Abstract
The huge increase in the generation of post-consumer plastic waste has produced a growing interest in eco-efficient strategies and technologies for their appropriate management and recycling. In response to this, PROQUIPOL Project is focused on developing, optimizing and adapting feedstock recycling technologies as an alternative for management for the treatment of complex plastic waste. Among the different plastic wastes studied, PROQUIPOL Project is working on providing a suitable treatment to the highly colored and complex multilayered post-consumer waste fractions of polyethylene terephthalate (PET) by chemical depolymerisation methods. Glycolysis and alkali hydrolysis processes have been studied with the aim of promoting the transformation of PET into the bis(2-hydroxyethyl) terephthalate monomer and terephthalic acid, respectively. In both cases operational conditions such as temperature, reaction time, catalyst to PET rate and solvent to PET rate have been considered to optimize product yield, achieving values near to 90 % and monomer purities over 95 % in both processes. This paper presents results obtained for each treatment as well as a simplified comparison of technical, economic and environmental issues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastic industry
Plastic production has increased from 1.5 million tonnes in 1950 to 230 million tonnes in 2009 (an average of 9 %). Just in Europe, plastic demand reached 45 million tonnes in 2009. By end of use applications, the packaging segment is the largest, representing 40 % of overall demand.
In relation to the end of life of plastic products, in 2009 more than a 50 % of consumed plastics became postconsumer waste which imply that around 25 million tonnes were waiting for suitable management. From that amount of plastic waste, 42 % was disposed in landfills while 24 % was recycled mechanically and 34 % was sent for energy recovery [1, 2]. It is true that every year the disposal ratio decreases; however, there remains much to be done. For this reason, European legislation is promoting the valorisation, recycling and recovering of plastic waste [3, 4]. Focusing on the packaging sector, then Europe is the largest plastic demander, the main source of plastic waste (63 %) and the sector with the highest ratio of recovery (66 %). Within this sector and covering 75 %, the most used plastics were low density polyethylene (LDPE), high density polyethylene (HDPE), polyethylene terephthalate (PET), polypropylene (PP) and polystyrene (PS), better known as the “big five”.
As regards the generation of plastic waste, the Spanish situation differ a bit from the European since in 2009 74 % of consumed plastics became postconsumer waste, ergo 2268 kt. From this amount of plastic waste, 1443 kt were generated in the packaging sector, with PET being the largest provider of waste with 483 kt and followed by HDPE and LDPE with 404 and 255 kt, respectively. Regarding recycling in the packaging sector, the Spanish collected data showing that in 2009, 383 kt (26.5 %) of this packaging plastic waste were recycled mechanically and of which 103 kt belonged to PET waste [5, 6].
PET in the packaging sector
Nowadays, the need of the packaging manufacture industry for adapting to new markets has promoted the development of new PET materials, designed to satisfy the requirements of conservation, packaging and marketing. The new PETs are complex multilayered and highly colored PET.
Complex multilayered PET is a material composed of different polymer layers with the aim of adding properties such as lower permeability to gases and vapours, migration avoidance, etc.; and it is commonly used in the packaging of natural products such as juices, soft drinks or beers. On the other hand, highly colored PET is in essence PET with pigments or colourings where the aim is just a question of marketing instead of technical performance.
The mechanical recycling of PET is fully established and developed; however, it is more suitable to natural or slightly colored PET waste. These new PETs, due to their varied nature, are a rejected stream in sorting plants sent to a landfill, since their mechanical recycling offers a final product with limitations on the colour, transparency and intrinsic viscosity and, therefore, restricting its applicability. Checking previous data about PET waste and its recycling, it can be detected that only in Spain are there nearly 380 kt of PET without treatment, not collected or disposed in landfills and composed mainly of these new PETs. According to this, chemical recycling based on depolymerisation processes is proposed as an emerging challenge, able to provide a suitable recycling treatment to the highly colored and complex multilayered post-consumer waste fractions of PET.
Chemical depolymerisation processes
The chemical recycling consists of the decomposition of polymers by chemical agents and catalysts to obtain the molecules that originated with them, with high value potential applications such as chemicals, monomers or new polymers [7]. Depending on the used agent, different types of chemical recycling (glycolysis, methanolysis, hydrolysis, etc.) can be carried out. Research in this area has demonstrated the efficiency of the PET recycling, which allows continuing investigation in this field with different types of PET materials to improve processes and technologies [8–10].
In accordance with this, among the PROQUIPOL Project objectives are to compare recycling processes based on chemical depolymerisation for improving the current PET recycling scheme. For this, glycolysis based on a catalytic reaction to promote the formation of BHET [11] and alkaline hydrolysis to promote the formation of terephthalic acid (TPA) [12], was considered as an alternative to management for these non-mechanically recoverable fractions.
Theory
PET glycolysis reaction
Glycolysis is a molecular depolymerisation process by transesterification between PET ester groups and a diol, usually ethylene glycol in excess, to obtain the monomer bis(2-hydroxyethyl terephthalate), according to the following reaction scheme:

BHET is widely used in the synthesis of polymeric materials, ranging from unsaturated polyester resins and PET to novel biocompatible systems. BHET is also used as a plasticiser in unsaturated polyester resins and rigid or flexible polyurethanes [13, 14].
The main advantage of this strategy is that it can be easily integrated into a conventional PET production plant and the recovered BHET can be blended with fresh BHET [15].
PET alkaline hydrolysis reaction
AN hydrolysis process is the reaction of PET with water to break down the polyester chains into TPA and EG [16] with an aqueous solution of sodium hydroxide according to the following reaction scheme:

As it can be observed in the previous chemical reaction, PET reacts with caustic soda, promoting at first the formation of the corresponding TPA disodium salt, which is soluble in water, and ethylene glycol. In a second step, hydrochloric acid is employed to promote the formation and precipitation of TPA. Nearly all purified terephthalic acid is consumed in polyester production including polyester fibre, PET bottle resin and polyester film. The remaining TPA is used in making cyclohexanedimethanol, terephthaloyl chloride, co-polyester-ether elastomers, plasticisers and liquid crystal polymers [17].
Taguchi optimization study
When an experiment has several factors that are relevant, the factorial experimental design should be used. Factorial experiments are the way to identify interactions between variables. Because of this, the glycolysis of PET experiments has been carried out using the Taguchi’s parameter design methodology.
The main goal of this study is to find the higher yield of BHET with optimum purity. The operation variables (or control factors) considered as the more influential were chosen based on previous studies [8, 10, 18, 19] and the experience of the research group in this field. The variables and the ranges used are:
-
The reaction temperature (T), between 195 and 220 °C, due to temperatures under 190 °C produce an excess of sub-products and high temperatures over 220 °C do not increase the rate or the amount of BHET obtained [19].
-
The reaction time (t) between 150 and 210 min, occurring in this range of time produces the maximum yield of the reaction. At reaction times under 90 min the reaction is not completed, after 120 min, the PET oligomer used disappears [20], and after 210 min, the changes of the yield of the reaction are constant.
-
The solvent to polymer mass ratio (EG/PET) is between 2 and 6 w/w. The solvent is used in excess due to the yield of the reaction increasing with the amount of EG.
-
The catalyst to polymer mass percentage ratio (ZnAc2/PET) which was between 0.2 and 1 %w/w. The catalyst chosen was zinc acetate due to it being proved that it produces the maximum amount of the BHET monomer [8, 10, 18].
In Table 1 appear the factors and levels used to perform an orthogonal array L9 to carry out nine experiments combining the different selected levels of each control factor considered. These experiments have been carried out firstly with virgin PET. Each experiment was repeated twice in order to observe the reproducibility of the glycolysis process. After finishing the experiments, the results are analysed in order to find the optimal level of each variable to have the optimal operational conditions.
Materials and methods
Materials
Virgin PET used was supplied by NOVAPET and samples of highly colored waste PET, complex multilayered waste PET and municipal solid waste PET (MSW) were provided by ECOEMBES, the Spanish integrated management system for light packaging waste. The types of post-consumed PET used were:
-
Highly colored PET: which content 90 % of PET and the presence of PE and paper in caps and labels which is used in water bottles and in mouthwash bottles.
-
Complex multilayered PET: 82–96 % of PET and the presence of polyamide (1–5 %) and PE/PP in caps (5–10 %), used as a container in beer and juice bottles.
-
Sorting plant: 92 % of PET followed by a 6.2 % of PE, 1.7 % of PP and the remains of polyamide, carton board, aluminium film and elastomers.
-
MSW: 92 % of PET and the presence of PE (4.5 %), PA (1.1 %) and mixtures of aluminium and PET (1.5 %). Remains of PP, PS, paper, aluminium film and EPS.
Glycolysis process
Glycolysis experiments were carried out in a 2 L stainless steel reactor. It has an electrical resistance to heat, a temperature control and a stirrer to ensure good mixing. An initial purge of nitrogen was used to prevent possible oxidation of the reaction products. The system can operate at a pressure of 6 bars. Ethylene glycol has been used as a solvent, and zinc acetate as a catalyst which was reported as the most active and suitable glycolysis catalyst [18–20].
In a first step, a factorial design of experiments was carried out with virgin PET to establish the best conditions for the process. Then, an optimum experiment was repeated with post-consumer PET. Factors under study in the factorial design of experiments were process temperature (195, 208 and 220 °C), reaction time (150, 180 and 210 min), EG/PET ratio (2, 4 and 6 w/w) and ZnAc2/PET ratio (0.2, 0.6 and 1.0 %w/w).
The starting time for the reaction was taken when the temperature was the desired one. Temperature was maintained constant during the whole reaction. Once the reaction was finished, boiling water was added in excess to the reaction products under vigorous agitation. Then, the mixture was filtrated to separate un-reacted PET and solid sub-products above the filter and a liquid phase composed of BHET, water and EG below the filter. This recovered liquid was refrigerated to ensure the crystallization of the BHET. A second filtration was performed to separate BHET crystals from EG. BHET was dried at 65 °C until at a constant weight. The BHET yield is calculated with Eq. 1.
where W PET,0 and W BHET,f refer to the initial weight of PET and the weight of BHET at the finish of the reaction, respectively. MWPET and MWBHET are the molecular weights of PET (192 g mol−1) and the BHET (254 g mol−1) repeating unit, respectively.
Besides assessing the feasibility of glycolysis for new PETs, the economically optimization of the process was studied. For that, and considering that the solvent used in the reaction was higher than the stoichiometric needed, EG obtained after the last filtration was subjected to evaporation at high temperatures (about 150 °C) to remove the water contained in the solution and thus reuse it in the following runs.
Alkaline hydrolysis process
Hydrolysis experiments were carried out in a 1.8 L batch operated stainless steel reactor with a maximum operating pressure and temperature of 250 bar and 500 °C, respectively. The reaction system includes automated control, real time parameter monitoring, gas feeding lines and manual sampling for liquids, vapors and gases. In all runs, 40 g of PET was charged to the reaction system. Firstly, a set of reactions with virgin PET were carried out to assess the influences of temperature, particle size and PET/NaOH ratio on TPA yield and select the appropriate conditions for the depolymerisation of post consumer PET waste.
After fFinishing the reaction, the mixture was filtered to remove non-reacted PET. After filtration, HCl was added to promote the formation and precipitation of TPA. The solution was filtered again where the solid phase, composed of TPA, was cleaned with water to remove possible solid elements. After filtration, TPA was dried at 80 °C for 2 h and weighed on an analytical balance to estimate the yield according to Eq. 2.
where W PET,0 and W TPA,f refer to the initial weight of PET and the weight of TPA at a specific reaction time, respectively. MWTPA and MWPET are the molecular weights of TPA (166 g mol−1) and the PET (192 g mol−1) repeating unit, respectively. This purified product was then subjected to various characterisation techniques.
Analysis of products
A FTIR spectrophotometer and a DSC thermal analysis were used to identify chemical structure and to determine purity of BHET and TPA. Commercial BHET and TPA were used for calibration purposes, in order to compare with the recovered products.
Glycolysis products
A FTIR spectrophotometer (Varian 670-IR with an ATR Pike accessory) was used to identify the chemical structure of BHET. DSC (TA Instruments DSC Q100 Model) was used for thermal analysis of BHET. Samples were heated from room temperature to 220 °C with a heating rate of 10 °C/min under a nitrogen stream of 50 mL/min.
Hydrolysis products
A FTIR spectrophotometer (Perkin Elmer Spectrum 100) was used to identify the chemical structure of TPA. DSC (Mettler Toledo DSC822e Model) was used for thermal analysis of TPA. Samples were heated from room temperature to 400 °C with a heating rate of 10 °C/min under a nitrogen stream of 15 mL/min.
Results and discussion
Glycolysis results
Analysis of variance: ANOVA
An analysis of variance of the results, using the ANOVA® commercial software, was completed to obtain the contribution of the factors and their interactions over the response variable (BHET yield), and to determine the levels that maximize the BHET production (see Table 1). This ANOVA was made mixing the contributions of no significant factors according to the significance test of Fisher with a confidence level of 99 %.
Table 2 shows the analysis of variance. The factor e1 is due to the analysis error, but in this case there are three recurrences so the error is zero. The factor e2 is due to the error of the factors and variables which have not been considered and they have an influence in the process (for example the quality of the waste PET). The factor <e> is due to the contribution of the experimental and analysis error (the sum of e1 and e2).
According to the results obtained by the analysis of variance (Table 2), it was observed that the EG to PET mass ratio was the most significant factor with an influence of 93.78 %. The reaction time was the second one with an influence of 2.78 % and finally, ZnAc2 to PET mass percentage ratio has only an influence of 0.65 %. The contribution of the experimental and analysis error was very low, only 0.42 %.
Influence of the conditions studied is shown in Fig. 1. It can be seen how level 3 for EG/PET factor (6 w/w) offers the highest BHET yield. The level 1 for time factor (150 min) had the optimum yield. The level 3 for the ZnAc2/PET was the best, but very similar to the level 1, so level 1 (0.2 %w/w) was selected as the best due to economic aspects. Finally, the temperature factor did not have a significant importance, but the second was the best level, 208 °C, so it was the level chosen.
According to Taguchi’s methodology, if these levels of control factors were used, a maximum BHET yield would be obtained whose value would be 89.09 ± 0.42. In order to demonstrate the results of Taguchi’s study, a confirmatory experiment was carried out with the optimum conditions to verify the BHET yield expected. In this last confirmatory experiment the BHET yield obtained was 87.56 %, so it was assumed that the conditions chosen were the optimal conditions.
Experiments with waste PET
As a result of the factorial design of the experiments, the influence of temperature, reaction time, EG/PET and ZnAc2/PET mass ratios was achieved. Considering optimal operational conditions (208 °C, 150 min, 6:1 w/w ratio EG/PET and 0.2 %w/w ratio ZnAc2/PET), experiments with post-consumer PET were carried out.
It was remarkable that in the experiments carried out with PET waste, a hard ball of polyolefin was formed, which could be easily separated from the rest of the reaction products. This amount of polyolefin represents 5–8 % of the total amount of PET waste. This is due to the fact that all PET waste contains a percentage of polyolefin.
The yield of waste PET was calculated by Eq. 1. But, in this case, the polyolefins obtained at the end of the reaction were not taken into account in calculating the yield. So the value of W PET,0 refers to the initial weight of PET less the weight of polyolefins obtained.
Figure 2 shows the effect of PET nature over BHET yield. The yield decrease from 88 % to nearly 77 %, being complex multilayered PET, has the worst percentage and the virgin PET the most favourable. The sorting plant PET and highly colored PET had a similar and intermediate yield. Anyway, the BHET yield obtained was very similar with the different experimental PET waste.
Characterization of BHET
A FTIR spectrophotometer and a DSC thermal analysis were used to identify chemical structure and to determine purity of BHET, respectively. Commercial BHET was used for calibration purposes, in order to compare with the recover products.
Figure 3 shows the thermograms obtained in the analysis of the recovered BHET, and the melting point and purity of these samples are summarized in Table 3. These thermograms show a reasonably sharp endothermic peak at 109 °C, which agrees very well with the known melting point of commercial BHET. The purity for these samples is very similar for all of them (around 97 %).
Besides, regarding FTIR studies in Fig. 4, it was confirmed that for a pure BHET, the absorptions at 3400, 3000–2800, 1700 and 1100 cm−1 were respectively due to –OH, C–H, C=O and C–O, verifying that infrared spectra of glycolysis products correspond with the BHET one.
From all these observations it was concluded that the product from glycolysis is highly pure BHET.
Alkaline hydrolysis results
Experiments with virgin PET
Temperature, PET particle size and PET/NaOH ratio were chosen as the independent variables for the hydrolysis study.
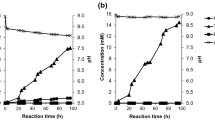
Runs #1, #2, #3, #4 and #5 in Table 4 were carried out at 150 °C to determine, on the one hand, the accuracy of the working procedure and, on the other hand, to confirm the similarity of taking samples at a given time in a unique reaction and achieving runs for different times. The first option about taking samples was selected for the whole research work. Runs #5, #6 and #7 were respectively completed at 150, 100 and 200 °C and showed the feasibility of operating at mild temperatures for achieving the highest TPA yield (see Fig. 5). Runs #5, #8 and #9 were carried out to assess the influence of the PET/NaOH molar ratio and verified that the 1/2.4 was the value required for getting a reasonable TPA yield as plotted at Fig. 5. Finally, runs #9 to #12 were completed at different PET particle size to ensure that the reaction rate was not limited by physical characteristics of the material. In accordance with the results, optimum operating conditions were fixed at 150 °C, a PET/NaOH molar ratio of 1/2.4 and a PET particle range between 0.2 and 0.5 mm.
Experiments with waste PET
At Fig. 5 are shown the yields of TPA (#13 to #16) obtained by the hydrolysis of waste PETs after 4 h at 150 °C, 1:2.4 PET/NaOH molar ratio. In the TPA yields it was found that PET nature and origin did not show a remarkable influence on TPA yield and that complex multilayered PET and PET from bulk MSW collection yields to TPA were close to 90 %, associated mainly to non-PET polymers and dirtiness.
Characterization of TPA
Characterization of alkaline hydrolysis products was analogous to that made in glycolysis, using a FTIR spectrophotometer and a DSC thermal analysis to identify chemical structure and to determine purity of TPA, respectively. Commercial TPA was used for calibration purposes, in order to compare with the recovered products.
DSC analysis of TPA produced after the hydrolysis of waste PETs at runs #13 to #16 is summarized in Table 5. Results are illustrated in Fig. 6, and compared with a commercial TPA.
On the other hand, regarding FTIR studies (Fig. 7) it was confirmed that, for a commercial TPA, the absorptions at 2800–3000, 1750, 700–800 cm−1, were respectively due to C–H phenyl group, C=O and C–H phenyl group verifying that infrared spectra of hydrolysis products correspond with the TPA one.
According to DSC and FTIR results, it can be confirmed that chemical structures are identical to the commercial one and purity values are over 95 % in all cases.
Economic processes comparison
After the technical feasibility of both processes was demonstrated, the economic viability was checked for a pilot plant (see Fig. 8) with a capacity of 200 t/year. The costs considered in viability were the depreciation, energy & utilities, personnel, chemicals, waste management and overheads.
After reacting 200 t of waste PET, then 166 t of TPA (99 % purity) were obtained in the alkaline hydrolysis process and 221 t of BHET (99 % purity) in the glycolysis process. In those conditions, the average production costs for the products were calculated and resulted in 2.71 € per 1 kg of TPA and 4.23 € per 1 kg of BHET. After the analysis of these first cost calculations it was detected that the highest costs in the processes were associated to the large excess of solvent used during the production of the BHET, and the waste water management during the production of TPA. The excess of solvent needed in the glycolysis of PET is six times greater than the amount of PET and represents 39.8 % of the total costs of the process. The waste water in the PET hydrolysis is due to the complex composition, which is an aqueous stream composed of organic phase (the EG generated during the PET depolymerisation), basic pH (due to the NaOH used in the reaction) and NaCl (formed by the neutralisation of the HCl excess used in the TPA precipitation).
For the economic optimisation of the processes, the plant capacity was increased, up to 8000 t/year, as well as alkaline hydrolysis or glycolysis improvement themselves were considered.
In the case of the glycolysis, the reduction of the EG/PET mass ratio from 6 to 4 was considered resulting in a slight decrease on BHET yield but not on BHET purity. In these new conditions, the cost of producing 1 kg of BHET was lowered till 3.31 € in comparison to the initial 4.23 €. Another alternative was the reuse of the EG in excess, after a distillation step to remove the water content proved that it could be reused four times, decreasing the cost up to 2.36 €/kgBHET (using EG/PET = 6) and up 1.99 €/kgBHET (using EG/PET = 4). So, finally it was elected to have a 4 EG/PET mass ratio and the reuse of EG to optimise the economics of the glycolysis process, as it can be seen at Table 6.
For the hydrolysis process, avoiding the external management of waste water, estimating a 40 % cost saving and operating one's own sewage treatment plant, the unitary cost was 1.02 €/kgTPA.
All these results are based on an economic study carried out considering all the variables and costs at an industrial scale. The results show that several socioeconomic aspects such as PET consumption, the types of PET consumed, the price of virgin and recycled PET or even a mechanical recycling situation will have a relevant effect on the economic feasibility and, therefore, on the process industrialization of these processes. The PET manufacturing industry is the key for moving to the implementation of the depolymerisation solutions.
Both processes still can be more optimized and scaled up for lowering manufacture costs but the final decision on the selection of one of them will need to consider the value of the raw material (waste PET) and the products (TPA or BHET).
Conclusions
There has been carried out a chemical recycling of highly colored and complex multilayered post-consumer PET by glycolysis to obtain BHET and by alkaline hydrolysis to obtain TPA.
Firstly, was defined the most suitable operating conditions (T = 208 °C, t = 150 min, EG/PET = 6 w/w, ZnAc2/PET = 0.2 %w/w in glycolysis process and T = 150 °C, t = 240 min, NaOH/PET = 2.4 mol/mol, dp = 0.5–1.0 mm in the hydrolysis process). The yields obtained close to 85 % of BHET and TPA yields up to 90 %.
These values were similar to that obtained when treating virgin PET, thereby suggesting that PET's nature did not show a remarkable influence on BHET and TPA production, except in runs with complex multilayered PET and PET from bulk MSW collection where the decrease was associated with non-PET polymers and dirtiness.
DSC and FTIR analysis verified that the BHET and TPA quality products derived from highly colored and complex multilayered post-consumer waste streams of PET were equivalent to those obtained from reference PET.
The economic analysis shows that the keys for the process optimization were, on the one hand, the addition of a distillation step in the glycolysis process to recover and reuse the EG and, on the other hand, the addition of a sewage treatment plant in the hydrolysis process to avoid the external waste water management.
Optimising the economic process with a capacity of a plant of 8000 t/year, the unitary cost of producing 1 kg of BHET is 1.99 €/kgBHET and of TPA is 1.02 €/kgTPA.
References
Mudgal S, Lyons L (2011) Plastic waste in the environment, final report. European Commission (DG Environment)
PlasticsEurope, EuPC, EuPR, EPRO and Consultic (2009). The compelling facts about plastics—an analysis of European plastics production, demand and recovery for 2008
European Parliament and Council Directive 94/62/EC of 20 December 1994 on packaging and packaging waste
Directive 2004/12/EC of the European Parliament and of the Council of 11 February 2004 amending Directive 94/62/EC on packaging and packaging waste
Cicloplast (2009). Estadísticas de consumo, residuos, reciclado y recuperación. Energética de los plásticos. España 2009
Centro español de Plásticos, CEP (2009) El sector de los plásticos
Arandes JM, Bilbao J, López Valerio D (2004) Reciclado de residuos plásticos. Revista Iberoamericana de Polímeros 5:1
Xi G, Li M, Sun C (2005) Study on depolymerization of waste polyethylene terephthalate into monomer of bis(2-hydroxyethyl terephthalate). Polym Degrad Stab 87(117):120
Ghaemy M, Mossaddegh K (2005) Depolymerization of poly(ethylene terephthalate) fibre wastes using ethylene glycol. Polym Degrad Stab 90(570):576
Baliga S, Wong WT (1989) Depolymerization of poly(ethylene terephthalate) recycled from post-consumer soft-drink bottles. J Polym Sci Part A Polym Chem 27(2071):2082
Kao CY, Cheng WH (1997) Investigation of catalytic glycolysis of polyethyelene terephthalate by differential scanning calorimetry. Thermochim Acta 292(95):104
Paszun D, Spychaj T (1997) Chemical recycling of poly(ethylene terephtalate). Ind Eng Chem Res 36(1373):1383
Alvarez A, Castano VM (1994) Semi-interpenetrating polymer networks produced with polyethylene terephthalate oligomer and unsaturated polyester resin. Polym Bull 32(447):453
Alvarez A, Castano VM (1995) Modification of polyester resins by an oligomeric additive. Polym Bull 35(187):194
Pang K, Kotek R, Tonelli V (2006) Review of conventional and novel polymerization processes for polyesters. Prog Polym Sci 31:1009–1037
Aguado J, Serrano D (1999) Feedstock recycling of plastic waste. In: Clark JH (ed) RSC clean technology monographs. York, United Kingdom, pp 31–58
http://www.icis.com/v2/chemicals/9076461/terephthalic-acid/uses.html
Chen JW, Chen LW, Cheng WH (1999) Kinetics of glycolysis of polyethylene terephthalate with zinc catalyst. Polym Int 48(885):888
Goje AS, Mishra S (2003) Chemical kinetics, simulation, and thermodynamics of glycolytic depolymerization of poly(ethylene terephthalate) waste with catalyst optimization for recycling of value added monomeric products. Macromol Mater Eng 288(326):336
Chen JW, Chen LW (1999) The glycolysis of poly(ethylene terephthalate). J Appl Polym Sci 73(35):40
Acknowledgments
The authors wish to acknowledge the confidence for and economic support of the Spanish Ministry of Education and Science to this work by financing the PROQUIPOL Project “Reference PID-560620-2009-4” and the supply of actual post-consumer waste samples of PET by ECOEMBES.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguado, A., Martínez, L., Becerra, L. et al. Chemical depolymerisation of PET complex waste: hydrolysis vs. glycolysis. J Mater Cycles Waste Manag 16, 201–210 (2014). https://doi.org/10.1007/s10163-013-0177-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-013-0177-y