Abstract
For the purpose of nationwide surveillance of the antimicrobial susceptibility of bacterial respiratory pathogens collected from patients in Japan, the Japanese Society of Chemotherapy conducted a third year of nationwide surveillance during the period from January to April 2008. A total of 1,097 strains were collected from clinical specimens obtained from well-diagnosed adult patients with respiratory tract infections. Susceptibility testing was evaluable with 987 strains (189 Staphylococcus aureus, 211 Streptococcus pneumoniae, 6 Streptococcus pyogenes, 187 Haemophilus influenzae, 106 Moraxella catarrhalis, 126 Klebsiella pneumoniae, and 162 Pseudomonas aeruginosa). A total of 44 antibacterial agents, including 26 β-lactams (four penicillins, three penicillins in combination with β-lactamase inhibitors, four oral cephems, eight parenteral cephems, one monobactam, five carbapenems, and one penem), three aminoglycosides, four macrolides (including a ketolide), one lincosamide, one tetracycline, two glycopeptides, six fluoroquinolones, and one oxazolidinone were used for the study. Analysis was conducted at the central reference laboratory according to the method recommended by the Clinical and Laboratory Standard Institute (CLSI). The incidence of methicillin-resistant S. aureus (MRSA) was as high as 59.8%, and those of penicillin-intermediate and penicillin-resistant S. pneumoniae (PISP and PRSP) were 35.5 and 11.8%, respectively. Among H. influenzae, 13.9% of them were found to be β-lactamase-non-producing ampicillin (ABPC)-intermediately resistant (BLNAI), 26.7% to be β-lactamase-non-producing ABPC-resistant (BLNAR), and 5.3% to be β-lactamase-producing ABPC-resistant (BLPAR) strains. A high frequency (76.5%) of β-lactamase-producing strains was suspected in Moraxella catarrhalis isolates. Four (3.2%) extended-spectrum β-lactamase-producing K. pneumoniae were found among 126 strains. Four isolates (2.5%) of P. aeruginosa were found to be metallo β-lactamase-producing strains, including three (1.9%) suspected multidrug-resistant strains showing resistance to imipenem, amikacin, and ciprofloxacin. Continual national surveillance of the antimicrobial susceptibility of respiratory pathogens is crucial in order to monitor changing patterns of susceptibility and to be able to update treatment recommendations on a regular basis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In order to investigate comprehensively the antimicrobial susceptibility and resistance of bacterial respiratory pathogens, the Japanese Society of Chemotherapy (JSC) established a nationwide surveillance network in 2006. The first and second surveys were conducted during the periods from January to August in 2006 and 2007, and we reported the trend of antimicrobial susceptibilities of bacterial species isolated from patients with respiratory tract infections (RTIs) (Niki et al. [14]). Here we report the study of the third year of nationwide surveillance conducted by the JSC during the period from January to April 2008. The results obtained from this survey will be used as a set of controls for those surveys to be conducted in future by the JSC and by other organizations as well.
Materials and methods
Strains and quality control
The causative bacteria from patients with RTIs were isolated from sputum, specimens collected by trans-tracheal aspiration, or bronchoscopy. Microbiological laboratory tests for respiratory pathogens were conducted by standard methods, including Gram staining and quantitative culture of various respiratory samples, at 46 medical institutions, as listed in Table 1. The isolated bacteria were identified at the species level in each institution’s laboratory. The isolates were suspended in Micro-bank tubes (Asuka Junyaku, Tokyo, Japan) and transferred to the central laboratory, the Research Center for Anti-infective Drugs of the Kitasato Institute (hereafter, the Center). Electronic uniform data sheets of each patient from whom these strains isolated were also completed at each institution and sent to the Center so that the microbiological data obtained were able to be stratified according to the settings and profiles of the patients and according to the diagnoses.
A total of 1,097 strains were received at the Center and kept at −80°C until the antimicrobial susceptibility testing was conducted. Re-identification and culture of them gave 987 evaluable strains, consisting of 189 Staphylococcus aureus, 211 Streptococcus pneumoniae, 6 Streptococcus pyogenes, 187 Haemophilus influenzae, 106 Moraxella catarrhalis, 126 Klebsiella pneumoniae, and 162 Pseudomonas aeruginosa.
Accuracy of determination of the minimum inhibitory concentration (MIC) of antibacterial agents was controlled according to the recommendations of the Clinical and Laboratory Standards Institute (CLSI), using the following control strains, respectively: S. aureus ATCC29213 and Escherichia coli ATCC35218 for clinical isolates of S. aureus and M. catarrhalis; S. pneumoniae ATCC49619 for those of S. pneumoniae and S. pyogenes; H. influenzae ATCC49247 for H. influenzae; E. coli ATCC25922 for K. pneumoniae and P. aeruginosa; and P. aeruginosa ATCC27853 for P. aeruginosa. E. coli ATCC35218 was used as a control strain for the MIC determination of β-lactam antibiotics combined with β-lactamase inhibitors.
Susceptibility testing and MIC determination
Susceptibility testing was performed according to CLSI (formerly NCCLS) standards M7-A7 [1] for the microbroth dilution method. In brief, cation-adjusted Mueller-Hinton broth (25 mg/L Ca++ and 12.5 mg/L Mg++; CA-MH broth) was used to measure the MICs against S. aureus, M. catarrhalis, K. pneumoniae, and P. aeruginosa. For the determination of the MIC of oxacillin, NaCl was added at 2% to CA-MH broth. For measuring the MICs against S. pneumoniae, S. pyogenes, and H. influenzae, 15 μg/mL nicotinamide, 5 mg/mL yeast extract, and horse blood at 5% were added to CA-MH broth.
A 0.005 mL portion of test organism solution, grown to turbidity of MacFarland Number 0.5 and diluted tenfold with saline, was inoculated to CA-MH broth to make a final volume of 0.1 ± 0.02 mL. This was poured into a well on a microplate (Eiken Kagaku, Tokyo, Japan) where a serially diluted freeze-dried test agent was placed, and the MIC was determined with the MIC2000 system (Eiken Kagaku).
Antibacterial agents
The susceptibilities of the bacterial strains were tested for the following 44 antimicrobial agents: four penicillins – benzylpenicillin (PCG; Meiji Seika Kaisha), oxacillin (MPIPC; Meiji), ampicillin (ABPC; Meiji), and piperacillin (PIPC; Toyama Chemical); three penicillins in combination with β-lactamase inhibitors – clavulanic acid-amoxicillin (CVA/AMPC; Glaxo SmithKline.), sulbactam-ABPC (SBT/ABPC; Pfizer Japan), and tazobactam-PIPC (TAZ/PIPC; Toyama); four oral cephems – cefaclor (CCL; Shionogi), cefdinir (CFDN; Astellas Pharma), cefcapene (CFPN; Shionogi), and cefditoren (CDTR; Meiji); eight parenteral cephems – cefazolin (CEZ; Astellas), cefoxitin (CFX; Banyu Pharmaceutical), cefmetazole (CMZ; Daiichi-Sankyo), cefotiam (CTM; Takeda Pharmaceutical), ceftazidime (CAZ; Glaxo SmithKline), ceftriaxone (CTRX; Chugai Pharmaceutical), cefepime (CFPM; Meiji), and cefozopran (CZOP; Takeda); a monobactam – aztreonam (AZT; Eisai); five carbapenems – imipenem (IPM; Banyu), panipenem (PAPM; Daiichi-Sankyo), meropenem (MEPM; Dainippon Sumitomo,), biapenem (BIPM;Meiji), and doripenem (DRPM; Shionogi); one penem – faropenem(FRPM; Astellas); three aminoglycosides – gentamicin (GM; Shionogi), amikacin (AMK;Banyu), and arbekacin (ABK; Meiji); four macrolides – erythromycin (EM; Dainippon Sumitomo), clarithromycin (CAM; Toyama), azithromycin (AZM; Pfizer), and telithromycin (TEL; Sanofi-Aventis); a lincosamide – clindamycin (CLDM; Dainippon Sumitomo.); a tetracycline – minocycline (MINO; Wyeth /Takeda); two glycopeptides – vancomycin (VCM; Shionogi) and teicoplanin (TEIC; Astellas); six fluoroquinolones – ciprofloxacin (CPFX; BayerYakuhin), levofloxacin (LVFX; Daiichi-Sankyo), tosufloxacin (TFLX; Toyama), gatifloxacin (GFLX; Kyorin Pharmaceutical), moxifloxacin(MFLX; Shionogi), and pazufloxacin (PZFX; Toyama); and an oxazolidinone – linezolide (LZD; Pfizer). These antimicrobial agents were serially diluted and placed in a freeze-dried state in the appropriate wells of microplates. The stability of the antimicrobial agent-containing microplates was guaranteed by the manufacturer (Eiken Kagaku) for 9 months.
Detection of β-lactamases
To detect β-lactamases in H. influenzae, tests with Nitrocefin disks (Kanto Chemical, Tokyo, Japan) were conducted according to the reference manual supplied by the manufacturer.
A recently established rapid detection method, the Cica-Beta Test 1® (Kanto Chemical, Tokyo, Japan), designed to detect extended-spectrum β-lactamase (ESBL) and metallo β-lactamase (MBL) directly in colonies of Gram-negative rods [2, 3], was employed to identify K. pneumoniae and P. aeruginosa strains which produce such β-lactamases.
Results
Staphylococcus aureus
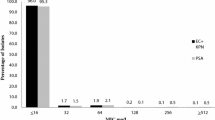
The in vitro antimicrobial susceptibilities, as MIC50/MIC90 values, and the range of MICs for S. aureus isolates are shown in Table 2. Among the total 189 strains of S. aureus, 113 strains (59.8%) were found to be methicillin-resistant S. aureus (MRSA; MIC of MPIPC ≥ 4 μg/mL).
Susceptibility of methicillin-susceptible S. aureus (MSSA)
The MIC90s of penicillins against 76 MSSA strains were 16–64 μg/mL; however, the MIC90s of penicillins in combinations with β-lactamase inhibitors (CVA/AMPC, SBT/ABPC, and TAZ/PIPC) decreased to 2.0–4.0 μg/mL. The MIC90s of CCL, CAZ, CTRX, CFPM, and CMZ ranged from 1.0 to 8.0 μg/mL, and those of the other seven cephems ranged from 0.25 to 1.0 μg/mL. Carbapenems showed the strongest activity, with MIC90s of ≤0.125 μg/mL. As for the aminoglycosides, GM, AMK, and ABK showed MIC90s of 8.0, 8.0, and 0.5 μg/mL, respectively. Among the macrolide-lincosamide antibiotics, TEL and CLDM showed relatively strong activity, with MIC90s of 0.25 and 0.5 μg/mL, respectively, but the rest of the macrolides showed weak activity, with MIC90s of ≥128 μg/mL. Relatively strong activities of MINO, VCM, TEIC, and LZD were shown, with MIC90s of 0.125–2.0 μg/mL. The MIC90s of the six fluoroquinolones were within the range of 2.0–8.0 μg/mL.
Susceptibility of MRSA
Only four agents—ABK, VCM, TEIC, and LZD—showed strong activity against MRSA, with an MIC90 of 2.0 μg/mL. MINO showed weak activity, with an MIC90 of 16 μg/mL. Other agents showed almost no activity, with MIC90s of ≥32 μg/mL.
Streptococcus pneumoniae
The susceptibilities of the 211 strains of S. pneumoniae to PCG revealed that 111 strains (52.6%), 75 strains (35.5%), and 25 strains (11.8%) were identified as penicillin-susceptible (PSSP), penicillin-intermediate (PISP), and penicillin-resistant strains (PRSP), respectively, with the breakpoint for PCG defined by the CLSI standards [1]. However, with the new Food and Drug Administration (FDA) criteria for breakpoint MICs for S. pneumoniae strains isolated from patients with pneumonia, 210 strains (99.5%), and 1 strain (0.5%), were classified as susceptible (MIC: ≤2 μg/mL), and intermediate (MIC: 4 μg/mL), respectively. No isolate among the stains we tested was found to be resistant (MIC: ≥8 μg/mL).
Among the β-lactams, CCL and CAZ showed high MIC90s (128 and 32 μg/mL, respectively) while many of the other β-lactams, except for the carbapenems, showed potent activities, with MIC90s of 2.0–4.0 μg/mL. All five carbapenems showed strong activities (MIC90: ≤0.5 μg/mL) against all S. pneumoniae strains, regardless of their different susceptibilities to PCG. Fluoroquinolones also showed potent activities against most of the strains, with MIC90s of 0.25–4 μg/mL, whereas 5 strains (2.4%) were found to be resistant to LVFX. The glycopeptides (VCM and TEIC), and TEL showed strong activities (MIC90: ≤0.5 μg/mL). Aminoglycosides were substantially less active, with MIC90s of 8.0–64.0 μg/mL. High frequencies of resistance to the macrolide antibiotics, EM, CAM, and AZM, were shown, with MIC90s of ≥128 μg/mL (Table 3).
Haemophilus influenzae
The susceptibilities of the 187 H. influenzae strains are summarized in Table 4. According to the CLSI breakpoint for ABPC [1], 101 strains (54.0%) were found to be ABPC-susceptible, 26 (13.9%) to be ABPC-intermediate, and 60 (32.1%) to be ABPC-resistant. With the use of the Nitrocephin disks, all ABPC-intermediate and 50 (26.7%) ABPC-resistant strains were found to be β-lactamase-non-producing, and they were defined as BLNAI and BLNAR, respectively. The other 10 (5.3%) ABPC-resistant strains were found to be β-lactamase-producing strains, designated as BLPAR. The MIC50 and MIC90 values of PCG and ABPC for BLPAR isolates were at least fivefold higher than those for BLNAR isolates. However, there were no differences in the MIC50 and MIC90 values of SBT/ABPC and CVA/AMPC among BLNAR isolates and BLPAR isolates. Regardless of susceptibility to ABPC, all of the H. influenzae strains were extremely susceptible to all six fluoroquinolones (MIC50s: ≤0.06 μg/mL). BLPAR strains showed high levels of resistance to PIPC, with MIC90 values of ≥256 μg/mL, whereas TAZ/PIPC showed strong activities, with MIC90s of ≤0.06 μg/mL. Among the cefems, CDTR and CTRX showed the most potent activities, with MIC90s of 0.25 μg/mL. Of the five carbapenem agents, MEPM showed the most potent acvitity against all types of H. influenzae strains. Among the macrolides, AZM showed the most potent acvitity, with an MIC90 of 2 μg/mL.
Moraxella catarrhalis
The susceptibilities of 106 M. catarrhalis strains are shown in Table 5. For the penicillins, β-lactamase inhibitors restored the activities of penicillins; e.g., SBT decreased the MIC90 of ABPC from 8 to 0.25 μg/mL and TAZ decreased the MIC90 of PIPC from 4 to ≤0.06 μg/mL. Carbapenems showed strong activities, with MIC90s of ≤0.25 μg/mL; in particular, MEPM and DRPM showed the most potent activities, with MICs for all isolates of ≤0.06 μg/mL. Fluoroquinolones also showed strong activities, with MIC90s of ≤0.06 μg/mL. Several cephems (CFDN, CFPN, CDTR, CTRX, CAZ, and CMZ), two aminoglycosides (GM and ABK), three macrolides (EM, CAM, and AZM), and the ketolide (TEL) also showed potent activities, with MIC90s of 0.125–1.0 μg/mL.
Klebsiella pneumoniae
The susceptibilities of 126 K. pneumoniae strains are shown in Table 6. Among 34 antimicrobial agents we tested, MEPM and DRPM showed the strongest activities, with MIC90s of ≤0.06 μg/mL. Of the cephems and the monobactam, CFDN, CAZ, CTRX CFPM, CZOP, and AZT showed strong activities, with MIC90s of 0.125–0.25 μg/mL. All fluoroquinolones we tested and two aminoglycosides (GM and ABK) also showed potent activities, with MIC90s of 0.25–0.5 μg/mL. β-Lactamase inhibitors apparently restored the activities of penicillins; e.g., SBT decreased the MIC90 of ABPC from ≥256 to 8 μg/mL and TAZ decreased the MIC90 of PIPC from 32 to 4 μg/mL. Among the 126 strains of K. pneumoniae, 4 strains (3.2%) were found to be ESBL producers.
Pseudomonas aeruginosa
A total of 162 P. aeruginosa strains were tested for antimicrobial susceptibility (Table 7). Among the β-lactams, three carbapenems (MEPM, BIPM, and DRPM) showed potent activities, with MIC50s of 0.25–0.5 μg/mL; however, these agents showed relatively higher MIC90 levels, of 8.0–16 μg/mL. Among the fluoroquinolones, CPFX showed the most potent activity, with MIC50s and MIC90s of 0.25 and 4.0 μg/mL, respectively. Other fluoroquinolones also showed strong activities, with MIC50s of 0.25–2.0 μg/mL, whereas the MIC90 levels (8.0 to ≥32 μg/mL) suggested partial resistance. Both PIPC and TAZ/PIPC showed potent activities, with MIC50s of 4 μg/mL; higher MIC90 levels (128 and 64 μg/mL) of these agents suggested resistance. The MIC50s of the three aminoglycosides (GM, AMK, and ABK), three cephems (CAZ, CFPM and CZOP), and the monobactam (AZT) were within the range of 1.0–4.0 μg/mL. Only one strain was identified as multidrug-resistant P. aeruginosa (MDRP) from its profile of resistance to IPM, AMK, and CPFX. Among the 162 P. aeruginosa strains, we found 4 (2.5%) MBL-producing strains and 3 multidrug-resistant strains (1.9%), and these 3 multidrug-resistant strains were found to be MBL-producers.
Discussion
The Japanese Society of Chemotherapy (JSC) established a nationwide surveillance network in 2006 to establish a resource for information about the antimicrobial susceptibility of bacterial pathogens in Japan. Our research focuses on seven major bacterial respiratory pathogens – S. aureus, S. pneumoniae, S. pyogenes, H. influenzae, M. catarrhalis, K. pneumoniae, and P. aeruginosa. It is desirable that analysis of antimicrobial susceptibility is done with the use of bacterial strains that actually cause the infections. To analyze the actual pathogens causing infections, we collected clinical isolates only from well-diagnosed adult patients with respiratory tract infections (RTIs).
Our surveillance was conducted for three consecutive years from 2006. The total number of strains collected for the surveillances conducted in 2006, 2007, and 2008 were 887, 1108, and 987, respectively. The species tested at surveillance in each of these years were as follows: S. aureus (205, 226, and 189), S. pneumoniae (200, 257, and 211), H. influenzae (165, 206, and 187), P. aeruginosa (143, 171, and 162), M. catarrhalis (91, 120, and 106), K. pneumoniae (74, 122, and 126), and S. pyogenes (9, 6, and 6). The numbers of each species in each year of surveillance may generally reflect the trend of pathogens of respiratory infections in Japan, but we think we should increase the scope of the survey by reflecting results with a greater number of pathogens.
With regard to S. aureus, in the present survey, 42 of 76 strains (55.2%) of MSSA were thought to be penicillinase-producing strains because of their resistance to ABPC and susceptibility to SBT/ABPC and CCL, and 11 of 76 strains (14.5%) of MSSA may be emr-harboring strains because of their resistance to the macrolides, EM, CAM, and AZM, and susceptibility to TEL (ketolide lacking emr resistance mechanism) [4]. The difference between MSSA resistance to GM (10.5%) and that to AMK (0%) implied the coexistence of aac(6′)/aph(2″)-harboring GM-resistant strains with aad(4′, 4″)-harboring AMK-resistant strains [5].
We found the incidence of MRSA to be as high as 59.8%, which is similar to the data reported by Mochizuki et al. [6] in a study analyzed with the microbiology laboratory database software WHONET 5. These MRSA strains are susceptible to ABK, VCM, TEIC, and LZD, except that a few strains are somewhat less susceptible (MIC: 8.0 μg/mL) to ABK; these strains may possess both aph(3′)-III and aac(6′)/aph(2″) genes, as reported recently [5]. Although the emergence of MRSA that is resistant to VCM, TEIC, or LZD has already been reported in Japan, such a resistant strain was not detected in the present survey.
Regarding S. pneumoniae, the proportion of PSSP/PISP/PRSP was found to be 53:35:12. The proportions of each group of strains in the first and second years of surveillance were at similar levels (61:35:4 and 65:30:5, respectively), but the results of the third year of surveillance suggest an increase in resistant strains of S. pneumoniae. Among PSSP, more than 55% are thought to be emr-harboring strains because of their resistance to macrolides (EM, CAM, and AZM) and CLDM and susceptibility to the ketolide TEL. As for PISP, their incidence (35%) in this survey of adult RTIs was much lower than that (50.8%) reported in pediatric infections [7–9]. The incidence of PRSP (12%) in our present survey was relatively low as compared with the data (16.9–49.0%) reported in pediatric infections [7–9]. This difference is thought to be attributable to the excess use of oral penicillins and cephems for the treatment of children, because the use of fluoroquinolones (except for norfloxacin) is contraindicated in children in Japan. Although TFLX has been permitted for the treatment for children in 2010, the present research was conducted in 2008. The pattern of susceptibility of PRSP somewhat resembled that of PISP; however, PRSP were substantially susceptible (MIC90s: ≤0.5 μg/mL) only to carbapenems, except for IPM, and they were not susceptible to TFLX, GFLX, MFLX, TEL, and VCM.
The FDA has raised the concentration at which S. pneumoniae is considered to be susceptible to penicillin for the treatment of pneumonia, although the susceptibility breakpoint for meningitis remains unchanged (0.06 μg/mL). With the new criteria for breakpoint MICs, only one of the 211 S. pneumoniae strains (0.5%) in the present survey was found to be intermediate and the other 210 strains (99.5%) were classified as susceptible. These results suggest that penicillin is still effective against community-acquired pneumonia caused by S. pneumoniae.
Concerning H. influenzae, half of the strains in the present survey showed decreased susceptibility to ABPC without production of β-lactamase; BLNAI (13.9%) and BLNAR (26.7%). The incidence of BLNAI in adults is thought to be somewhat lower (30.4%) than that in children [10]. All six fluoroquinolones demonstrated extremely strong activity (MIC90: ≤0.06 μg/mL) against H. influenzae strains, regardless of their ABPC susceptibility. Among the rest of the agents, PIPC, TAZ/PIPC, CDTR, CTRX, and MEPM showed strong activities (MIC90s of 0.125–0.5 μg/mL) against BLNAS, BLNAI, and BLNAR strains. TAZ markedly restored the activity of PIPC against BLPAR (MIC90 decreased from ≥256 to ≤0.06 μg/mL).
The susceptibilities of M. catarrhalis in the present survey showed that β-lactamase inhibitors restored the activities of penicillins against these strains: SBT decreased the MIC90 of ABPC from 8 to 0.25 μg/mL. The data suggest that most of the strains were resistant to penicillins because of β-lactamase production. For the treatment of M. catarrhalis infections, carbapenems, macrolides, and fluoroquinolones may be recommended because these drugs showed strong activities, with MIC90s of ≤0.06–0.25 μg/mL.
The prevalence of extended-spectrum β-lactamase (ESBL) strains has become a concern in recent years. Yagi et al. conducted a survey of ESBLs among 9,794 K. pneumoniae clinical isolates in Japan during the period January 1997 to January 1998 and they reported that 34 isolates (0.3%) had been found to produce ESBLs [11]. However, an increase in the number of ESBL-producing strains has been suggested; Yamaguchi et al. [12] reported the results of a nationwide surveillance of antibacterial activity of clinical isolates in 2006, and 3.3% (3 of 91) K. pneumoniae strains were found to be ESBL-producing strains. In our study, 4 of 126 K. pneumoniae strains (3.2%) were found to be ESBL-producing strains, and these results were consistent with the previous report.
In the present survey, 4 (2.5%) metallo-β-lactamase (MBL)-producing strains and 3 (1.9%) multidrug-resistant strains were found in 162 P. aeruginosa isolates. Yamaguchi et al. compared the frequencies of multidrug-resistant strains of P. aeruginosa between isolates from urinary tract infections and those from RTIs. They reported that 5.6 and 1.8% of multidrug-resistant strains were found from the urinary isolates and the respiratory isolates, respectively. Therefore, a low incidence of multidrug-resistant P. aeruginosa may be limited to respiratory infections [13].
The present study has revealed, in comparison to that of 2007, that the incidence of S. pneumoniae isolation was higher in 2008, while the frequencies of other bacterial species were comparable to those in 2007. The total frequency of S. pneumoniae isolation, including PISP and PRSP, increased from 35.5% in 2007 to 47.3% in 2008; the difference was statistically significant, at P < 0.001. Because the frequencies of PISP in 2007 and 2008 showed no significant difference, the contribution of the PRSP isolation frequency must have been markedly high in 2008. In fact, the frequency of PRSP isolation in 2007 was 5.1% and that in 2008 was 11.8%; this is a statistically significant difference, at P < 0.001. Thus, careful watching of the trend of PRSP may be needed [14].
We think our surveillance data will be a useful reference for the treatment of respiratory infections in our country. There is substantial evidence that the overuse of antibiotics is a major cause of the emergence of resistance in respiratory pathogens. To prevent the further spread of antimicrobial resistance in respiratory pathogens, proper antibiotic use is needed. We should also continue the surveillance to determine the actual situation of the resistance shown by bacterial respiratory pathogens to antimicrobial agents.
References
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, 16th informational supplement M100-S16 and M45-P. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. Document M7-A7. Wayne: Clinical and Laboratory Standards Institute; 2006.
Hanaki H, Kubo R, Nakano T, Kurihara M, Sunagawa K. Characterization of HMRZ-86: a novel chromogenic cephalosporin for the detection of extended-spectrum beta-lactamases. J Antimicrob Chemother. 2004;53(5):888–9.
Colodner R, Reznik B, Gal V, Yamazaki H, Hanaki H, Kubo R. Evaluation of a novel kit for the rapid detection of extended-spectrum beta-lactamases. Eur J Clin Microbiol Infect Dis. 2006;25(1):49–51.
Jenkins SG, Farrell DJ, Patel M, Lavin BS. Trends in anti-bacterial resistance among Streptococcus pneumoniae isolated in the USA, 2000–2003: PROTEKT US years 1–3. J Infect. 2005;51(5):355–63.
Barada K, Hanaki H, Ikeda S, Yamaguchi Y, Akama H, Nakae T, Inamatsu T, Sunakawa K. Trends in the gentamicin and arbekacin susceptibility of methicillin-resistant Staphylococcus aureus and the genes encoding aminoglycoside-modifying enzymes. J Infect Chemother. 2007;13(2):74–8.
Mochizuki T, Okamoto N, Yagishita T, Takuhiro K, Mashiko K, Ogawa F, Tosaka N, Kurokawa A, Yamamoto Y. Analysis of antimicrobial drug resistance of Staphylococcus aureus strains by WHONET 5: microbiology laboratory database software. J Nippon Med Sch. 2004;71(5):345–51.
Kamiya H, Kato T, Togashi T, Iwata S, Kurosaki T, Baba S, Masuda S, Sato S, Yoshimura O, Fujii M, Shimada A, Yagi K, Yano H, Sugita R, Fujimaki Y, Komatsu N, Tango T, The Research Group on Streptococcus Pneumoniae Serotypes among Children. Epidemiological survey of pneumococcus serotypes in pediatric patients with acute suppurative otitis media. Kansenshogaku Zasshi. 2007;81(1):59–66. (in Japanese; abstract in English).
Chiba N, Kobayashi R, Hasegawa K, Morozumi M, Nakayama E, Tajima T, Iwata S, Ubukata K, Acute Respiratory Diseases Study Group. Antibiotic susceptibility according to genotype of penicillin-binding protein and macrolide resistance genes, and serotype of Streptococcus pneumoniae isolates from community-acquired pneumonia in children. J Antimicrob Chemother. 2005;56(4):756–60.
James A, Karlowsky JA, Thornsberry C, Critchley IA, Jones ME, Evangelista AT, Noel GJ, Sahm DF. Susceptibilities to levofloxacin in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis clinical isolates from children: results from 2000–2001 and 2001–2002 TRUST Studies in the United States. Antimicrob Agents Chemother. 2003;47(6):1790–7.
Hasegawa K, Kobayashi R, Takada E, Ono A, Chiba N, Morozumi M, Iwata S, Sunakawa K, Ubukata K. Nationwide surveillance for bacterial meningitis. High prevalence of type b beta-lactamase-non-producing ampicillin-resistant Haemophilus influenzae in meningitis: the situation in Japan where Hib vaccine has not been introduced. J Antimicrob Chemother. 2006;57(6):1077–82.
Yagi T, Kurokawa H, Shibata N, Shibayama K, Arakawa Y. A preliminary survey of extended-spectrum beta-lactamases (ESBLs) in clinical isolates of Klebsiella pneumoniae and Escherichia coli in Japan. FEMS Microbiol Lett. 2000;184:53–6.
Yamaguchi K, Ishii Y, Iwata M, Watanabe N, Uehara N, Yasujima M, Kasai T, Suwabe A, Yamahata K, Kaku M, Kanemitsu K, Imafuku Y, Nishiyama K, Murakami M, Yomoda S, Taniguchi N, Yamada T, Nomura F, Watanabe M, Kanno H, Aihara M, Maesaki S, Hashikita G, Kondo S, Misawa S, Horiuchi H, Tazawa Y, Nakashima H, Takemura H, Okada M, Yamazaki F, Horii T, Maekawa M, Baba H, Ishigo S, Fujita N, Komori T, Ichiyama S, Iinuma Y, Maeda S, Yamanaka K, Murata Y, Matsuo S, Kohno H, Kinoshita S, Fujita J, Negayama K, Murase M, Miyamoto H, Kusano N, Mihara E, Itaha H, Ono J, Yoshimura H, Yanagihara K, Matsuda J, Saikawa T, Hiramatsu K. Nationwide surveillance of parenteral antibiotics containing meropenem activities against clinically isolated strains in 2006. Jpn J Antibiot. 2007;60:344–77.
Yamaguchi K, Ohno A, Ishii Y, Tateda K, Iwata M, Kanda M, Akizawa K, Shimizu C, Kon S, Nakamura K, Matsuda K, Tominaga M, Nakagawa T, Sugita A, Ito T, Kato J, Suwabe A, Yamahata K, Kawamura C, Tashiro H, Horiuchi H, Katayama Y, Kondou S, Misawa S, Murata M, Kobayashi Y, Okamoto H, Yamazaki K, Okada M, Haruki K, Kanno H, Aihara M, Maesaki S, Hashikita G, Miyajima E, Sumitomo M, Saito T, Yamane N, Kawashima C, Akiyama T, Ieiri T, Yamamoto Y, Okamoto Y, Okabe H, Moro K, Shigeta M, Yoshida H, Yamashita M, Hida Y, Takubo T, Kusakabe T, Masaki H, Heijyou H, Nakaya H, Kawahara K, Sano R, Matsuo S, Kono H, Yuzuki Y, Ikeda N, Idomuki M, Soma M, Yamamoto G, Kinoshita S, Kawano S, Oka M, Kusano N, Kang D, Ono J, Yasujima M, Miki M, Hayashi M, Okubo S, Toyoshima S, Kaku M, Sekine I, Shiotani J, Horiuchi H, Tazawa Y, Yoneyama A, Kumasaka K, Koike K, Taniguchi N, Ozaki Y, Uchida T, Murakami M, Inuzuka K, Gonda H, Yamaguchi I, fujimoto Y, Iriyama J, Asano Y, Genma H, Maekawa M, Yoshimura H, Nakatani K, Baba H, Ichiyama S, Fujita S, Kuwabara M, Okazaki T, Fujiwara H, Ota H, Nagai A, Fujita J, Negayama K, Sugiura T, Kamioka M, Murase M, Yamane N, Nakasone I, Okayama A, Aoki Y, Kusaba K, Nakashima Y, Miyanohara H, Hiramatsu K, Saikawa T, Yanagihara K, Matsuda J, Kohno S, Mashiba K. In vitro susceptibilities to levofloxacin and various antibacterial agents of 12,919 clinical isolates obtained from 72 centers in 2007. Jpn J Antibiot. 2009;62:346–70.
Niki Y, Hanaki H, Matsumoto T, Yagisawa M, Kohno S, Aoki N, Watanabe A, Sato J, Hattori R, Terada M, Koashi N, Kozuki T, Maruo A, Morita K, Ogasawara K, Takahashi Y, Watanabe J, Takeuchi K, Fujimura S, Takeda H, Ikeda H, Sato N, Niitsuma K, Saito M, Koshiba S, Kaneko M, Miki M, Nakanowatari S, Honda Y, Chiba J, Takahashi H, Utagawa M, Kondo T, Kawana A, Konosaki H, Aoki Y, Ueda H, Sugiura H, Ichioka M, Goto H, Kurai D, Okazaki M, Yoshida K, Yoshida T, Tanabe Y, Kobayashi S, Okada M, Tsukada H, Imai Y, Honma Y, Nishikawa K, Yamamoto T, Kawai A, Kashiwabara T, Takesue Y, Wada Y, Nakajima K, Miyara T, Toda H, Mitsuno N, Sugimura H, Yoshioka S, Kurokawa M, Munekawa Y, Nakajima H, Kubo S, Ohta Y, Mikasa K, Maeda K, Kasahara K, Koizumi A, Sano R, Yagi S, Takaya M, Kurokawa Y, Kusano N, Mihara E, Kuwabara M, Fujiue Y, Ishimaru T, Matsubara N, Kawasaki Y, Tokuyasu H, Masui K, Negayama K, Ueda N, Ishimaru M, Nakanishi Y, Fujita M, Honda J, Kadota J, Hiramatsu K, Aoki Y, Nagasawa Z, Suga M, Muranaka H, Yanagihara K, Fujita J, Tateyama M, Sunakawa K, Totsuka K. Nationwide surveillance of bacterial respiratory pathogens conducted by the Japanese Society of Chemotherapy in 2007: general view of the pathogens’ antibacterial susceptibility. J Infect Chemother. 2009;15(3):156–67.
Acknowledgments
This investigation was supported by grants from the following pharmaceutical companies (in alphabetical order): Abbott Japan Co., Ltd.; Astellas Pharma Inc.; Banyu Pharmaceutical Co., Ltd.; Bayer Yakuhin, Ltd.; Chugai Pharmaceutical Co., Ltd.; Daiichi Sankyo Company Limited; Dainippon Sumitomo Pharma Co., Ltd.; Glaxo SmithKline K. K.; Kyorin Pharmaceutical Co., Ltd.; Meiji Seika Kaisha, Ltd.; Pfizer Japan Inc.; Sanofi-Aventis K.K., Shionogi & Co., Ltd.; Taiho Pharmaceutical Co., Ltd.; Taisho Pharmaceutical Co., Ltd.; Takeda Pharmaceutical Company Limited; and Toyama Chemical Co., Ltd. We are grateful to T. Nakae and C. Yanagisawa at the Kitasato Institute (Tokyo, Japan) for their encouragement with the microbiological testing and Y. Suzuki, H. Endo, and Y. Yamaguchi for their technical assistance in this surveillance.
Author information
Authors and Affiliations
Corresponding author
Additional information
The members of The Japanese Society of Chemotherapy (JSC) Surveillance Committee are listed in the Appendix.
Appendix
Appendix
The Japanese Society of Chemotherapy Surveillance Committee
Y. Niki, T. Matsumoto, S. Kohno, N. Aoki, A. Watanabe, J. Sato, R. Hattori, N. Koashi, M. Terada, T. Kozuki, A. Maruo, K. Morita, K. Ogasawara, Y. Takahashi, K. Matsuda, K. Nakanishi, and K. Totsuka
About this article
Cite this article
Niki, Y., Hanaki, H., Matsumoto, T. et al. Nationwide surveillance of bacterial respiratory pathogens conducted by the Japanese Society of Chemotherapy in 2008: general view of the pathogens’ antibacterial susceptibility. J Infect Chemother 17, 510–523 (2011). https://doi.org/10.1007/s10156-011-0214-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10156-011-0214-5