Abstract
Introduction
Though uncommon, the collecting duct carcinoma (CDC) of Bellini is a very aggressive primary renal tumour occurring in less than 1% of all renal cell carcinoma (RCC) cases. This rare subtype was always excluded from the prospective trials with targeted therapies. Few data so far available concern the subgroup analyses from the expanded access programs with sorafenib and sunitinib, and from temsirolimus randomized study.
Patients and methods
From December 2004 to May 2010, 333 patients with advanced RCC have been treated in our Institution with targeted therapies: of these, 7 (2.6%) were affected by CDC. General characteristics, symptoms, pathological features, treatments and patients’ outcome were recorded.
Results
All patients affected by CDC received targeted agents as first-line therapy: more precisely, 4 patients were treated with sorafenib, 2 with temsirolimus and 1 with sunitinib. After progression 2 patients received a second-line treatment with sunitinib. No patients were alive at 5 years. Five patients developed early progression of disease with a very short 4-month survival, while 2 cases had a long-lasting disease control with an overall survival time accounting for 49 and 19 months, respectively. Treatment-related adverse events were manageable consisting of fatigue, diarrhoea, hand–foot syndrome, hypertension and anemia, the latter being the most frequent. No treatment discontinuations due to adverse event were needed.
Conclusions
This investigation shows that targeted agents are safe, displaying some degree of activity in CDCs: therefore, they could be considered as an alternative in patients not eligible to chemotherapy regimens. Further studies including biomarkers as predictive factors of tumour biology and clinical features are required to improve the management of this challenging disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accounting for less than 1% of all renal malignancies, collecting duct carcinoma (CDC) represents a rare tumour deriving from cells of Bellini collecting duct. Over 100 cases have been described in literature in patients ageing between 13 and 83 years (median 55 years), with a male-to-female predominance of approximately 2:1 [1]. Usually, patients with CDC display abdominal pain, flank mass and hematuria: imaging of the upper tract often suggests the presence of urothelial carcinoma, and patients may occasionally show positive urine cytology [2]. Tokuda et al reported the largest series of CDC, represented by 81 cases, treated with surgical and medical approach. All cases were identified between a retrospective survey undertaken in Japan from August 2001 to April 2003. The median age at diagnosis was 58.2 years and males comprised 71.6% of all population. At diagnosis 32.1% of cases had metastatic disease and 44.2% regional lymphnodes involvement. Surgery was performed in 80 cases, while 51 cases received immunotherapy and/or chemotherapy for their advanced disease. The 1 and 5 years cancer specific survival was 69 and 34.3% respectively [3].
Collecting duct carcinoma has an aggressive biologic behaviour and in about one third of patients spread of disease can be observed as from diagnosis [4]. Metastases at lungs, liver and adrenal glands are common; bony metastases are often osteoblastic, and lymph-node involvement is extremely frequent especially at the level of cervical lymph-nodes [5]. Generally, patients with CDC are characterized by unfavourable prognosis and approximately two third of them die within 2 years of diagnosis [6].
On account of its origin in the distal nephron which makes this tumour more similar to urothelial carcinoma rather than to clear-cell carcinoma, so far treatment of these patients was based on a number of chemotherapy regimens including cisplatin–gemcitabine, or on immunotherapy: however, results of these experiences have been somewhat disappointing.
Here below, we report the data of a retrospective analysis on efficacy observed in 7 consecutive patients with CDC treated with targeted therapies in our Institution.
Patients and methods
From December 2004 to May 2010, a series of 333 patients with advanced RCC was treated with targeted therapies in our Institution. Data such as gender, race, symptoms, pathological features and patients’ outcome reported in the hospital case history forms have been recorded and processed. All patients underwent adequate pre- or post-surgical staging of disease carried out with CT and bone scan. Staging assessments were repeated every 2 or 3 months during the treatment and at disease progression. Response to treatment was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST v 1.1) [7]. As regard patients diagnosed CDC, all histological specimens were reviewed and confirmed by an internal pathologist, and tumour was staged according to TNM classification.
Results
The median age of the entire cohort of 333 patients with RCC treated in our Institution with targeted agents was 62 years (range 55–69) and the majority of patients were male with a male/female ratio of 3:1. Clear-cell tumour was the most frequent histology (86%), and only 14% were non clear-cell tumours including 7 (2.6%) cases of CDC.
Overall, the majority of patients (163; 53.9%) received one line of treatment, 113 (36.5%) received two lines, 30 (9.7%) three, and 4 (1.3%) received four lines.
The main characteristics of patients with CDC tumours included in this report are shown in Table 1. All patients were Caucasian and most of them were male. The median age at diagnosis was 51 years (range 33–69). Disease was symptomatic in 70% of patients, with gross haematuria and pain as the most frequent symptoms. Nephrectomy was performed in 6 patients, while in the only one not amenable to surgery because of local spread of the disease, the diagnosis was performed through the biopsy of renal mass. All the nephrectomies were undertaken as the first treatment approach and were followed by the targeted therapies. In all tumours, histological examination revealed high-grade CDCs. All patients were metastatic at diagnosis: nodal involvement was observed in 6 (86%) patients, and the most frequent metastatic sites were lung and bone. Fifty-seven percent of patients had only one metastatic site, while the remaining 43% had two or more sites.
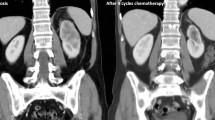
At the beginning of treatment 5 patients showed 0–1 ECOG performance status, and none of them had previously been treated with chemotherapy. Four patients received as first-line treatment sorafenib, 1 sunitinib and 2 temsirolimus: following progression, two patients received a second-line with sunitinib. Two patients, one treated with sorafenib and another one treated with temsirolimus, attained a disease control lasting 33 and 6 months, respectively. At progression these patients received a second-line treatment with sunitinib which yielded further 10- and 9-month disease control. Therefore, the overall survival for both patients was 49 and 19 months, respectively. The remaining five patients (3 who received sorafenib, 1 sunitinib and 1 temsirolimus as first-line treatment) developed early progression of disease with a 4-month survival.
Treatment-related adverse events were manageable and consisted of fatigue, diarrhoea, hand–foot syndrome, hypertension and anemia, being the latter the most frequent. No patient discontinued therapy because of adverse events.
Discussion
Due to its uncommonness, it becomes very difficult to define the real incidence, prognosis and the best treatment modalities of CDCs. As far as our data are concerned, we confirm the prevalence in males and the aggressiveness of this tumour which in the majority of patients is diagnosed in advanced stage. Moreover, compared to literature data, in our series of RCC treated with targeted agents we have observed a higher incidence of CDCs likely due to the large and diversified array of patients referring to our Institution for tumour diagnosis, treatment and care.
Patients with CDCs have been always excluded from clinical trials evaluating targeted therapies in RCC: however, we have been able to carry out subgroup analyses, and therefore to collect information supporting a clinical activity of these drugs in CDCs, in a limited sample of patients treated in our Institution during the everyday clinical practice.
Currently, no unequivocal indications exist for surgical and medical treatment of metastatic disease: for instance, the role of radical nephrectomy as treatment of choice in advanced stages has been recently strongly criticized giving rise to heated debates. Méjean et al. [8] in fact, reported that none of 5 who underwent nephrectomy for metastatic disease at diagnosis was alive 21 months after surgery; specifically, the observation that three patients died immediately after surgery suggests that surgical excision alone not only doesn’t improve prognosis but also could give rise to peri-operative and early post-operative complications [9].
In the light of these proofs, it appears that all possible efforts should be made to anticipate the diagnosis of CDCs.
Presence of several pathological conditions has been established to define CDCs; however sometimes the differential diagnosis can turn out difficult since the pathological cells characteristics are not exclusive but could belong to other tumor types. The typical CDC has a tubular or tubule-papillary growth pattern where irregular angulated glands infiltrating renal parenchyma are associated with a desmoplastic stroma. The edge of the tumour is often ill-defined and there is extensive permeation of renal parenchyma. Moreover, the cells of CDC usually display high grade (Fuhrman 3 and 4) nuclear features [1, 10, 11] (Table 2).
Immuno-histochemistry could be a sound support for differential diagnosis: CDC cells usually stain for low molecular weight and broad spectrum keratins, whereas high molecular weight keratins as 34βE12 and CK19 are commonly co-expressed with vimentin. If the CD10 and villin stains are negative the expression of CD15 and epithelial membrane antigen may be variable as for Ulex europaeus agglutinin-1 and peanut lectin [1, 2].
The optimal medical therapeutic approach for metastatic CDC has not yet been established: despite striking responses to cytokines have been reported in the past, [12] currently immunotherapy has only an historical role. Even if trials comparing immunotherapy with chemotherapy have not been carried out, presently the latter represents the most used therapeutic approach, likely on account of the fact that CDCs show biological similarities with urothelial cell carcinoma. Generally chemotherapy consists of combinations several drugs such as methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC), or the two-drug regimen cisplatin–gemcitabine. As regards MVAC, no data from prospective studies are available: the largest retrospective series including 12 patients treated at the M. D. Anderson Cancer Center reports only a minor response lasting 5 months [4] (Table 1).
Conversely, a prospective phase II study seems to indicate a better activity of the cisplatin–gemcitabine regimen. In this multicentre trial, 20 patients with metastatic CDCs have been enrolled in the space of 46 month: the primary end-point was the overall response rate and the secondary end-points were progression free survival (PFS) and overall survival (OS). Platinum-gemcitabine induced 65% clinical benefit with 25% partial responses and 40% stable diseases, whereas disease progression was reported in 20% of patients. Median PFS and OS were 7.9 and 9.5 months, respectively, with only 48% of patients alive after 1 year from the beginning of treatment [12]. Even if the response rate was about half as compared to that achieved in patients with transitional-cell carcinoma of the urothelium treated with the same regimen (response rate 49%), thus confirming the poor prognosis of these patients, this study for the first time supplies in a prospective way evidence of some activity of the chemotherapeutic approach in CDCs. [13].
Based on these considerations, it appears that CDC is an orphan disease where specific targets for treatment are urgently needed. However, whereas on the one hand CDC has been found cytogenetically different from other renal malignancies, on the other hand, with the exclusion of the amplification of the gene c-erbB-2 which has been related to poorer prognosis, no specific markers have been identified [14, 15]. Furthermore, the expression and the activity of the receptors for the vascular endothelial growth factor (VEGFR) and for the platelet derived growth factor (PDGFR) as well as the role of mTOR, which represent the main targets of the current available anti-angiogenic agents, have not been so far reported.
In this study we report the activity and the safety of anti-angiogenic agents in CDCs. All patients had advanced disease and did not receive chemotherapy or immunotherapy for their metastatic disease. Six cases received a radical nephrectomy as upfront therapy and only two received 2 targeted therapies as sequential therapy. These last patients having a longer disease control as compared to other one, presented a good performance status and lymph nodes metastases at diagnosis. Both received a previous cytoreductive nephrectomy and presented only lymph nodes disease. For unknown reasons these 2 patients had a long-term disease control while the remaining 5 showed a very short survival. Considering the few number of patients treated and the characteristic heterogeneity of the disease, it is impossible to identify some clinical or pathological factors associated with a better outcome. Notwithstanding our results suggest that targeted agents may play an important role inhibiting angiogenesis or other pathways in this tumour. Moreover anti-angiogenic agents could represent a safe alternative in patients not eligible to chemotherapy regimens.
To conclude, targeted agents may represent an interesting treatment modality in this tumour, but further studies are urgently needed to identify possible predictive markers and their exact role in a possible sequential treatment of CDCs.
References
Srigley JR, Eble JN. Collecting duct carcinoma of kidney. Semin Diagn Pathol. 1998;15:54–67.
Eble JN, Sauter G, Epstein JI, Martignoni G. World Health Organization classification of tumours. Pathology and genetics of tumours of the urinary system and male genital organs. IARC Press: Lyon; 2004. http://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb7/index.php, accessed September 11, 2011.
Tokuda N, Naito S, Matsuzaki O, Nagashima Y, Ozono S, Igarashi T. Collecting duct (Bellini duct) renal cell carcinoma: a nationwide survey in Japan. J Urol. 2006;176:43–9.
Dimopoulos MA, Logothetis CJ, Markowitz A, Sella A, Amato R, Ro J. Collecting duct carcinoma of the kidney. Br J Urol. 1993;71:388–91.
Matz LR, Latham BI, Fabian VA, Vivian JB. Collecting duct carcinoma of the kidney: a report of three cases and review of the literature. Pathology. 1997;29:354–9.
Levin HS, Myles JL. The Pathology of Renal Neoplasms. Renal cell carcinoma: molecular biology, immunology, and clinical management. 2nd ed. 2009; Totowa: Humana Press. p. 341–406.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Méjean A, Rouprêt M, Larousserie F, Hopirtean V, Thiounn N, Dufour B. Is there a place for radical nephrectomy in the presence of metastatic collecting duct (Bellini) carcinoma? J Urol. 2003;169:1287–90.
Fleming S, Lewi HJ. Collecting duct carcinoma of the kidney. Histopathology. 1986;10:1131–41.
Kennedy SM, Merino MJ, Linehan WM, Roberts JR, Robertson CN, Neumann RD. Collecting duct carcinoma of the kidney. Hum Pathol. 1990;21:449–56.
Rumpelt HJ, Störkel S, Moll R, Schärfe T, Thoenes W. Bellini duct carcinoma: further evidence for this rare variant of renal cell carcinoma. Histopathology. 1991;18:115–22.
Culine S, Oudard S, Duclos B, Banu E, Priou F, Rousseau F, et al. A phase II prospective study of gemcitabine and platin-based combination as first-line chemotherapy for metastatic Bellini duct carcinoma patients. Results of GETUG study. J Clin Oncol. 2005;23:4543.
von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–77.
Füzesi L, Cober M, Mittermayer C. Collecting duct carcinoma: cytogenetic characterization. Histopathology. 1992;21:155–60.
Selli C, Amorosi A, Vona G, Sestini R, Travaglini F, Bartoletti R, et al. Retrospective evaluation of c-erbB-2 oncogene amplification using competitive PCR in collecting duct carcinoma of the kidney. J Urol. 1997;158:245–7.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Procopio, G., Verzoni, E., Iacovelli, R. et al. Is there a role for targeted therapies in the collecting ducts of Bellini carcinoma? Efficacy data from a retrospective analysis of 7 cases. Clin Exp Nephrol 16, 464–467 (2012). https://doi.org/10.1007/s10157-012-0589-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-012-0589-3