Summary
In this short review article we discuss three key oral presentations from the European Society for Medical Oncology (ESMO) Congress 2021 concerning localised, as well as advanced/metastatic renal cell carcinoma, highlighting their potential implications for the improvement of therapeutic modalities in affected patients. (1) Conditional survival and 5‑year follow-up of CheckMate 214 currently represent the longest available phase III follow-up data in the first-line (combination) treatment of clear cell renal cell carcinoma patients with nivolumab + ipilimumab vs. sunitinib. This analysis demonstrated durable efficacy benefits with the respective combination vs. sunitinib. Moreover, conditional survival results predict an increased probability of durable overall survival, progression-free survival, and response rates with nivolumab + ipilimumab at 2‑ and 3‑year landmarks. (2) The randomised, double-blind, phase III KEYNOTE-564 study, presented as a highlight late-breaking abstract at the ASCO Congress 2021, met its primary endpoint of disease-free survival with post nephrectomy adjuvant pembrolizumab vs. placebo in clear cell renal cell carcinoma patients. At ESMO 2021, the authors presented patient-reported outcomes, whereby no clinically meaningful changes from baseline in health-related quality of life or symptom scores were observed with adjuvant pembrolizumab or placebo. These findings suggest that adjuvant pembrolizumab was tolerable from a patient perspective. (3) A phase II prospective trial of frontline cabozantinib in metastatic collecting ducts carcinoma, namely the BONSAI trial (Meeturo 2), met its primary endpoint objective response rate, showing promising efficacy and acceptable tolerability of cabozantinib in respective patients. Since metastatic collecting ducts carcinoma is biologically poorly characterised and heavily underrepresented in prospective randomised trials, BONSAI gains particular importance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
This short review article covers potential therapeutic improvements regarding localised, as well as advanced/metastatic renal cell carcinoma (RCC), wherefore three outstanding oral presentations from the European Society for Medical Oncology (ESMO) Congress 2021 shall be highlighted.
Advanced or metastatic RCC (aRCC) still represents an incurable disease in the long run, despite enormous improvements concerning systemic therapies over the past decades, mainly the introduction of immune checkpoint inhibitors (ICIs) [1, 2]. In particular, dual checkpoint inhibition with nivolumab (N) and ipilimumab (I), as well as the combination of a PD-(L)-1 ICI and a vascular endothelial growth factor receptor (VEGFR)-tyrosine kinase inhibitor (TKI) were shown to improve response rates, progression-free survival (PFS), and/or overall survival (OS) when compared with sunitinib (S), the long-time gold standard in the first-line setting [2,3,4,5]. Despite these advances, an optimised treatment selection for the individual aRCC patient remains challenging, since no direct head-to-head trials comparing these novel strategies have been conducted so far. In addition, no RCC biomarkers for the daily routine clinical practice are available yet [2].
Thus, we provide a brief summary of the above-mentioned presentations concerning the complex and rapidly evolving field of aRCC.
Conditional survival and 5-year follow-up in CheckMate 214: first-line N + I vs. sunitinib in aRCC
(Motzer R.J. et al./661P)
Robert J. Motzer (Memorial Sloan Kettering Cancer Center, New York, NY, USA) presented conditional survival- and 5‑year follow-up data of CheckMate 214—currently representing the longest available phase III follow-up data in the first-line treatment of clear cell advanced renal cell carcinoma (aRCC) patients—with nivolumab + ipilimumab (N + I) vs. sunitinib (S) [6].
Conditional survival, used to predict sustained treatment benefit, accounts for the time since treatment initiation and provides improved prognostic information at landmark time points [7]. Conditional survival in clear cell aRCC patients was estimated in CheckMate 214 with a minimum 5‑year follow-up (median: 67.7 months). Clear cell aRCC patients stratified by International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk group and region were randomised to N (3 mg/kg) + I (1 mg/kg) Q3Wx4, followed by N (3 mg/kg) Q2W vs. S (50 mg) QD for 4 weeks on, 2 weeks off (6-week cycle). Trial endpoints included OS, PFS and objective response rate (ORR); both per independent radiology review using RECIST v1.1 in IMDC intermediate/poor risk (IP; primary), intent-to-treat (ITT; secondary), and favorable risk (FAV; exploratory) patients. Conditional survival—the probability of remaining alive (cOS), progression-free survival (cPFS), or in response (cDOR) 2 years beyond landmark time points of 2 and 3 years—was analysed.
Superior OS, PFS, ORR and complete response (CR) benefits with N + I vs. S were maintained in ITT and IP patients. Consistently higher cOS, cPFS, and cDOR rates were observed with N + I vs. S in ITT and IP patients at all time points. In the N + I arm, the probability of remaining alive 2 years beyond the 3‑year landmark (cOS) was 81% (ITT), 79% (IP), and 85% (FAV). The probability of remaining progression-free (cPFS) for an additional 2 years beyond the 3‑year landmark was 89% (ITT), 90% (IP), and 85% (FAV). For N + I patients who were in response at 3 years, the probability of remaining in response (cDOR) for an additional 2 years was 89% (ITT), 90% (IP), and 85% (FAV), whereby no new safety signals emerged with longer follow-up time.
Thus, the long-term follow-up in this 5‑year analysis demonstrated durable efficacy benefits with N + I vs. S, whereby N + I should only be given to IMDC IP patients. Moreover, conditional survival results predicted an increased probability of durable OS, PFS, and response with N + I at 2‑ and 3‑year landmarks, and highlight the long-term clinical benefit with N + I in patients with clear cell aRCC.
Regarding the toxicities of treatment-related adverse events (trAE), it has to be emphasized that the equally presented phase II PRISM trial demonstrated a clinically significant reduction in G3/4 trAE rates, giving I 12-weekly (instead of Q3W), in combination with N [8].
Pembrolizumab vs. placebo as adjuvant therapy for patients with RCC: patient-reported outcomes in KEYNOTE-564
(Choueiri T.K. et al./653O)
Partial/radical nephrectomy still represent the standard-of-care treatment for locoregional renal cell carcinoma (RCC) [9], as explained by Toni K. Choueiri (Medical Oncology Department, Dana Farber Cancer Institute, Harvard Medical School, Boston, MA, USA) in his virtual presentation. The background of KEYNOTE-564 [10] is constituted by a bunch of controversies: (1) Currently, there is no globally accepted standard adjuvant RCC therapy that is supported by high levels of evidence. (2) Various studies of adjuvant immunotherapy with cytokines have yielded negative results [11]. (3) VEGFR-targeted therapy has not shown a consistent benefit in the adjuvant RCC setting so far [12]. Another challenge is the fact that depending on various risk factors, such as tumour stage, tumour size, nodal involvement, and nuclear grade, nearly half of patients eventually experience disease recurrence after surgery, not to mention that M1 stage patients and no evidence of disease (NED) after resection of oligometastatic sites are also at a high risk of relapse [11,12,13].
Patient-reported outcomes (PRO) were evaluated in all randomised patients with ≥ 1 dose study treatment and ≥ 1 completed assessment for the specific outcome. FKSI-DRS and EORTC QLQ-C30 were administered electronically. Prespecified secondary endpoints included least square (LS) mean change in symptom scores as measured by FKSI-DRS and health-related quality of life (QoL) as measured by the QLQ-C30 global health status/quality of life (GHS/QoL) and physical functioning (PF) scales from baseline to week 52. LS mean change in FKSI-DRS score was −1.12 (95% confidence interval [CI] −1.53 to −0.71) with pembrolizumab (pembro) vs. −0.45 (95%CI −0.84 to −0.05) with placebo (P); both were below the threshold of ≥ 3 for a clinically meaningful change in FKSI-DRS. LS mean change in QLQ-C30 GHS/QoL score was −4.25 (95%CI −6.32 to −2.19) with pembro vs. −1.68 (95%CI −3.69 to 0.32) with P. LS mean change in QLQ-C30 PF score was −1.81 (95%CI −3.19 to −0.43) with pembro vs. −0.90 (95%CI −2.23 to 0.44) with P. Mean score change for both arms in both scales was below the clinically meaningful change threshold of ≥ 10 for QLQ-C30. Health-related QoL and symptom scores were maintained across all evaluated time points.
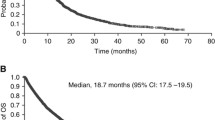
The authors conclude that no clinically meaningful changes from baseline in health-related QoL or symptom scores were observed with adjuvant pembro or P, whereby these scores remained stable over time. PRO findings of KEYNOTE-564 suggested that adjuvant pembro was tolerable from a patient perspective. These findings are particularly important since KEYNOTE-564 currently represents the first positive phase III study of an adjuvant immunotherapy in RCC, the observed disease-free survival (DFS) benefit having been consistent across subgroups, including the M1 NED population (Fig. 1). Therefore, pembro represents a potential new standard-of-care for clear cell RCC patients in the adjuvant setting.
Disease-free survival (DFS) by investigator in the intention-to-treat (ITT) population in the KEYNOTE-564 trial. aChoueiri TK, et al. (2021) (suppl 15; abstr LBAS). bCross prespecified p‑value boundary for statistical significance of 0.0114. DFS was estimated by the Kaplan–Meier method; hazard ratios (HRs) and 95% confidence intervals (Cls) were estimated using a stratified Cox proportional hazard model. Between-arm differences assessed with stratified log-rank test. ITT population included all randomized participants. NR not reached. Data cutoff date December 14, 2020. Presented at: European Society for Medical Oncology (ESMO) Congress 2021; September 16–20, 2021. With kind permission from Dr. Toni Choueiri
Phase II prospective trial of frontline cabozantinib in metastatic collecting ducts RCC: the BONSAI trial (Meeturo 2)
(Procopio G. et al./654MO)
Collecting ducts carcinoma (CDC) represents a rare (approximately 1% of renal tumours) albeit highly aggressive malignant epithelial tumour arising from the principal cells of the distal segment of the collecting ducts of Bellini in the renal medulla [14, 15]. Due to the rarity of CDC and complexity in diagnostic criteria, affected patients usually present in advanced (bad) clinical conditions due to symptomatic disease with synchronous metastases and a dismal prognosis with a median OS of 11 months (even after a doublet chemotherapy with platinum salt plus gemcitabine) [15]. Self-explanatorily, this rare renal malignancy is biologically poorly characterised and largely underrepresented in prospective randomised trials.
BONSAI represents a prospective, monocentric, phase II trial that tested cabozantinib (cabo) 60 mg in treatment-naïve metastatic CDC patients. Primary endpoint was ORR per RECIST v1.1. Secondary endpoints included PFS, OS, and safety profile. Exploratory objectives were to identify somatic mutations by targeted DNA sequencing and to define molecular subtypes by RNA sequencing. From 01/2018 to 11/2020, 25 patients were enrolled, of whom 23 started treatment; median age was 66 years, 19 patients were male; the most common metastatic sites were lymph nodes and bones, followed by lung and liver; median follow-up was 8 months, ORR was 35% (1 complete response [CR] and 7 partial responses [PR]); median PFS was 6 months; all patients reported at least one grade 1–2 AE: the most common were fatigue (43%), hypothyroidism (28%), stomatitis (28%), anorexia (26%), hand–foot syndrome (13%), hypertension (17%), and diarrhoea (13%). Five patients reported G3 AEs (2 × thromboembolic events, 2 × arterial hypertension, 1 × fatigue), while no G4–5 AEs were reported; 17% of patients required a dose reduction. DNA sequencing was successful in 21 (91%) patients, whereby all tumours were microsatellite stable and no association between tumour mutational burden and response to cabo was observed. Non-responders were frequently mutated in chromatin remodelling, transcriptional regulation and (Wingless and Int-1)-WNT pathways. BONSAI clearly met its primary endpoint showing promising efficacy and acceptable tolerability of cabo in metastatic CDC patients (Fig. 2; Table 1). Thus, this important study is able to provide a contemporary treatment guidance in the rare but complex field of advanced CDC, where current available treatment options and results from prospective trials are limited.
Summary of tumour response in the BONSAI (Meeturo 2) trial. a Summary of tumor response; b Kaplan–Meier estimates of progression-free survival (PFS). ORR objective response rate; CR complete response; PR partial response; SD stable disease; PD progressive disease, NA not available. Presented at: European Society for Medical Oncology (ESMO) Congress 2021; September 16–20, 2021. With kind permission from Dr. Guiseppe Procopio
Take-home message
-
Long-term clinical benefit with nivolumab + ipilimumab in advanced/metastatic clear cell renal cell carcinoma.
-
KEYNOTE-564 represents the first positive phase III study of adjuvant immunotherapy in renal cell carcinoma.
References
Cited literature
Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
Quhal F, Mori K, Bruchbacher A, et al. First-line immunotherapy-based combinations for metastatic renal cell carcinoma: a systematic review and network meta-analysis. Eur Urol Oncol. 2021;4(5):755–65.
Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90.
Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–15.
Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–27.
ClinicalTrials.gov. Nivolumab combined with ipilimumab versus sunitinib in previously untreated advanced or metastatic renal cell carcinoma (checkmate 214). 2021. https://clinicaltrials.gov/ct2/show/NCT02231749. Accessed 26 Jan 2022.
Eloranta S, Smedby KE, Dickman PW, et al. Cancer survival statistics for patients and healthcare professionals—a tutorial of real-world data analysis. J Intern Med. 2021;289(1):12–28.
EU Clinical Trials Register. A randomised phase II trial of nivolumab in combination with alternatively scheduled ipilimumab in first-line treatment of patients with advanced or metastatic renal cell carcinoma (PRISM). 2018. https://www.clinicaltrialsregister.eu/ctr-search/trial/2017-001476-33/GB. Accessed 26 Jan 2022.
Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):706–20.
ClinicalTrials.gov. Safety and efficacy study of pembrolizumab (MK-3475) as monotherapy in the adjuvant treatment of renal cell carcinoma post nephrectomy (MK-3475-564/KEYNOTE-564). 2021. https://clinicaltrials.gov/ct2/show/NCT03142334. Accessed 26 Jan 2022.
Smaldone MC, Fung C, Uzzo RG, et al. Adjuvant and neoadjuvant therapies in high-risk renal cell carcinoma. Hematol Oncol Clin North Am. 2011;25(4):765–91.
Sun M, Marconi L, Eisen T, et al. Adjuvant vascular endothelial growth factor-targeted therapy in renal cell carcinoma: a systematic review and pooled analysis. Eur Urol. 2018;74(5):611–20.
Correa AF, Jegede O, Haas NB, et al. Predicting renal cancer recurrence: defining limitations of existing prognostic models with prospective trial-based validation. J Clin Oncol. 2019;37(23):2062–71.
Ciszewski S, Jakimow A, Smolska-Ciszewska B. Collecting (Bellini) duct carcinoma: a clinical study of a rare tumour and review of the literature. Can Urol Assoc J. 2015;9(9–10):E589–E93.
Pagani F, Colecchia M, Sepe P, et al. Collecting ducts carcinoma: an orphan disease. Literature overview and future perspectives. Cancer Treat Rev. 2019;79:101891.
Further reading
ClinicalTrials.gov. caBozantinib in cOllectiNg ductS Renal Cell cArcInoma (BONSAI). 2021. https://clinicaltrials.gov/ct2/show/NCT03354884. Accessed 26 Jan 2022
Acknowledgements
None of the contributing authors have any conflicts of interest, including specific financial interests, relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
G.C. Hutterer and M. Pichler declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hutterer, G.C., Pichler, M. Renal cell carcinoma—presentation highlights from the ESMO Congress 2021. memo 15, 102–106 (2022). https://doi.org/10.1007/s12254-022-00798-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-022-00798-6