at −1.3, −1.0, and −0.2 V. The total electric charge transferred during electrolysis was 310, 1200, and 4760 C, respectively. The composition of the neodymium magnet electrolyzed at each potential is shown in Table 2. The change in current over time is shown in Fig. 4. Changes in the SEM images and XRD patterns are shown in Figs. 5 and 6, respectively.
The boundary phase consists of concentrated dysprosium and neodymium. At −1.3 V, the rare-earth element content and the XRD peak intensity of the rare-earth elements decreased, whereas the space was left between each main phase. These results suggest that rare-earth elements were leached from the boundary phase during stage I. At −1.0 V, the rare-earth content of neodymium magnet decreased and XRD peaks attributable to Nd2Fe14B alloy and rare-earth elements were not observed. The surface morphology of stage I was similar to that of stage II. However, in the case of the sample electrolyzed at −0.2 V, the iron content decreased and the surface morphology differed from that of the sample electrolyzed at −1.0 V, even though the XRD patterns of the samples electrolyzed at −0.2 and −1.0 V were similar.
Figure 4 shows a noisy period during the early stages of electrolysis. This noise was generated by a change in the surface area of the electrode upon decomposition of the neodymium magnets. The rear-earth elements in the boundary phase were leached first, followed by leaching of the rare-earth elements from the main phase. The early stage of the time-dependent change in current at −0.2 V exhibited the same behavior as that at −1.0 V, and the end of this stage was noisy. This noise was a consequence of the rough surface that resulted from iron leaching from the main phase. The change in surface morphology changed the surface area of the electrode, which generated noise. The leaching flow of the rare-earth elements from neodymium magnets was the same, irrespective of the oxidation potential. The difference in oxidation potential was controlled by the oxidation flow.
Potentiostatic electrolysis of mixed magnets
The change in current over time during electrolysis at −1.0 V for a mixed-type neodymium magnet in the tantalum bucket is shown in Fig. 7. The total magnet weight was 2.0 g: 1.0 g each of magnet A and magnet B. The curve in Fig. 7 is similar to that in Fig. 4b. These results suggest that rare-earth elements were leached within 4000 s, which led to substantial decomposition of the magnets. The decomposition of the neodymium magnets generated noise as a result of the change in current resulting from the change of the electrode surface area. The composition of the neodymium magnets electrolyzed as a mixture is shown in Table 3. The rare-earth element content of both neodymium magnets electrolyzed at −1.0 V decreased to 2.0% and the estimated leaching ratio of rare-earth elements was approximately 90%. These results demonstrate that this leaching process could be used to treat different neodymium magnets simultaneously.
Conclusion
Rare-earth elements were leached from neodymium magnets using electrolysis in a molten eutectic mixture of LiCl and KCl. The use of potentiostatic electrolysis enabled the selective leaching of rare-earth elements from the neodymium magnets in the potential range from −1.8 to −0.8 V. Control of the oxidation potential enabled control of the oxidation stage during potentiostatic electrolysis. In the first phase, rare-earth elements were leached from the boundary phase of neodymium magnets. In the second phase, rare-earth elements were leached from the interior of the main phase. Because the oxidation stage depends on the oxidation potential, rare-earth elements could be simultaneously leached from mixed neodymium magnets.
References
Rabatho JP, Tongamp W, Takasaki Y, Haga K, Shibayama A (2013) Recovery of Nd and Dy from rare earth magnetic waste sludge by hydrometallurgical process. J Mater Cycles Waste Manag 15:171–178
Hoogerstraete TV, Wellens S, Verachtert K, Binnemans K (2013) Removal of transition metals from rare earths by solvent extraction with an undiluted phosphonium ionic liquid: separations relevant to rare-earth magnet recycling. Green Chem 15:919–927
Kikuchi Y, Matsumiya M, Kawakami S (2014) Extraction of rare earth ions from Nd–Fe–B magnet wastes with TBP in tricaprylmethylammonium nitrate. Solv Extr Res Dev Jpn 21:137–145
Itakura T, Sasai R, Itoh H (2006) Resource recovery from Nd–Fe–B sintered magnet by hydrothermal treatment. J Alloy Compd 408–412:1382–1385
Takeda O, Okabe TH, Umetsu Y (2006) Recovery of neodymium from a mixture of magnet scrap and other scrap. J Alloy Compd 408–412:387–390
Okabe TH, Takeda O, Fukuda K, Umetsu Y (2003) Direct extraction and recovery of neodymium metal from magnet scrap. Mater Trans 44:798–801
Sekimoto H, Kubo T, Yamaguchi K (2014) Development of a new recycling process for neodymium permanent magnet using B2O3 flux. J MMIJ 130:494–500 (in Japanese)
Hoshi H, Miyamoto Y, Furusawa K (2014) Technique for separating rare earth elements from R–Fe–B magnets by carbothermal reduction method. J Jpn Inst Met Mater 78:258–266 (in Japanese)
Takeda O, Nakano K, Sato Y (2014) Recycling of rare earth magnet waste by removing rare earth oxide with molten fluoride. Mater Trans 55:334–341
Itoh M, Miura K, Machida K (2009) Novel rare earth recovery process on Nd–Fe–B magnet scrap by selective chlorination using NH4Cl. J Alloy Compd 477:484–487
Saito T, Sato H, Ozawa S, Yu J, Motegi T (2003) The extraction of Nd from waste Nd–Fe–B alloys by the glass slag method. J Alloy Compd 353:189–193
Kamimoto Y, Yoshimura G, Itoh T, Kuroda K, Ichino R (2015) Leaching of rare earth elements from neodymium magnet using electrochemical method. TMRS-J 40:343–346
Acknowledgements
This work was supported by the Environment Research and Technology Development Fund of the Ministry of the Environment, Japan 3K143005, JSPS KAKENHI Grant No. 24656457, Japan Oil, Gas and Metals National Corporation, the Hori Sciences and Arts Foundation, and the Tokai Foundation for Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamimoto, Y., Itoh, T., Kuroda, K. et al. Recovery of rare-earth elements from neodymium magnets using molten salt electrolysis. J Mater Cycles Waste Manag 19, 1017–1021 (2017). https://doi.org/10.1007/s10163-016-0563-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-016-0563-3