Abstract
Background
Renal interstitial fibrosis is the common pathway in progressive renal diseases, where oxidative stress promotes inflammation and macrophage infiltration. Febuxostat is a novel nonpurine xanthine oxidase (XO)-specific inhibitor for treating hyperuricemia. While some reports suggest a relationship between hyperuricemia and chronic kidney disease (CKD), the renoprotective mechanism of an XO inhibitor in CKD remains unknown. Recent reports have focused on XO as a source of oxidative stress.
Methods
Here, we investigate the potential of febuxostat to reduce fibrogenic and inflammatory responses in an established interstitial fibrosis model—unilateral ureteric obstruction (UUO). Male Sprague–Dawley rats were divided into three groups: sham-operated group, vehicle-treated UUO group, and febuxostat-treated UUO group.
Results
Treatment with febuxostat diminished XO activity in obstructed kidneys, and suppressed nitrotyrosine, a marker of oxidative stress. Consequently, febuxostat inhibited early proinflammatory cytokine expression, followed by a reduction of interstitial macrophage infiltration. In addition, febuxostat suppressed transforming growth factor-β messenger RNA expression, thereby ameliorating smooth muscle alpha actin and type I collagen expression.
Conclusion
Our results provide evidence for the renoprotective action of febuxostat against the formation of interstitial fibrosis. A decrease in macrophage infiltration and interstitial fibrosis, along with a decrease of the oxidative stress marker, strongly suggests the existence of a causal relationship between them. Febuxostat may have therapeutic value in slowing or preventing interstitial fibrosis in patients with CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accumulating evidence has shown a positive relationship between serum uric acid (UA) levels and cardiovascular mortality in patients with chronic kidney disease (CKD) [1, 2]. Xanthine oxidase/dehydrogenase (XOR) converts hypoxanthine and xanthine into xanthine and UA, respectively. The reduction in serum UA levels by administering the xanthine oxidase (XO) inhibitor allopurinol has been shown to slow the progression of renal dysfunction and decrease the risk of cardiovascular disease (CVD) in patients with CKD [3]. The renoprotective effects of an XO inhibitor were also shown in animal experimental models, including models using 5/6 nephrectomized rats [4] or diabetic mice [5]. This beneficial effect is thought to originate from a lowering of the plasma UA level, because UA itself has been shown to generate oxidative stress in adipocytes, vascular endothelial cells, and vascular smooth muscle cells [6–8].
Besides these hyperuricemia-related adverse effects, several studies have focused on XO as a source of oxidative stress. McCord [9] demonstrated that XOR functions in either a xanthine dehydrogenase (XDH) form, which transfers an electron to nicotinamide adenine dinucleotide (NAD+) and generates NAD+ hydrogen (NADH), or an XO form, which transfers an electron to oxygen and generates oxidative stress. Calcium overload promotes conversion of the protein structure from XDH to XO. Due to this conversion, XO has been demonstrated to act as the major source of oxidative stress during ischemia reperfusion injury or acute renal-allograft rejection [10]. Angiotensin II-induced endothelial dysfunction also shows the significant role of XO in oxidative injury [11]. Renal interstitial fibrosis is one of the common histopathological features of progressive renal disease with diverse etiology. The unilateral ureteral obstruction (UUO) is a well-characterized experimental model of renal injury leading to tubulointerstitial fibrosis; however, the significant role of an increase in XO-dependent oxidative stress has been shown only after the release of obstructed ureter [12], and little information is available about the role of XO-induced oxidative stress in renal interstitial fibrosis.
The aim of this study was to investigate the role of XO activity on the progression of renal interstitial fibrosis in the UUO model. We used rats to minimize the influence of any UA-dependent action, based on the fact that the serum UA level in rodents is much lower than in humans. The effect of XO inhibition on renal interstitial fibrosis was tested by the administration of febuxostat, a newly developed XO inhibitor. In contrast to allopurinol, febuxostat does not inhibit other enzymes in the purine and pyrimidine metabolism pathway, and a sufficient dosage to inhibit XOR activity can be safely used even in subjects with damaged kidney function [13].
Materials and methods
Animals
Healthy male Sprague–Dawley rats (196–206 g body weight; Japan SLC Inc., Shizuoka, Japan) were maintained at the Institute of Experimental Animal Sciences of Osaka University Graduate School of Medicine, an accredited specific pathogen-free facility. The rats were housed in a constant-temperature room with a 12-h:12-h dark–light cycle, and fed food pellets, with ad libitum access to water.
Pretreatment and medication
In all animal experiments, the rats were anesthetized by intraperitoneal injection of pentobarbital (50 mg/kg) and handled in a humane manner in accordance with the guidelines of the Animal Committee of Osaka University. The rats were then assigned to one of three treatment groups: the UUO operation group (U); the UUO group receiving febuxostat (Teijin Pharma Ltd., Tokyo, Japan) medication (F); and the sham operation group (S).
The left ureter of each rat in groups U and F was ligated at the proximal end with 3-0 silk at two points and cut off at the middle. The sham operation consisted of a similar suprapubic incision and identification of the left ureter, but ligation of the ureter was not performed. All of the rats that underwent surgery were given intraperitoneal ampicillin.
Febuxostat (10 mg/kg/day) oral gavages were started at 24 h and 1 h before the operation, and continued every 24 h until sacrifice. This dosage was chosen as the maximal dosage that can be administered without forming the deposition of xanthine crystallizes in renal tubules [14]. The F group was administered 0.5% (w/v) methyl cellulose solution, given through a tube under anesthesia, whereas the respective U and S groups received solution without febuxostat.
The rats were sacrificed at days 1 (n = 5 for each group), 4 (n = 8 for each group), and 14 (n = 3 for each group) after surgery. A median incision was performed under anesthesia, blood samples were drawn from the aortic bifurcation, and urine samples were taken from the bladder (unaffected side) and obstructed renal pelvis (affected side). Subsequently, the obstructed kidneys in groups U and F, and the corresponding left kidneys in group S, were harvested. The kidneys were perfused with cold physiological salt solution and immediately decapsulated and cut into several pieces for the XO/XDH activity assay, nitro-oxidative stress assay, histological analysis, and RNA preparation.
XO/XDH activity assay
Measurement of XO and XDH activity in kidney tissue was based on the pterin-based assay [15]. In brief, approximately 100 mg of kidney tissues was homogenized in 1 mL assay buffer (50 mM K-phosphate, 1 mM ethylenediaminetetraacetic acid, 0.5% dimethyl sulfoxide, and protease inhibitor cocktail, pH 7.4). The supernatant (150 μL) was co-incubated with 50 μL pterin solution (final concentration of 50 μM) or pterin with methylene blue solution (final concentration of 50 μM) to assay XO or both XO and XDH activity, respectively. Before and after an 120-min incubation at 37°C, fluorometric assays were performed to calculate the production of isoxanthopterin. Protein concentration was measured by Pierce BCA Protein Assay Kit (Thermo Scientific Inc., Billerica, MA, USA).
Antibodies
Specific polyclonal antibodies for anti-smooth muscle α actin antibody (SMαA; 1:400, clone 1A4; Sigma-Aldrich, St. Louis, MO, USA), and, for macrophage staining, anti-rat CD68 (1:200, clone ED1, MCA341R; AbD Serotec, Kidlington, Oxfordfordshire, UK) were used in this study.
Morphology and immunohistochemical staining
Following fixation with 4% paraformaldehyde, the kidneys were processed to paraffin. Histological sections (2 μm) of the kidneys were used for periodic acid–Schiff (PAS) and Picrosirius red staining, or for immunohistochemical staining. Immunohistochemical staining was carried out by the standard avidin-biotinylated peroxidase complex method (Vectastain® ABC Kit; Vector Laboratories Inc., Burlingame, CA, USA) with diaminobenzidine as the chromogen.
We scored and calculated the percentage of fibrosis, macrophage infiltration, and myofibroblasts in the interstitial area according to Sirius red-positive areas, the number of ED-1-positive cells in the interstitial space, and the percentage of SMαA staining-positive areas. All of the slides were highlighted on digitized images using a computer-aided manipulator (light microscopy; Nikon Eclipse 80i (Nikon, Tokyo, Japan), and pictures were taken with the Nikon ACT-1 ver. 2.63) Glomeruli and large vessels were excluded in the microscopic fields for image analysis. The scores of ten fields per each kidney section were averaged and used as the scores for the individual rats.
Real-time quantitative polymerase chain reaction (PCR)
Total RNA was extracted from whole kidneys using TRIzol® (Invitrogen, Carlsbad, CA, USA), and was reverse transcribed to complementary DNA (cDNA). Gene expression was measured by real-time quantitative PCR using an Applied Biosystems Prism 7500 (Applied Biosystems, Foster City, CA, USA) with cDNA, SYBR® Green PCR Core Reagents (Invitrogen), and a set of primers. The primers for rat monocyte chemotactic protein-1 (MCP-1), interleukin-1 (IL-1), IL-12, transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), SMαA, type I collagen, and 18S ribosome were as follows: MCP-1, 5′-atgcagttaatgccccactc-3′ (forward), 5′-ttccttattggggtcagcac-3′ (back); IL-1, 5′-gcctcgtgctgtctgacccat-3′ (forward), 5′-gggatccacactctccagctgcag-3′ (back), 5′-ggcagttgggcaggtgacgt-3′ (back); TGF-β, 5′-tgcttcagctccacagagaa-3′ (forward), 5′-tggttgtagagggcaaggac-3′ (back); TNF-α, 5′-agatgtggaactggcagagg-3′ (forward), 5′-cccatttgggaacttctcct-3′ (back); SMαA, 5′-tccctggagaagagctacga-3′ (forward), 5′-tgaaagatggctggaagagg-3′ (back); type I collagen, 5′-ggccaggcagttctgattgg-3′ (forward), 5′-tcggctcatgcgtggcctca-3′ (back); and 18S ribosome, 5′-cggctaccacatccaaggaa-3′ (forward), 5′-agctggaattaccgcggc-3′ (back).
Nitrotyrosine enzyme-linked immunosorbent assay (ELISA)
Nitrotyrosine levels were quantified by ELISA using a nitrotyrosine ELISA kit (Northwest Life Science Specialties, LLC, Vancouver, WA, USA) according to the manufacturer’s instructions. Nitrotyrosine standard or kidney homogenates were incubated with nitrotyrosine antibody in the microplate for 1 h; this was followed by incubation with streptavidin peroxidase for 1 h. The samples were incubated with tetramethylbenzidine substrate for 30 min, and the reaction was stopped by 2.0 mol/L citric acid. The formation of yellow product was measured at 450 nm.
Statistical analysis
All values are expressed as mean ± SE. Statistical analysis was evaluated using the Dunnett method by JMP version 9.0.0 (SAS Institute Inc., Cary, NC, USA), and P < 0.05 was considered to be statistically significant.
Results
Febuxostat suppressed renal XO and XDH activity
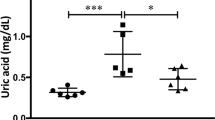
At day 1, renal tissue XO and XDH activity was not changed in the vehicle-treated obstructed kidneys compared with the sham-operated kidneys, but activity of both XO and XDH was induced on day 4. Treatment with febuxostat almost completely eliminated both XO and XDH activity on days 1 and 4 (Fig. 1a). Concomitant with the reduction in XO/XDH activity, febuxostat significantly reduced UA levels compared with vehicle treatment on day 4 (Fig. 1b). Compared with the sham-operated rats, the UUO rats exhibited impaired renal function, assessed by serum urea nitrogen (UN) and creatinine. Febuxostat had no significant effects on the elevated serum UN and creatinine levels (Fig. 1b).
Effects of UUO and treatment with febuxostat on renal XDH and XO activity, serum uric acid, urea nitrogen, and creatinine concentration. sham sham operation, UUO + V UUO operation without treatment, UUO + Fx UUO operation with febuxostat. Each parameter was assessed at days 1 and 4 after UUO. a XDH and XO activity in whole kidney extracts. ***P < 0.001 compared with reference. b Serum concentration of uric acid, urea nitrogen, and creatinine. **P < 0.01, ***P < 0.001 compared with reference
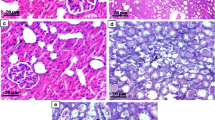
Febuxostat ameliorated tubular damage and interstitial infiltration
Although both the vehicle- and febuxostat-treated obstructed kidneys showed ureteral dilatation to a similar extent, the vehicle-treated obstructed kidneys exhibited tubular damage, showing the rupture of the brush border and increased interstitial infiltration. Treatment with febuxostat ameliorated the rupture of the brush border and suppressed interstitial infiltration in the obstructed kidneys (Fig. 2a). Because we observed a protective effect of febuxostat on tubular damage, we examined the effect of febuxostat on proinflammatory cytokine expression in the obstructed kidneys. Real-time reverse transcriptase-PCR revealed that messenger RNA (mRNA) expression of MCP-1, TNF-α, and IL-1β was increased on day 1 in the obstructed kidneys. In contrast, febuxostat suppressed the increase in these cytokine expressions (Fig. 2b). We next examined macrophage infiltration in the interstitium. The number of ED-1 positive macrophages was significantly increased in the interstitial area of the vehicle-treated obstructed kidneys on day 4. Parallel with the significant reduction of MCP-1 in the febuxostat-treated obstructed kidneys, febuxostat suppressed the infiltration of ED-1-positive macrophages, which was consistent with the observation from PAS staining (Fig. 2c, d). Concomitant with macrophage infiltration, the macrophage-derived cytokine IL-12β was increased in the vehicle-treated obstructed kidneys on day 4, but reversed in the febuxostat-treated kidneys (Fig. 2e).
Effects of UUO and treatment with febuxostat on renal morphology, inflammatory cytokines, and macrophage infiltration. a Periodic acid-Schiff staining at 4 days after operation. b mRNA expression levels of MCP-1, TNF-α, and IL-1β at day 1 after operation, as analyzed by real-time PCR. Results are expressed as relative expression against the expression of corresponding genes in sham. *P < 0.05, **P < 0.01, ***P < 0.001 compared with reference. Immunohistological staining for ED-1 (c) and the number of ED-1 positive cells in interstitial space per ×400 magnification field (d) on day 4 after operation. ***P < 0.001 compared with reference. e mRNA expression levels of IL-12β at days 1 and 4 after operation, as analyzed by real-time PCR. Results are expressed as relative expression against the expression of corresponding genes in sham. **P < 0.01, ***P < 0.001 compared with reference
Febuxostat inhibits interstitial fibrosis
We then examined the therapeutic effect of febuxostat on interstitial fibrosis in the obstructed kidneys. Similar to inflammatory cytokine expression, real-time reverse transcriptase-PCR demonstrated that TGF-β, SMαA, and type I collagen mRNA levels were increased in the obstructed kidneys compared with the sham-operated kidneys, while febuxostat suppressed the increase in mRNA expression on day 4 (Fig. 3a–c). Phenotypic alteration, assessed by immunohistological positive areas for SMαA on day 4, was augmented in the obstructed kidneys, but was mostly limited to the vessels in the febuxostat-treated kidneys (Fig. 3d, e). On day 14, Picrosirius red staining demonstrated that the febuxostat-treated obstructed kidneys exhibited significantly less interstitial fibrosis than the vehicle-treated kidneys (Fig. 4a, b).
Effects of UUO and treatment with febuxostat on renal fibrosis-related genes and SMαA. a–c mRNA expression levels of TGF-β, SMαA, and type I collagen at days 1 and 4 after operation, as analyzed by real-time PCR. Results are expressed as relative expression against the expression of corresponding genes in sham. **P < 0.01, ***P < 0.001 compared with reference. Immunohistological staining for SMαA (d), and the percentage of SMαA stained positive areas in interstitial space (e) on day 4 after operation. ***P < 0.001 compared with reference
Effects of UUO and treatment with febuxostat on renal fibrosis and nitrotyrosine concentration. Picrosirius red staining (a), and the percentage of Picrosirius red staining-positive fibrotic areas in interstitial space (b) on day 14 after UUO. **P < 0.01 compared with reference. c Renal concentration of nitrotyrosine at days 1 and 4 after UUO was evaluated by ELISA. ***P < 0.001 compared with reference
Febuxostat inhibits oxidative stress
To investigate the therapeutic mechanism of febuxostat, we examined oxidative stress. On day 1, the nitrotyrosine concentration, a marker of nitro-oxidative stress, of the febuxostat-treated obstructed kidneys was lower than that of the vehicle-treated kidneys (Fig. 4c). Compared with the corresponding sham kidneys, the nitrotyrosine concentration of the obstructed kidneys did not change at day 1, but was reduced at day 4. (Day 1: sham kidneys, 2.74 ± 0.44 pmol/mg protein; obstructed kidneys, 2.58 ± 0.28 pmol/mg protein. Day 4: sham kidneys, 1.05 ± 0.26 pmol/mg protein; obstructed kidneys, 0.21 ± 0.04 pmol/mg protein, P < 0.01 vs. sham.)
Discussion
In the present study, we tested the hypothesis that febuxostat has therapeutic effects on tubulointerstitial injury in a rat UUO model, in which the plasma UA concentration is lower than in humans due to the presence of uricase. The obstructed kidneys exhibited increased proinflammatory and fibrogenic cytokines, thereby inducing macrophage infiltration and interstitial fibrosis. The administration of febuxostat ameliorated these manifestations by inhibiting the induction of proinflammatory and fibrogenic cytokines. Of interest is that febuxostat reduced nitro-oxidative stress, as assessed by nitrotyrosine, a marker of oxidative stress. We propose a new strategy for treating progressive kidney diseases using febuxostat as an anti-oxidant drug in addition to its effect on the reduction of UA.
We first demonstrated that febuxostat suppressed oxidative stress by assessing the nitrotyrosine level. Nitrotyrosine is a tyrosine nitration product mediated by reactive nitrogen species under proinflammatory conditions. Peroxynitrite anion is one of the most powerful reactive oxygen species that is produced by the reaction of nitric oxide (NO) and superoxide radicals, and is considered to be a marker of reactive nitrogen species induced by inducible nitric oxide synthase (iNOS), accompanied by oxidative stress [16]. We identified a lower production of nitrotyrosine in the febuxostat-obstructed kidneys; this may originate from both a complete blockade of XO activity and diminished induction of iNOS [7]. Several studies have focused on XO as a source of reactive oxygen species (ROS) production. XDH, which is unable to generate ROS, is converted to XO by cellular calcium overload [9]. XO can produce ROS, such as superoxide, hydrogen peroxide, and hydroxyl radicals [9, 17]. Because we showed that febuxostat diminished XO activity compared with the vehicle-treated obstructed kidneys, the reduction in XO activity might therefore have suppressed the production of nitrotyrosine in the febuxostat-treated obstructed kidneys. The concentration of nitro-tyrosine in sham kidney was identical or higher than that of UUO kidney. Although the precise mechanism of this unanticipated finding is not apparent, it may be explained by the decreased substrate of nitro-tyrosine in kidney. A previous report demonstrated that tyrosine escaped from damaged tissue to blood stream in a gut ischemia reperfusion model [18]. The formation of nitro-tyrosine depends on oxidative stress, NO and tyrosine. Therefore, the reduced concentration of tyrosine in damaged UUO kidney may be a possible cause of reduced production of nitro-tyrosine in UUO compared to sham kidney.
In the present study, we found a simultaneous increase in nitro-oxidative stress and MCP-1 mRNA induction on day 1, prior to the occurrence of macrophage infiltration on day 4. A previous report showed a positive interaction between ROS and macrophage infiltration. Oxidative stress promotes the expression of various inflammation-related molecules, including MCP-1, which, in turn, promotes inflammatory cell infiltration [19]. Together with the reduction in XO activity, febuxostat treatment clearly demonstrated anti-inflammatory effects, even at 1 day after ureteral obstruction. TNF-α and IL-1β mRNA expression were also suppressed in the febuxostat-treated obstructed kidneys on day 1. Furthermore, the inhibition of macrophage infiltration contributed to the reduction in the expression of IL-12β, one of the macrophage-derived cytokines, which otherwise promoted tubulointerstitial inflammation. Therefore, febuxostat may halt the vicious cycle involving tubules and macrophages.
We also demonstrated that febuxostat suppressed interstitial fibrosis. Febuxostat suppressed TGF-β, type I collagen and SMαA expression on day 4, resulting in significant interstitial fibrosis on day 14, as assessed by Picrosirius red staining. These results suggest that one aspect of the protective mechanism of febuxostat in a UUO kidney is the reduction of nitro-oxidative stress, which, in turn, might suppress the proinflammatory and fibrogenic cytokines, followed by a reduction in macrophage infiltration and tissue fibrosis. Interestingly, Landmesser et al. [11] demonstrated the crosstalk between angiotensin II signaling and conversion of XDH to XO in endothelial cells. Angiotensin II is known to activate nicotinamide adenine dinucleotide phosphate (NADPH) oxidase; Landmesser et al. showed that the oxidative stress created by NADPH oxidase activation promotes the conversion of the XDH form to the XO form, which generates further oxidative stress in endothelial cells. Angiotensin II signaling was also reported to contribute to the progression of fibrosis in UUO [20]. Angiotensin II and TGF-β coordinately promote interstitial fibrosis [21]. Thus, the XOR system could possibly increase nitro-oxidative stress in the process of forming interstitial fibrosis. The amelioration of this XOR system may be a therapeutic target for the treatment of interstitial fibrosis.
The present study supports the current pathological concept that XO activity itself, rather than hyperuricemia, may play an important role in causing progressive tissue fibrosis. Several reports have suggested a UA-independent therapeutic effect of XO inhibitor. A clinical study by Ogino et al. [22] showed that benzbromarone lowered the level of UA, but had no effect on hemodynamic impairment in patients with chronic heart failure. A study by Sanchez-Lozada et al. [4] showed that an XO inhibitor provided a renoprotective effect in 5/6 nephrectomized rats without hyperuricemia [4]. Since XOR is expressed ubiquitously, the targeting of XO activity can be applied to a variety of tissue and disease conditions. Patients with CKD have been shown to have high oxidative stress [23]; a high protein conversion rate from XOR to XO is assumed to occur in those patients. The use of an XOR inhibitor in treating patients with CKD has been restricted due to the lack of appropriate agents, but there is now the novel agent febuxostat, which can be used effectively even in the presence of CKD. Further investigation is needed into the role of febuxostat in the progression of CKD. Although the reduction of UA itself may have a protective effect for patients with CKD, the UA-independent actions of an XOR inhibitor may play a significant role against the progression of CKD or CVD.
In conclusion, the results obtained from our non-hyperuricemic obstructed kidney model showed that XOR activity contributes to the progression of renal interstitial fibrosis by modulating oxidative stress and proinflammatory cell infiltration. Our observations support the current pathological concept that, in addition to hyperuricemia, an increase in XO activity itself may play an important role in the progression of tissue fibrosis. A novel XOR inhibitor, febuxostat, may be a therapeutic tool for treating progressive interstitial fibrosis.
References
Madero M, Sarnak MJ, Wang X, Greene T, Beck GJ, Kusek JW, Collins AJ, Levey AS, Menon V. Uric acid and long-term outcomes in CKD. Am J Kidney Dis. 2009;53:796–803.
Suliman ME, Johnson RJ, Garcia-Lopez E, Qureshi AR, Molinaei H, Carrero JJ, Heimburger O, Barany P, Axelsson J, Lindholm B, Stenvinkel P. J-shaped mortality relationship for uric acid in CKD. Am J Kidney Dis. 2006;48:761–71.
Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincon A, Arroyo D, Luno J. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5:1388–93.
Sanchez-Lozada LG, Tapia E, Soto V, Avila-Casado C, Franco M, Wessale JL, Zhao L, Johnson RJ. Effect of febuxostat on the progression of renal disease in 5/6 nephrectomy rats with and without hyperuricemia. Nephron Physiol. 2008;108:69–78.
Kosugi T, Nakayama T, Heinig M, Zhang L, Yuzawa Y, Sanchez-Lozada LG, Roncal C, Johnson RJ, Nakagawa T. Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am J Physiol Renal Physiol. 2009;297:F481–8.
Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens. 2008;26:269–75.
Kawada N, Moriyama T, Ando A, Fukunaga M, Miyata T, Kurokawa K, Imai E, Hori M. Increased oxidative stress in mouse kidneys with unilateral ureteral obstruction. Kidney Int. 1999;56:1004–13.
Yu MA, Sanchez-Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. 2010;28:1234–42.
McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–63.
Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293:C584–96.
Landmesser U, Spiekermann S, Preuss C, Sorrentino S, Fischer D, Manes C, Mueller M, Drexler H. Angiotensin II induces endothelial xanthine oxidase activation: role for endothelial dysfunction in patients with coronary disease. Arterioscler Thromb Vasc Biol. 2007;27:943–8.
Young MR, Young IS, Johnston SR, Rowlands BJ. Lipid peroxidation assessment of free radical production following release of obstructive uropathy. J Urol. 1996;156:1828–32.
Takano Y, Hase-Aoki K, Horiuchi H, Zhao L, Kasahara Y, Kondo S, Becker MA. Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenase. Life Sci. 2005;76:1835–47.
Horiuchi H, Ota M, Kobayashi M, Kaneko H, Kasahara Y, Nishimura S, Kondo S, Komoriya K. A comparative study on the hypouricemic activity and potency in renal xanthine calculus formation of two xanthine oxidase/xanthine dehydrogenase inhibitors: TEI-6720 and allopurinol in rats. Res Commun Mol Pathol Pharmacol. 1999;104:307–19.
Beckman JS, Parks DA, Pearson JD, Marshall PA, Freeman BA. A sensitive fluorometric assay for measuring xanthine dehydrogenase and oxidase in tissues. Free Radic Biol Med. 1989;6:607–15.
Mohiuddin I, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C. Nitrotyrosine and chlorotyrosine: clinical significance and biological functions in the vascular system. J Surg Res. 2006;133:143–9.
Greene EL, Paller MS. Xanthine oxidase produces O −.2 in posthypoxic injury of renal epithelial cells. Am J Physiol. 1992;263:F251–5.
Contrin LM, Lobo SM, Navegantes LC, Orrico SP, Queiroz MM, Cury PM, Lira EC, Carta A, Yamamoto AE, Vincent JL. Tyrosine: a possible marker of severe intestinal injury during ischemia. J Surg Res. 2009;155:268–72.
Paul-Clark MJ, McMaster SK, Sorrentino R, Sriskandan S, Bailey LK, Moreno L, Ryffel B, Quesniaux VF, Mitchell JA. Toll-like receptor 2 is essential for the sensing of oxidants during inflammation. Am J Respir Crit Care Med. 2009;179:299–306.
Moriyama T, Kawada N, Akagi Y, Ando A, Horio M, Yamauchi A, Nagata K, Imai E, Hori M. TCV-116 inhibits interstitial fibrosis and HSP47 mRNA in rat obstructive nephropathy. Kidney Int Suppl. 1997;63:S232–5.
Bascands JL, Schanstra JP. Obstructive nephropathy: insights from genetically engineered animals. Kidney Int. 2005;68:925–37.
Ogino K, Kato M, Furuse Y, Kinugasa Y, Ishida K, Osaki S, Kinugawa T, Igawa O, Hisatome I, Shigemasa C, Anker SD, Doehner W. Uric acid-lowering treatment with benzbromarone in patients with heart failure: a double-blind placebo-controlled crossover preliminary study. Circ Heart Fail. 2010;3:73–81.
Aslam S, Santha T, Leone A, Wilcox C. Effects of amlodipine and valsartan on oxidative stress and plasma methylarginines in end-stage renal disease patients on hemodialysis. Kidney Int. 2006;70:2109–15.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Omori, H., Kawada, N., Inoue, K. et al. Use of xanthine oxidase inhibitor febuxostat inhibits renal interstitial inflammation and fibrosis in unilateral ureteral obstructive nephropathy. Clin Exp Nephrol 16, 549–556 (2012). https://doi.org/10.1007/s10157-012-0609-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-012-0609-3