Abstract
The selective recovery of rare earth elements from Nd-Fe-B magnets through a novel selective chlorination process using zinc chloride was investigated. A Nd-Fe-B magnet powder and zinc chloride mixture in an alumina crucible was positioned in a gas-tight quartz tube. This quartz tube was placed in an electric furnace preheated to 1000 K for 1.5 h for the reactions. After the experiments, a mixture of metallic iron and neodymium chloride was produced owing to the selective chlorination of rare earth elements in the magnet powder. In addition, the chlorination efficiencies of neodymium, dysprosium, and praseodymium were 96.5%, 57.2%, and 97.6%, respectively, under certain conditions. Therefore, it was demonstrated that the novel selective chlorination using zinc chloride developed in this study is feasible for the efficient recycling of Nd-Fe-B magnets.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Neodymium (Nd)–iron (Fe)–boron (B) magnets with addition of small amount of dysprosium (Dy) have excellent properties such as high coercivity even at high temperatures [1]. Because of these properties, Nd magnets can be used in various applications such as hybrid and electric cars. The demand for green cars is expected to increase in the future to meet the need of carbon dioxide (CO2) gas emission reduction; therefore, the utilization of Nd magnets will increase.
Rare earth elements (REEs) are extracted from several mineral resources such as bastnaesite, monazite, and ion adsorption deposits. Among REEs, light rare earth elements (LREEs), such as Nd and praseodymium (Pr), are extracted from bastnaesite and monazite. These mineral resources are abundant in nature and also distributed around the world [2]. However, heavy rare earth elements (HREEs), such as Dy and terbium (Tb), can only be extracted economically from ion adsorption deposits in the southern parts of China [2]. This is the one reason that China monopolizes the world production of REEs.
During the extraction of LREEs from monazite, environmental threats and radiation risks are encountered, because monazite contains radioactive elements such as uranium (U) and thorium (Th) [2]. Therefore, proper treatments of these radioactive elements generated during the extraction are necessary to preserve the environment. In contrast, ion adsorption deposits contain very little amount of radioactive elements because these elements are washed out because of weathering [2]. However, there are still environmental concerns during the extraction of REEs because they are extracted via elution using ammonium sulfate ((NH4)2SO4).
To produce REEs with preservation of environment, recycling of spent Nd magnets is required. However, only Nd scraps generated in magnet production plants are commercially used as feedstock for recycling because these scraps contain a large amount of REEs and processing costs are minimum [2, 3]. Although spent Nd magnets are not recycled at present, it is necessary to develop an efficient and environmentally sound process for the recovery of REEs from spent magnets when the increase of the use of Nd magnets is considered.
Numerous methods have been developed to recover REEs from spent magnets [4,5,6,7,8,9]. Among those, the selective recovery of REEs through the chlorination of spent magnets using metal chloride is promising [8, 9]. When the selective chlorination of spent magnets using ferrous chloride (FeCl2) or magnesium chloride (MgCl2) is utilized, only REEs are recovered as chloride without chlorination of Fe in magnets. Shirayama reported that Nd in a spent magnet was selectively chlorinated to neodymium chloride (NdCl3) by reacting with MgCl2 at 1273 K [8]. In addition, approximately 80% of Nd and Dy was recovered from the initial magnet after the reactions for 12 h [8]. Hua reported that the rate-determining step of the selective chlorination using MgCl2 is the diffusion of MgCl2 into an ash layer consisting of Fe metal remained [10].
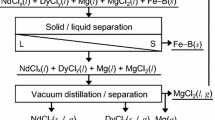
In this study, a selective chlorination process using zinc chloride (ZnCl2) was investigated to recover REEs from Nd magnets, as shown in Fig. 1 [11]. When ZnCl2 is used as the chlorinating agent, reaction temperature can be decreased because of its low melting point. In addition, the reaction rate can be increased because low melting temperature of zinc (Zn) metal produced by the chlorination reaction is alloyed with the ash layer of Fe metal. Furthermore, rare earth chlorides such as NdCl3 can be separated from the residue obtained after selective chlorination by utilizing different vapor pressures at high temperatures.
Chemical potential diagrams of the a Nd–Zn–Cl and b Fe–Zn–Cl systems at 1000 K (the boiling point of ZnCl2 is 999.5 K [12] or 1005 K [14] depending on the reference consulted, Reprinted by permission from Springer Nature: Springer, Journal of Sustainable Metallurgy, Selective Chlorination of Rare Earth Elements from a Nd–Fe–B Magnet Using Zinc Chloride [15]
Mechanisms of Selective Chlorination of Nd-Fe-B Magnet Using ZnCl2
Figure 2a, b show the chemical potential diagrams of Nd–Zn–Cl and Fe–Zn–Cl systems, respectively, at 1000 K [12, 13]. ZnCl2 (l,g) equilibrates with Zn (l) and NdCl3 (s) at point a in Fig. 2a while Nd (s) does not coexist with ZnCl2 (l,g) at 1000 K. Therefore, when a sufficient amount of ZnCl2 is used as a chlorinating agent, Nd in the magnets will be chlorinated to NdCl3 by reacting with ZnCl2 at 1000 K, as shown in Eq. (1). In addition, Dy and Pr in the magnets also will be chlorinated to dysprosium chloride (DyCl3) and praseodymium chloride (PrCl3) by reacting with ZnCl2 at 1000 K, as shown in Eqs. (2) and (3), respectively. On the other hand, the chemical equilibrium point for FeClx (x = 2,3, l,g), ZnCl2 (l,g), and Zn (l) does not exist, as shown in Fig. 2b. As a result, even though a sufficient amount of ZnCl2 is used for the reactions, Fe in magnets will not be chlorinated
Experimental
Figure 3 shows a schematic of the experimental apparatus, and Table 1 lists the experimental of the selective chlorination process using ZnCl2 at 1000 K for 1.5 h. A mixture of Nd-Fe-B magnet powder and anhydrous ZnCl2 placed in an alumina (Al2O3) crucibles was positioned in a quartz holder inside a glove box. Afterward, the quartz holder was removed from the glove box and placed at the bottom of a gas-tight quartz reactor. The gas inside the reactor was replaced with Ar gas through evacuation and filling of Ar gas. The pressure inside the reactor was maintained at 1 atm during the experiments by flowing Ar gas. After the completion of atmosphere control, the reactor was placed in an electric furnace, which was preheated to 1000 K. When the reaction time was finished, the reactor was immediately removed from the furnace and cooled to room temperature. The samples obtained after the selective chlorination reaction were subjected to deionized (DI) water leaching at room temperature for 0.5 h, and the residues obtained after the leaching were dried at 343 K for 0.5 h.
Results and Discussion
Table 2 lists the composition of the Nd magnet used as feedstock. In addition, Fig. 4 shows the results of XRD analysis of the residues obtained after the selective chlorination reactions without water leaching. As expected from the thermodynamic analysis, NdCl3 and Fe (Zn) were identified after the experiments. DyCl3 and PrCl3 were not identified because the feedstock contains low concentrations of these elements, as shown in Table 2.
Further, Fig. 5 shows the influence of the particle size of the feedstock on the chlorination efficiencies of Nd, Dy, and Pr after the experiments. When the particle size of the feedstock used for selective chlorination using ZnCl2 increased from <75 μm to 300–600 μm, the chlorination efficiencies of Nd, Dy, and Pr increased from 93.8%, 36.3%, and 96.0% to 96.5%, 57.2%, and 97.6%, respectively. In addition, the total chlorination efficiency of Nd, Dy, and Pr was 92.1% when the particle size of the feedstock was 300–600 μm. As the particle size decreased, the chlorination efficiencies of REEs decreased because the concentration of oxygen in the feedstock increased from 0.188 mass% at 300–600 μm to 0.467 mass% at <75 μm. REEs in the magnet powder feedstock easily react with oxygen during processing because these elements are reactive. However, the oxides of REEs could not be chlorinated to rare earth chlorides by reacting with ZnCl2 at 1000 K. For example, when the oxides of Nd and Dy react with ZnCl2 at 1000 K, neodymium oxychloride (NdOCl) and dysprosium oxychloride (DyOCl) are produced, as shown in Eqs. (4) and (5), respectively [12, 15]. As a result, the chlorination efficiencies of REEs increased when the particle size of the feedstock was increased.
Results of XRD analysis of the residues obtained after the selective chlorination reactions (Reprinted by permission from Springer Nature: Springer, Journal of Sustainable Metallurgy, Selective Chlorination of Rare Earth Elements from a Nd-Fe-B Magnet Using Zinc Chloride, (Kyung-Hwan Lim et al. 2021)
Conclusions
The selective chlorination process using ZnCl2 to recover Nd, Dy, and Pr from Nd magnets was investigated in this study. When the Nd magnet reacted with ZnCl2 at 1000 K, Nd in the feedstock chlorinated to NdCl3; however, Fe in the feedstock did not. In addition, the chlorination efficiencies of Nd, Dy, and Pr reached 96.5%, 57.2%, and 97.6%, respectively, when the particle size of the feedstock used for the chlorination reaction at 1000 K for 1.5 h was 300–600 μm. As a result, 92.1% of REEs was recovered from the Nd magnets through the selective chlorination process.
References
Haider SK, Lee J-Y, Kim D, Kang YS (2020) Eco-friendly facile three-step recycling method of (Nd-RE)2Fe14B magnet sludge and enhancement of (BH)max by ball milling in ethanol. ACS Sustainable Chem Eng 8:8156–8163
Takeda O, Okabe TH (2014) Current status on resource and recycling technology for rare earths. Metall Mater Trans E 1:160–173
Akahori T, Miyamoto Y, Saeki T, Okamoto M, Okabe TH (2017) Optimum conditions for extracting rare earth metals from waste magnets by using molten magnesium. J Alloys Compd 703:337–343
Okabe TH, Takeda O, Fukuda K, Umetsu Y (2003) Direct extraction and recovery of neodymium metal from magnet scrap. Mater Trans 44:798–801
Takeda O, Okabe TH, Umetsu Y (2006) Recovery of neodymium from a mixture of magnet scrap and other scrap. J Alloys Compd 408–412:387–390
Kobayashi S, Kobayashi K, Nohira T, Hagiwara R, Oishi T, Konishi H (2011) Electrochemical formation of Nd-Ni alloys in molten LiF-CaF2-NdF3. J Electrochem Soc 158:E142–E146
Saito T, Sato H, Ozawa S, Yu J, Motegi T (2003) The extraction of Nd from waste Nd-Fe-B alloys by the glass slag method. J Alloys Compd 353:189–193
Shirayama S, Okabe TH (2018) Selective extraction and recovery of Nd and Dy from Nd-Fe-B magnet scrap by utilizing molten MgCl2. Metall Mater Trans B 49:1067–1077
Uda T (2002) Recovery of rare earths from magnet sludge by FeCl2. Mater Trans 43:55–62
Hua Z, Wang J, Wang L, Zhao Z, Li X, Xiao Y, Yang Y (2014) Selective extraction of rare earth elements from NdFeB scrap by molten chlorides. ACS Sustainable Chem Eng 2:2536–2543
Lim K-H, Choi C, Moon G, Lee T-H, Kang J (2021) Selective Chlorination of Rare Earth Elements from a Nd-Fe-B Magnet Using Zinc Chloride. J Sustain Metall 7:794–805
Barin I (1995) Thermochemical data of pure substances. VCH Verlagsgesellschaft mbH, Weinheim
Hatada N (2014) Chesta: Software for Creating Chemical Potential Diagram, version 3.2.6.9, http://www.aqua.mtl.kyoto-u.ac.jp/wordpress/chestaEng.html
Windholz M, Budavari S, Blumetti RF, Otterbein E.S (1983) The Merch Index – An encyclopedia of chemicals, drugs, and biologicals, 3rd ed. Rahway N.J. USA, pp. 9932
Jacob KT, Dixit A, Rajput A (2016) Stability field diagrams for Ln-O-Cl systems. Bull Mater Sci 39:603–611
Acknowledgements
This research was supported by the Korea Evaluation Institute of Industrial Technology, the Korean Ministry of Industry (Project No. 20000970, 21-9805)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Lim, KH., Choi, C., Moon, G., Lee, TH., Kang, J. (2022). Recovery of Rare Earth Elements from Nd-Fe-B Magnet Through Selective Chlorination Using Zinc Chloride. In: Lazou, A., Daehn, K., Fleuriault, C., Gökelma, M., Olivetti, E., Meskers, C. (eds) REWAS 2022: Developing Tomorrow’s Technical Cycles (Volume I). The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-92563-5_77
Download citation
DOI: https://doi.org/10.1007/978-3-030-92563-5_77
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92562-8
Online ISBN: 978-3-030-92563-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)