Abstract
Background
Granulocyte colony-stimulating factor (G-CSF) is commonly administered to cancer patients undergoing myelosuppressive chemotherapy, especially when incidence rate of febrile neutropenia (FN) surpasses 20%. While primary prophylaxis with G-CSF has been proven effective in preventing FN in patients with cancer, there is limited evidence regarding its efficacy in specifically, lung cancer. Our systematic review focused on the efficacy of G-CSF primary prophylaxis in lung cancer.
Methods
We extracted studies on non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC) using the PubMed, Ichushi Web, and Cochrane Library databases. Two reviewers assessed the extracted studies for each type of lung cancer and conducted quantitative and meta-analyses of preplanned outcomes, including overall survival, FN incidence, infection-related mortality, quality of life, and musculoskeletal pain.
Results
A limited number of studies were extracted: two on NSCLC and six on SCLC. A meta-analysis was not conducted owing to insufficient data on NSCLC. Two case–control studies explored the efficacy of primary prophylaxis with G-CSF in patients with NSCLC (on docetaxel and ramucirumab therapy) and indicated a lower FN frequency with G-CSF. For SCLC, meta-analysis of five studies showed no significant reduction in FN incidence, with an odds ratio of 0.38 (95% confidence interval 0.03–5.56, P = 0.48). Outcomes other than FN incidence could not be evaluated due to low data availability.
Conclusion
Limited data are available on G-CSF prophylaxis in lung cancer. Primary prophylaxis with G-CSF may be weakly recommended in Japanese patients with NSCLC undergoing docetaxel and ramucirumab combination therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the predominant cause of mortality in many countries [1] and presents a significant public health challenge. Most lung cancer cases are diagnosed at an advanced stage, necessitating comprehensive and systematic pharmacotherapy as the cornerstone of treatment. Over the past two decades, pharmacotherapy for lung cancer has undergone remarkable advancements, particularly with the introduction of molecular-targeted agents [2,3,4] and immune checkpoint inhibitors [5,6,7]. These breakthroughs have reshaped therapeutic approaches and offered new hope to patients with this formidable disease. Despite the strides made in targeted therapies and immunotherapy, cytotoxic chemotherapy remains a crucial component of the treatment paradigm for advanced lung cancer. Myelosuppression is a challenge associated with cytotoxic chemotherapy, with febrile neutropenia (FN) emerging as a serious adverse event. FN can cause treatment-related mortality. In response, the development of granulocyte colony-stimulating factor (G-CSF) has provided a valuable tool for mitigating the risk of FN in individuals undergoing cytotoxic chemotherapy [8]. Guidelines such as those endorsed by the American Society of Clinical Oncology and the National Comprehensive Cancer Network recommend the use of G-CSF for chemotherapeutic regimens where the incidence rate of FN is projected to exceed 20% [9,10,11,12]. However, this threshold lacks a solid scientific foundation, raising questions regarding the clinical benefits of G-CSF in individuals undergoing chemotherapy. Moreover, the efficacy of G-CSF in the treatment of lung cancer remains unclear. To address these critical gaps in knowledge, we conducted a comprehensive systematic review on the use of G-CSF in lung cancer. Our primary objective was to investigate the efficacy of G-CSF in lung cancer treatment. By scrutinizing available evidence from relevant studies, we aimed to provide valuable insights into the role of G-CSF in mitigating the risks associated with cytotoxic chemotherapy in patients with lung cancer. Through this endeavor, we sought to enhance our understanding of optimal strategies for managing myelosuppression and improving outcomes in individuals confronting the challenges of advanced lung cancer treatment.
This systematic review was conducted to develop the Japan Society of Clinical Oncology Clinical Practice Guidelines for the Use of G-CSF (2022).
Methods
Literature search
We referred to the “Medical Information Network Distribution Service (Minds) Handbook for Clinical Practice Guideline Development 2014″ [6] and “Minds Clinical Practice Guideline Development Guide 2017″ [7] to develop guidelines and conduct a literature search for non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC), respectively, using PubMed, Ichushi Web (Japanese medical bibliographic database), and Cochrane Library databases. The search terms used for the literature search were "non-small-cell lung cancer” (for NSCLC), “small-cell lung cancer” (for SCLC), “neutropenia,” “granulocyte colony-stimulating factor,” “pegfilgrastim,” “prevention,” and ”control.” Two members of the systematic review team conducted an initial screening of all articles based on their titles and abstracts (K.K. and D. H. for NSCLC and G. M. and D. H. for SCLC) and then proceeded to full-text screening (i.e., secondary screening) based on the inclusion and exclusion criteria. In case of disagreements among the reviewers regarding the inclusion/exclusion of the literature, a resolution was achieved through discussion between the reviewers. The papers were scrutinized for quality-reported data relevant to the selection criteria outlined below. The selection criteria included studies with a randomized controlled trial (RCT), non-RCT, cohort, or case–control design. The exclusion criteria comprised guidelines, reviews, letters, abstracts without papers, laboratory studies, systematic reviews, meta-analyses, and gray literature.
Data extraction and quality assessment
The reviewers of the systematic review team (K.K. for NSCLC and G.M. for SCLC) reevaluated the articles after the second screening and extracted data using standardized data abstraction forms. Evidence from the studies was evaluated according to the outcomes of the clinical questions listed by the guidelines team. The outcomes included overall survival (OS), FN incidence rate, infection-related mortality, quality of life (QOL), and musculoskeletal pain. The positive and negative outcomes of prophylactic G-CSF were evaluated using the population, intervention, comparator, and outcome (PICO) frameworks. The quality of the literature and the overall body of evidence were examined using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach, subsequently categorized into four levels: "strong," "medium," "weak," and "very weak."
Statistical methodology for meta-analysis
The risk ratio (RR) for each endpoint was computed and the effect size was indicated by a 95% confidence interval (CI) for each study. These calculations were performed using either fixed- or random-effects models depending on the extent of heterogeneity. A Forest plot was used to visually depict the calculated RR for individual studies and comprehensive meta-analyses. The level of heterogeneity was evaluated using the I2 test and Chi-square-based Q test, and a corresponding p-value was determined. We used Review Manager (RevMan), version 5.41 software, developed by the Cochrane Collaboration (London, UK) for statistical analyses.
Results
Literature search
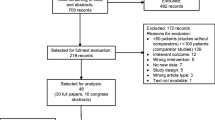
The initial search for NSCLC yielded 109 results distributed across different databases as follows: 93 from PubMed, none from the Cochrane Library, and 16 from Ichushi Web (search conducted on March 27, 2020). Following the screening process, which involved filtering for human subjects, publication dates between January 1, 1990 and December 31, 2019, publications in either English or Japanese, and applying the selection criteria outlined in the preceding section, a significant number of articles were excluded. Finally, only two articles that met the inclusion criteria were selected [13, 14] (Fig. 1). Similarly, the initial search for SCLC yielded 80 results distributed across different databases as follows: 59 from PubMed, 1 from the Cochrane Library, and 20 from Ichushi Web (search conducted on March 27, 2020). Following the screening process, which involved filtering for human subjects, publication dates between January 1, 1990, and December 31, 2019, publications in either English or Japanese, and applying the selection criteria outlined in the preceding section, a significant number of articles were excluded. Finally, only six articles met the inclusion criteria and were selected [15,16,17,18,19,20] (Fig. 2).
Studies employed in the meta-analysis
Only two studies with small sample sizes included our preplanned outcomes for NSCLC [13, 14]. Therefore, a meta-analysis was not conducted for the NSCLC data. For SCLC, six studies (no RCT, one case–control and five cohort studies) were included in the descriptive qualitative analysis [15,16,17,18,19,20]. The case–control study was excluded from the meta-analysis because of a lack of information regarding the actual FN incidence rate [20]. Five studies [15,16,17,18,19] were examined in the meta-analysis with regard to the FN incidence rate. Meta-analyses of other preplanned outcomes such as OS, QOL, infection-related mortality, and musculoskeletal pain were not feasible because of insufficient data measurements in these studies.
Primary prophylaxis with G-CSF and FN incidence rate
For NSCLC, two case–control studies were available for descriptive qualitative analysis of the FN incidence rate [13, 14]. Both studies investigated the efficacy of primary prophylaxis with G-CSF in Japanese patients with NSCLC, treated with docetaxel and ramucirumab. In one study, the incidence of FN was 0% (0/29) when PEG-G-CSF was administered and 50% (2/4) when not, while in the other study, it was 0% (0/10) with PEG-G-CSF and 40% (4/10) without its administration. A meta-analysis of the FN incidence rate was not performed because of the small sample size. Owing to the small number of studies and lack of meta-analyses, the quality and certainty of evidence on this outcome were rated as “very weak.”
Six SCLC-related studies (one case–control study and five cohort studies) were available for descriptive qualitative analyses. After excluding one case–control study due to a lack of data on the FN incidence rate [20], the remaining five cohort studies were included in the analyses [15,16,17,18,19]. Among these, three were single-arm cohort studies that did not include a control group without G-CSF [16,17,18]. Therefore, a meta-analysis was performed using incidence rates in the intervention group [15,16,17,18,19] (Fig. 3A and 3B). The meta-analysis showed that the FN incidence rate in the control group was 9/50 (18%), while that in the intervention group was 53/232 (22.8%), with an odds ratio of 0.38 (95% CI 0.03–5.56, p = 0.48), which was not statistically significant. Owing to the small number of studies and wide CI, the quality/certainty of evidence on this outcome was rated as “very weak.” One reason for the wide CI was attributable to the difference in the regimens used in each study.
Primary prophylaxis with G-CSF and outcomes other than FN incidence rate
None of the studies included in this analyses investigated the efficacy of G-CSF on OS, infection-related mortality, QOL, or musculoskeletal pain in patients with NSCLC or SCLC. Therefore, the effectiveness of G-CSF in relation to these outcomes has been inconclusive in NSCLC and SCLC.
Discussion
We conducted a systematic review and meta-analysis of the effectiveness of primary prophylaxis with G-CSF in lung cancer to establish a Clinical Practice Guidelines for the Use of G-CSF 2022. During this systematic review, it became evident that there is little research elucidating the utility of G-CSF in the treatment of lung cancer. Based on this review, a weak recommendation is made for the primary prophylactic administration of G-CSF in chemotherapy (only with a combination of docetaxel and ramucirumab) for advanced NSCLC, whereas a weak recommendation is made against the primary prophylactic administration of G-CSF in chemotherapy for SCLC.
In many clinical guidelines, primary prophylaxis with G-CSF is recommended for chemotherapeutic regimens with an FN incidence rate > 20%, regardless of the cancer type[9,10,11,12]. In the treatment of lung cancer, regimens with an FN incidence rate exceeding 20% are relatively uncommon, and this scarcity may contribute to the limited evidence for G-CSF efficacy in lung cancer.
For NSCLC treatment, various regimens, such as platinum and taxanes have been employed. A regimen associated with a relatively high risk of FN is the combination therapy of docetaxel and ramucirumab. The FN incidence rate with docetaxel and ramucirumab therapy in the Japanese population exceeds 20% [21]. In clinical practice, primary prophylactic administration of G-CSF is often performed when using docetaxel and ramucirumab combination therapy. Our systematic review shows that primary prophylactic administration of G-CSF reduces the incidence rate of FN in patients receiving docetaxel and ramucirumab combination [13, 14]. However, no clear evidence was observed for other regimens. Therefore, our guidelines recommending the use of G-CSF for NSCLC are limited to docetaxel and ramucirumab therapy.
Platinum-based agents and topoisomerase inhibitors are used to treat advanced SCLCs. Regimens such as nogitecan and cisplatin + irinotecan + etoposide therapy are considered to have a relatively high risk of FN [18], and in clinical practice, primary prophylactic administration of G-CSF is considered an option. There is no special mention of primary prophylactic administration of G-CSF for patients with advanced SCLC in the American Society of Clinical Oncology [9], European Society for Medical Oncology [11], and National Comprehensive Cancer Network [10] guidelines, and it should be considered according to the risk of FN occurrence for each general-use regimen. The usefulness of primary prophylaxis of G-CSF has not been clearly demonstrated for each regimen in our study. Primary prophylactic administration of G-CSF is weakly discouraged in our clinical guidelines for SCLC because the meta-analysis did not show a significant reduction in the FN incidence rate. Evidence of the efficacy of G-CSF in SCLC is scarce, and the accumulation of evidence is awaited. Primary prophylactic administration of G-CSF may also be considered in high-risk regimens.
In this systematic review, there was no evidence regarding primary prophylactic G-CSF efficacy in chemotherapy for advanced NSCLC/SCLC in relation to OS, infection-related mortality, QOL, and musculoskeletal pain.
In conclusion, this systematic review and meta-analysis of primary prophylactic G-CSF administration for lung cancer treatment, suggests that primary prophylaxis with G-CSF may reduce the incidence rate of FN in patients with NSCLC, treated with docetaxel and ramucirumab combination therapy, while showing no significant reduction in the FN incidence rate in patients with SCLC. Thus, primary prophylaxis with G-CSF may be weakly advisable in NSCLC treatment with docetaxel and ramucirumab combination therapy, while not in SCLC treatment. Further studies with larger sample sizes need to be undertaken in future, for testing the veracity of these recommendations.
Data availability
Data associated with this systematic review can be accessed from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/CAAC.21660
Soria J-C, Ohe Y, Vansteenkiste J et al (2018) Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 378:113–125. https://doi.org/10.1056/NEJMOA1713137
Peters S, Camidge DR, Shaw AT et al (2017) Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 377:829–838. https://doi.org/10.1056/NEJMOA1704795
Shaw AT, Ou S-HI, Bang Y-J et al (2014) Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 371:1963–1971. https://doi.org/10.1056/NEJMOA1406766
Reck M, Rodríguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 375:1823–1833. https://doi.org/10.1056/NEJMOA1606774
Garassino MC, Gadgeel S, Speranza G et al (2023) Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J Clin Oncol 41:1992–1998. https://doi.org/10.1200/JCO.22.01989
Hellmann MD, Paz-Ares L, Bernabe Caro R et al (2019) Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 381:2020–2031. https://doi.org/10.1056/NEJMOA1910231
De Oliveira BC, Lewis S, Sandschafer D et al (2023) Two decades of pegfilgrastim: what have we learned? Where do we go from here? Curr Med Res Opin 39:707–718. https://doi.org/10.1080/03007995.2023.2196197
Smith TJ, Bohlke K, Lyman GH et al (2015) Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 33:3199–3212. https://doi.org/10.1200/JCO.2015.62.3488
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Hematopoietic Growth Factors (Version 3. 2024). https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf. Accessed 2 Feb 2024
Klastersky J, de Naurois J, Rolston K et al (2016) Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann Oncol 27:v111–v118. https://doi.org/10.1093/ANNONC/MDW325
Aapro MS, Bohlius J, Cameron DA et al (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47:8–32. https://doi.org/10.1016/J.EJCA.2010.10.013
Mouri A, Kaira K, Shiono A et al (2019) Clinical significance of primary prophylactic pegylated-granulocyte-colony stimulating factor after the administration of ramucirumab plus docetaxel in patients with previously treated non-small cell lung cancer. Thorac Cancer 10:1005–1008. https://doi.org/10.1111/1759-7714.13022
Takase M, Shibata K, Iwasa K, et al (2019) Measures to Ensure Safety of Docetaxel plus Ramucirumab for Advanced Non-Small-Cell Lung Cancer as the Second- or Later-Line (Article in Japanese). In: Gan To Kagaku Ryoho. https://pubmed.ncbi.nlm.nih.gov/31273171/. Accessed 23 Nov 2023
Schiller JH, Kim K, Hutson P et al (1996) Phase II study of topotecan in patients with extensive-stage small-cell carcinoma of the lung: an Eastern Cooperative Oncology Group Trial. J Clin Oncol 14:2345–2352. https://doi.org/10.1200/JCO.1996.14.8.2345
Goto K, Sekine I, Nishiwaki Y et al (2004) Multi-institutional phase II trial of irinotecan, cisplatin, and etoposide for sensitive relapsed small-cell lung cancer. Br J Cancer 91:659–665. https://doi.org/10.1038/SJ.BJC.6602056
Hata A, Katakami N, Fujita S et al (2011) Amrubicin at a lower-dose with routine prophylactic use of granulocyte-colony stimulating factor for relapsed small-cell lung cancer. Lung Cancer 72:224–228. https://doi.org/10.1016/J.LUNGCAN.2010.08.009
Goto K, Ohe Y, Shibata T et al (2016) Combined chemotherapy with cisplatin, etoposide, and irinotecan versus topotecan alone as second-line treatment for patients with sensitive relapsed small-cell lung cancer (JCOG0605): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 17:1147–1157. https://doi.org/10.1016/S1470-2045(16)30104-8
Yamaguchi T, Kurita Y, Saito R et al (1994) Clinical effect of recombinant human G-CSF on neutropenia induced by chemotherapy in small-cell lung cancer patients. Biotherapy 8:1423–1429
Negoro Y, Yano R, Yoshimura M et al (2019) Influence of UGT1A1 polymorphism on etoposide plus platinum-induced neutropenia in Japanese patients with small-cell lung cancer. Int J Clin Oncol 24:256–261. https://doi.org/10.1007/S10147-018-1358-4
Yoh K, Hosomi Y, Kasahara K et al (2016) A randomized, double-blind, phase II study of ramucirumab plus docetaxel vs placebo plus docetaxel in Japanese patients with stage IV non-small cell lung cancer after disease progression on platinum-based therapy. Lung Cancer 99:186–193. https://doi.org/10.1016/J.LUNGCAN.2016.07.019
Acknowledgements
We thank Natsuki Narita for her support of our systematic review. We would like to thank Editage (www.editage.jp) for English language editing.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the conception and design of this study. E.I. wrote the draft of the manuscript, and all authors reviewed and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Eiki Ichihara received honoraria from Eli Lilly and research funding from MSD, Ono Pharmaceutical, Jansen Pharma, and Takeda Pharmaceutical. Go Makimoto received honoraria from Kyowa Kirin. Yukinori Ozaki received honoraria from Daiichi-Sankyo, Pfizer, Chugai, Lilly, and Kyowa Kirin. Kenji Tsuchihashi received honoraria from Ono Pharmaceutical, Chugai Pharmaceutical, Taiho Pharmaceutical, and Novartis Pharma. Yuji Miura received honoraria from Ono Pharmaceutical, MSD, Takeda Pharmaceuticals, Eisai, Bristol Myers Squibb, and research funding from Ono Pharmaceutical and MSD. Shingo Yano received research funding from Otsuka Pharmaceutical. Dai Maruyama received honoraria from Ono Pharmaceutical, Janssen Pharma, Nippon Shinyaku, Eisai, Mundipharma, Kyowa Kirin, Chugai Pharmaceutical, Zenyaku, MSD, SymBio, Sanofi, AbbVie, Takeda Pharmaceutical, AstraZeneca, Bristol Myers Squibb, and Genmab, and research funding from Amgen, Astellas Biopharma, Novartis, Kyowa Kirin, Ono Pharmaceutical, Chugai Pharmaceutical, Janssen Pharma, Takeda Pharmaceutical, Otsuka Pharmaceutical, Sanofi, Astellas, Bristol Myers Squibb, AbbVie, Eisai, MSD, Taiho Pharmaceutical, AstraZeneca, Eli Lilly, and Genmab. Tetsuhiro Yoshinami received honoraria from Kyowa Kirin, Pfizer, Chugai Pharmaceutical, Eli Lilly, MSD, AstraZeneca, and Eizai. Takashi Motohashi received honoraria from AstraZeneca, Chugai Pharmaceutical, and Myriad Genetics. Eishi Baba received honoraria from Chugai Pharmaceutical and Daiichi-Sankyo, and research funding from Taiho Pharmaceutical and Chugai Pharmaceutical. Takahiro Kimura received honoraria from Sanofi. Shinji Nakao received honoraria from Kyowa Kirin. Atsushi Sato received honoraria from Chugai Pharmaceutical, and Taiho Pharmaceutical. Atsushi Sato received research funding from Chugai Pharmaceutical, and Taiho Pharmaceutical. Toshimi Takano received honoraria from Daiichi-Sankyo, Chugai Pharmaceutical, and Eli Lilly. Toshio Kubo received honoraria from Chugai Pharmaceutical.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ichihara, E., Ochi, N., Makimoto, G. et al. Effectiveness and safety of primary prophylaxis with G-CSF for lung cancer: a systematic review and meta-analysis to develop clinical practice guidelines for the use of G-CSF 2022. Int J Clin Oncol 29, 355–362 (2024). https://doi.org/10.1007/s10147-024-02469-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-024-02469-4