Abstract
Background
A few previous clinical studies have shown that chloride (Cl) contributes to the progression and development of hypertension or proteinuria. Therefore, we aimed to determine whether hyperchloremia is associated with hypertension or proteinuria in patients with chronic kidney disease (CKD) and to define the relationships between the reduction in serum Cl concentration associated with CKD treatment and improvements in hypertension and/or proteinuria.
Methods
We performed a retrospective observational study of new or referred patients with CKD who had hyperchloremia, moderate proteinuria, renal dysfunction, and hypertension. Patients taking medication for metabolic acidosis or with a history of dialysis were excluded. The participants’ systolic and diastolic blood pressure (BP), serum sodium (Na) and Cl concentrations, and urinary protein (UP) concentration were measured at baseline and after 1 month of CKD treatment.
Results
Fifty-one patients with CKD were included in the study. Their serum Cl concentration independently correlated with sBP and UP at baseline (P = 0.022 and P = 0.033, respectively). After 1 month’s CKD treatment, their serum Na and Cl concentrations, sBP, and UP were significantly lower. The change in sBP during the month (ΔsBP) correlated with the change in serum Cl (ΔCl) (P = 0.012) but not with the change in serum Na. Multivariate analysis showed that ΔsBP was independently associated with ΔCl (P = 0.029).
Conclusions
Hyperchloremia is an independent predictor of hypertension and proteinuria for patients with CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension is a risk factor for the onset and progression of chronic kidney disease (CKD) [1,2,3]. It is also present in most patients with CKD, in whom it increases the risks of cardiovascular disease and death [4]. The presence of proteinuria is similarly associated with higher risks of the progression of CKD and death [5]. Furthermore, a recent study showed that patients with CKD and extremely high systolic and diastolic blood pressure (BP) had the highest mortality rates, and similar findings were made in subgroups of patients with high urinary microalbumin/creatinine ratios [6].
Both genetic and environmental factors play significant roles in the development of hypertension. Of the environmental factors that affect BP, salt consumption has been the subject of intense scientific research. Although salt is composed primarily of sodium chloride (NaCl), only sodium (Na) has been thought to be associated with salt-sensitive hypertension. However, in contrast to the effect of NaCl loading, some previous studies have shown that non-chloride sodium salt loading, for example, using sodium bicarbonate (NaHCO3), sodium citrate, or sodium phosphate, is associated with low BP [7,8,9,10]. Furthermore, chloride (Cl) loading is associated with progressive renal vasoconstriction and a reduction in glomerular filtration rate (GFR) [11].
The relationship between dietary salt intake and proteinuria has also been studied in patients with CKD. Dietary salt restriction significantly reduces not only BP but also proteinuria [12]. Although only the Na present in dietary salt has been reported to be associated with proteinuria [13, 14], we have previously shown that in aldosterone-infused rats, the inclusion of NaCl in the drinking water is associated with greater hypertension and proteinuria than the inclusion of NaHCO3 [15], which suggests that Cl contributes to the progression of these defects. However, very few studies, and especially clinical studies, have shown a relationship between Cl and BP or proteinuria. Therefore, to test the hypothesis that Cl has an important role in hypertension and renal damage/proteinuria, we performed a retrospective study of the relationships of hyperchloremia with hypertension and proteinuria in patients with CKD. Specifically, we aimed to define the relationship between the reduction in serum Cl concentration associated with the treatment of CKD and improvements in hypertension and/or proteinuria.
Materials and methods
Study sample
We performed a retrospective observational study of 51 new or referred patients with CKD who were inpatients or outpatients at the Department of Nephrology, Hiroshima University Hospital, Hiroshima, Japan, between 1 April 2011 and 31 March 2019.
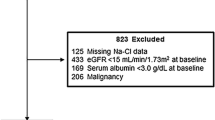
The inclusion of patients in the study was based on the following criteria at the time of their initial visit: (1) serum Cl concentration ≥ 105 mEq/L; (2) moderate proteinuria (urinary protein (UP) of 0.15–3.5 g/gCr); (3) renal dysfunction (estimated GFR (eGFR) of 15–90 ml/min/1.73 m2); and (4) hypertension (clinic sBP ≥ 130 mmHg). We excluded patients who were taking medication for the treatment of metabolic acidosis, such as NaHCO3, and those with a history of dialysis at their initial visit.
The patients underwent routine treatment of their CKD, which comprised lifestyle modification, nutritional education, appropriate consumption of water, and the appropriate use of medication for approximately 1 month. During this treatment period, the patients who demonstrated worsening of their renal function, requiring renal replacement therapy, and those for whom corticosteroid and immunosuppressive treatment for nephritis was started or adjusted were excluded. The patients underwent a further physical examination and laboratory testing approximately 1 month after their initial visit.
This study was approved by the Ethics Committee of Hiroshima University Hospital (approval number E-1752) and was performed in compliance with the principles of the Declaration of Helsinki.
Clinical data
The data were collected from the participants’ medical records, and these included age, sex, systolic and diastolic BP, the presence of diabetes mellitus (DM), laboratory data (hemoglobin, albumin, urea nitrogen, creatinine (Cr), eGFR, uric acid, Na, Cl, potassium, total cholesterol (TC), triglyceride, high-density lipoprotein (HDL)-cholesterol, non-HDL-cholesterol, hemoglobin A1c, and UP), the use of medications (antihypertensive agents, diuretics, anti-lipemic agents, medications for metabolic acidosis, corticosteroids, immunosuppressive agents, and other medications for CKD), CKD stage, the primary cause of the CKD, and the length of time between the initial and subsequent visits. Both systolic and diastolic BP were measured in the sitting position using a mercury sphygmomanometer after a 5 min rest. We calculated eGFR using the Modification of Diet in Renal Disease equation (eGFR = 194 × Cr−1.094 × age−0.287, multiplied by 0.739 for women) developed by the Japanese Society of Nephrology. The non-HDL-cholesterol concentration was calculated by subtracting the HDL-cholesterol from the TC concentration. UP was evaluated as a spot urinary protein/creatinine ratio. Patients with DM were identified as those with diabetic kidney disease (DKD) as the primary cause of their CKD. We defined a 1-month period as being between 2 and 6 weeks.
Statistical analysis
Normally distributed continuous data are expressed as median (interquartile range) and categorical data as percentages. Continuous datasets were compared using paired t tests. The statistical significance level was set as P < 0.05. Univariate and multivariate linear regression analyses were used to identify independent predictors of sBP, UP, the change in sBP, and the change in UP. Data were analyzed using JMP ver. 14 (SAS Institute, Inc., Cary, NC, USA).
Results
A total of 51 patients were enrolled in the study. The principal clinical characteristics of the participants at their first visit are summarized in Table 1. The participants comprised 27 men and 24 women, with a median age of 70 (58–78) years. Thirty-five of the 51 participants (68.6%) were already taking renin–angiotensin system (RAS) inhibitors; and 14 (27.5%) were taking diuretics, including loop, thiazide, and potassium-sparing diuretics. Almost all participants with diuretics (13 participants; 92.9%) were also taking RAS inhibitors. The participants were in stage 4 (20 participants; 39.2%), stage 3b (14 participants; 27.5%), stage 3a (seven participants; 13.7%), or stage 2 (10 participants; 19.6%) CKD. The most frequent nephropathy underlying their CKD was nephrosclerosis (28 participants; 54.9%), followed by DKD (11 participants; 21.6%), nephritis (7 participants; 13.7%), and autosomal dominant polycystic kidney disease (2 participants; 3.9%). The cause in three of the participants (5.9%) was unclear. The median systolic and diastolic BP of the entire sample were 140 (134–150) mmHg and 70 (60–76) mmHg, respectively. The median UP of the entire sample was 0.83 (0.43–1.87) g/gCr. However, the median serum Cl concentration of the entire sample was relatively high because all the participants had high serum Cl concentrations (≥ 105 mEq/L).
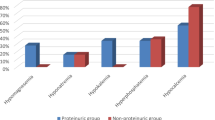
The results of the univariate and multivariate analyses of sBP are shown in Table 2. sBP significantly correlated with serum Cl concentration at baseline (P = 0.004), and multivariate analysis also showed an independent association between serum Cl and sBP (P = 0.022). Similarly, there was a significant association between UP and serum Cl in these analyses (P = 0.002 and P = 0.033, respectively) (Table 3).
The characteristics of the participants 1 month after their initial visit are shown in Table 4. The median length of time between the initial and subsequent visits was 28 (21–34) days. The first RAS inhibitor had been started, the dose of RAS inhibitor had increased, the inhibitor had been discontinued, or an alternative RAS inhibitor had been prescribed in 17 participants (33.3%). A thiazide diuretic had been added or the original had been changed to another thiazide diuretic in 20 participants (39.2%). Two participants (3.9%) were newly prescribed NaHCO3. There was no significant change in the CKD stage, although one participant had progressed to stage G5. The serum concentrations of Na and Cl, sBP, UP, and eGFR had significantly decreased from 141 (139–142) to 139 (138–141) mEq/L (P < 0.001), from 107 (106–109) to 105 (103–108) mEq/L (P < 0.001), from 140 (134–150) to 124 (120–144) mmHg (P < 0.001), from 0.83 (0.43–1.87) to 0.76 (0.28–1.28) g/gCr (P < 0.001), and from 34 (23–51) to 33 (23–46) mL/min/1.73 m2 (P < 0.001), respectively.
To identify parameters that may be involved in the reduction in BP with CKD treatment, we analyzed the correlations between the change in sBP (ΔsBP) and the changes in the serum concentrations of Na (ΔNa) and Cl (ΔCl) (Table 5). ΔsBP correlated with ΔCl (P = 0.012), but not with ΔNa, and multivariate analysis showed that ΔsBP was independently associated with ΔCl (P = 0.029). The change in UP (ΔUP) did not correlate with ΔCl, but did correlate with the change in eGFR (ΔeGFR) (P = 0.043) (Table 6), and multivariate analysis did not reveal significant associations between ΔUP and any of the clinical parameters.
Discussion
We performed a retrospective observational study of clinical data, focusing on hyperchloremia, collected for 51 Japanese patients with CKD. We found that their serum Cl concentration correlated with their sBP and UP at their initial examination, and that ΔCl correlated with ΔsBP 1 month after the initial visit. These findings imply that hyperchloremia is associated with hypertension and proteinuria in patients with CKD.
The kidneys play a vital role in the control of BP and UP that involves the maintenance of salt and water balance. Dietary salt restriction is well known to reduce both BP and UP; however, the detailed mechanisms whereby each one is reduced by dietary salt restriction remain unclear. There is evidence for the benefits of Na restriction on hypertension [13], proteinuria [16], and arterial stiffness [17] in patients with CKD, but there is also some evidence of a role for Cl in the regulation of BP and UP that indicates it may be even more important than Na.
Dietary salt is the principal source of Cl, and it is lost in sweat and through gastric and renal excretion. A high circulating concentration of Cl, referred to as hyperchloremia, introduces renal vasoconstriction and reduces GFR [11, 18, 19]. Delivery of excess Cl to the macula densa activates tubuloglomerular feedback, which induces afferent arteriolar vasoconstriction, mesangial contraction, and an associated reduction in GFR [20,21,22], resulting in an increase in systemic arterial BP.
Although no previous clinical studies have shown a direct relationship between hyperchloremia and BP, some have shown that hypochloremia is associated with higher incidences of mortality and cardiovascular disease. For example, Grodin et al. reported that serum Cl concentration is independently and inversely associated with mortality in patients with heart failure [23]. Furthermore, Mandai et al. and Kubota et al. reported that low serum Cl concentration is an independent predictor of death and cardiovascular events in patients with CKD [24, 25], which suggests that hypochloremia has effects on blood vessels, but the mechanisms remain unclear.
In our previous study, we showed that aldosterone-infused rats develop more severe hypertension and renal inflammation if they drink water containing NaCl than if they drink water containing NaHCO3 [15]. Conversely, dietary Cl restriction slows the development of hypertension and reduces thiazide-sensitive sodium–chloride cotransporter activation. Moreover, we found that Cl overload is associated with the activation of T lymphocytes, which is involved in the development of both hypertension and renal damage. Furthermore, we found evidence that hyperchloremia causes renal damage, which is indicated by proteinuria, through overactivation of the renin–angiotensin–aldosterone system (RAAS), although a detailed mechanism has not yet been identified.
In the present study, we identified a reduction in serum Cl concentration over a 1-month period. Although the treatment of CKD, such as through a reduction in dietary salt intake, appropriate consumption of water, and the use of diuretics, may have contributed to the reduction in serum Cl concentration, the identity of the most important cause remains unclear. We believe that appropriate water consumption is important for a reduction in serum Cl to be achieved. Cl reabsorption can occur via the sodium–potassium–2 chloride co-transporter (NKCC2) in the thick ascending limb of the loop of Henle, the sodium–chloride co-transporter (NCC) in the distal convoluted tubule, and/or the thiazide-sensitive apical sodium-dependent chloride–bicarbonate exchanger (NDCBE) in the collecting duct [26]. We hypothesize that an amelioration of dehydration through appropriate consumption of water induces a reduction in urinary Cl concentration, leading to an inhibition of Cl reabsorption via a downregulation of one or more of these transporters/exchangers, and reducing serum Cl concentration.
The present study had several limitations. First, the sample size was relatively small because of the use of a number of inclusion and exclusion criteria. Second, because the follow-up period was only 1 month, we do not know what the long-term trends might be: a longer study period may have been required to identify trends with respect to proteinuria. Finally, owing to a lack of clinical data, including the results of blood gas analysis and urinary Na and Cl concentrations, we could not evaluate hyperchloremic metabolic acidosis or the daily NaCl intake. We also could not exclude the presence of secondary hypertension; therefore, it is unclear whether the hypertension of all the participants was secondary to their CKD. Moreover, the measurement of BP alone is insufficient to evaluate CKD treatment; in the future, body fluid volume should be evaluated by measuring body mass and composition.
In conclusion, we have shown that high serum Cl concentration is associated with high sBP and UP. Furthermore, we have shown that sBP decreases when serum Cl concentration decreases, and that these associations are independent of serum Na concentration. However, further clinical studies are required to investigate whether the management of serum Cl concentration would ameliorate hypertension in patients with CKD.
References
Coresh J, Wei GL, McQuillan G, Brancati FL, Levey AS, Jones C, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third national health and nutrition examination survey (1988–1994). Arch Intern Med. 2001;161:1207–16.
Jacobsen P, Rossing K, Tarnow L, Rossing P, Mallet C, Poirier O, et al. Progression of diabetic nephropathy in normotensive type 1 diabetic patients. Kidney Int Suppl. 1999;71:S101–5.
Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of diet in renal disease study group. N Engl J Med. 1994;330:877–84.
Chang AR, Lóser M, Malhotra R, Appel LJ. Blood pressure goals in patients with CKD: a review of evidence and guidelines. Clin J Am Soc Nephrol. 2019;14:161–9.
Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238–52.
Kovesdy CP, Bleyer AJ, Molnar MZ, Ma JZ, Sim JJ, Cushman WC, et al. Blood pressure and mortality in US veterans with chronic kidney disease: a cohort study. Ann Intern Med. 2013;159:233–42.
Schorr U, Distler A, Sharma AM. Effect of sodium chloride- and sodium bicarbonate-rich mineral water on blood pressure and metabolic parameters in elderly normotensive individuals: a randomized double-blind crossover trial. J Hypertens. 1996;14:131–5.
Luft FC, Zemel MB, Sowers JA, Fineberg NS, Weinberger MH. Sodium bicarbonate and sodium chloride: effects on blood pressure and electrolyte homeostasis in normal and hypertensive man. J Hypertens. 1990;8:663–70.
Kurtz TW, Al-Bander HA, Morris RC. Salt-sensitive essential hypertension in men. Engl J Med. 1987;317:1043–8.
Shore AC, Markandu ND, MacGregor GA. A randomized crossover study to compare the blood pressure response to sodium loading with and without chloride in patients with essential hypertension. J Hypertens. 1988;6:613–7.
Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Investig. 1983;71:726–35.
Garofalo C, Borrelli S, Provenzano M, de Stefano T, Vita C, Chiodini P, et al. Dietary salt restriction in chronic kidney disease: a meta-analysis of randomized clinical trials. Nutrients. 2018;10:732.
Campbell KL, Johnson DW, Bauer JD, Hawley CM, Isbel NM, Stowasser M, et al. A randomized trial of sodium-restriction on kidney function, fluid volume and adipokines in CKD patients. BMC Nephrol. 2014;15:57.
de Vries LV, Dobrowolski LC, van den Bosch JJON, Riphagen IJ, Krediet CTP, Bemelman FJ, et al. Effects of dietary sodium restriction in kidney transplant recipients treated with renin–angiotensin–aldosterone system blockade: a randomized clinical trial. Am J Kidney Dis. 2016;67:936–44.
Yamauchi T, Doi S, Nakashima A, Doi T, Sohara E, Uchida S, et al. Na+–Cl− cotransporter-mediated chloride uptake contributes to hypertension and renal damage in aldosterone-infused rats. Am J Physiol Renal Physiol. 2018;315:F300–12.
McMahon EJ, Bauer JD, Hawley CM, Isbel NM, Stowasser M, Johnson DW, et al. A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol. 2013;24:2096–103.
Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103:987–92.
Yunos N, Bellomo R, Story D, Kellum J. Bench-to-bedside review: Chloride in critical illness. Crit Care. 2010;14:226.
Bullivant EM, Wilcox CS, Welch WJ. Intrarenal vasoconstriction during hyperchloremia: role of thromboxane. Am J Physiol. 1989;256:F152–7.
Schnermann J, Ploth DW, Hermle M. Activation of tubulo-glomerular feedback by chloride transport. Pflugers Arch. 1976;362:229–40.
Salomonsson M, Gonzalez E, Kornfeld M, Persson AEG. The cytosolic chloride concentration in macula densa and cortical thick ascending limb cells. Acta Physiol Scand. 1993;147:305–13.
Hashimoto S, Kawata T, Schnermann J, Koike T. Chloride channel blockade attenuates the effect of angiotensin II on tubuloglomerular feedback in WKY but not spontaneously hypertensive rats. Kidney Blood Press Res. 2004;27:35–42.
Grodin JL, Simon J, Hachamovitch R, Wu Y, Jackson G, Halkar M, et al. Prognostic role of serum chloride levels in acute decompensated heart failure. J Am Coll Cardiol. 2015;66:659–66.
Mandai S, Kanda E, Iimori S, Naito S, Noda Y, Kikuchi H, et al. Association of serum chloride level with mortality and cardiovascular events in chronic kidney disease: the CKD-ROUTE study. Clin Exp Nephrol. 2017;21:104–11.
Kubota K, Sakaguchi Y, Hamano T, Oka T, Yamaguchi S, Shimada K, et al. Prognostic value of hypochloremia versus hyponatremia among patients with chronic kidney disease-a retrospective cohort study. Nephrol Dial Transplant. 2020;35:987–94.
Nagami GT. Hyperchloremia—why and how. Nefrologia. 2016;36:347–53.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Takahashi, A., Maeda, K., Sasaki, K. et al. Relationships of hyperchloremia with hypertension and proteinuria in patients with chronic kidney disease. Clin Exp Nephrol 26, 880–885 (2022). https://doi.org/10.1007/s10157-022-02229-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-022-02229-6